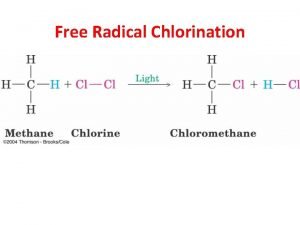
Free Radical Reactions Radical Reactions Chapter 10 A





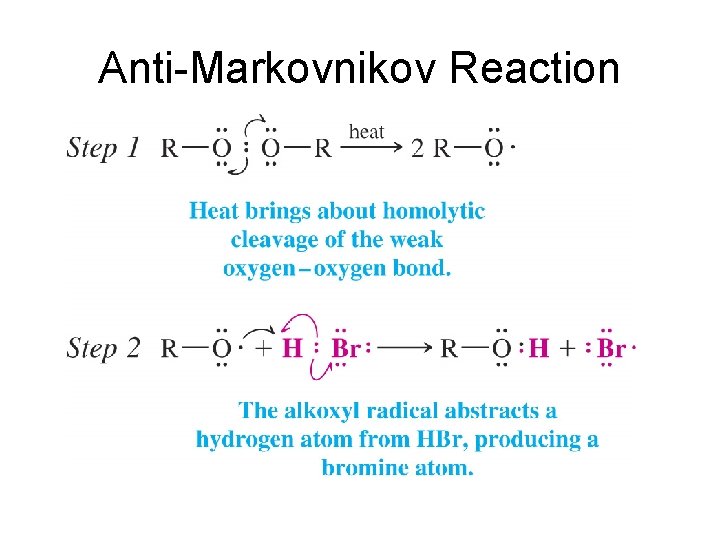


- Slides: 30

Free Radical Reactions

Radical Reactions Chapter 10 A. B. C. D. E. F. G. Description Causes of Free Radical Formation Importance in Biology, Medicine & Industry Mechanism Selectivity of Halogens Anti-Markovnikov Reaction Ozone Depletion & CFC’s

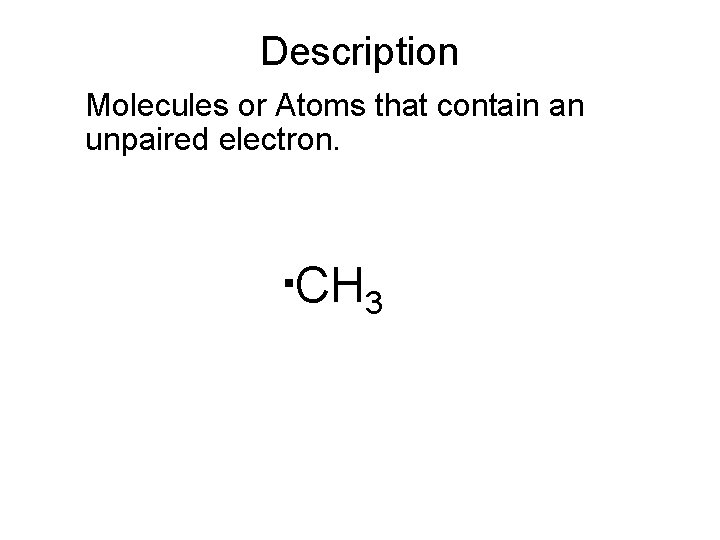
Description Molecules or Atoms that contain an unpaired electron. . CH 3

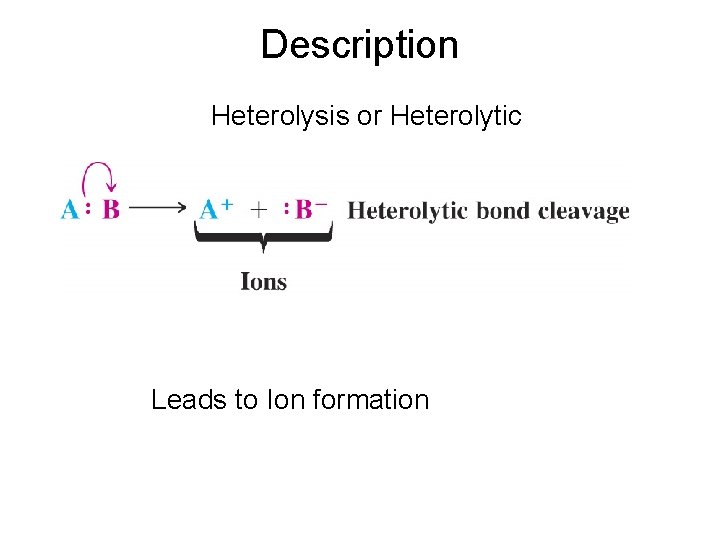
Description Heterolysis or Heterolytic Leads to Ion formation

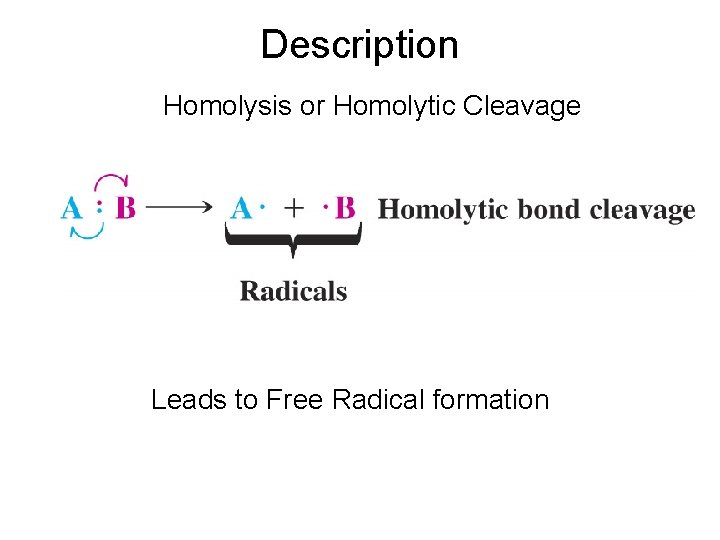
Description Homolysis or Homolytic Cleavage Leads to Free Radical formation

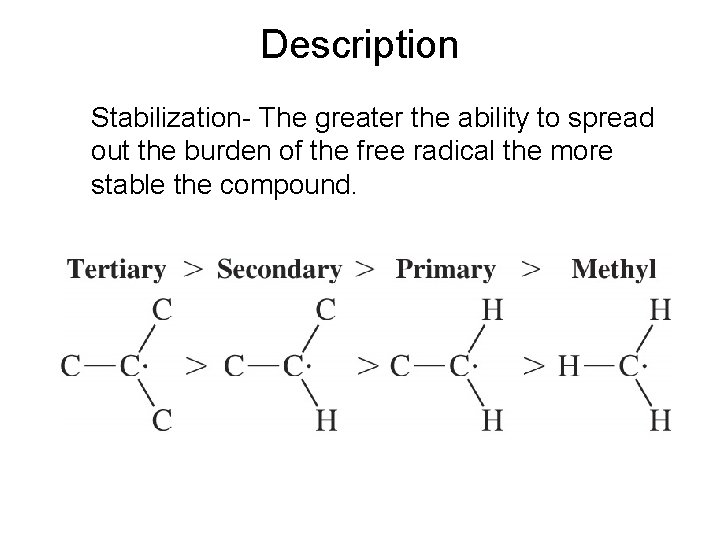
Description Stabilization- The greater the ability to spread out the burden of the free radical the more stable the compound.

Causes of Free Radical Formation • • • Metabolism Immune Response Combustion Radiation UV (Sunlight) Environmental Pollutants

Importance in Biology & Medicine • • Aging (Link 2) Cancer Emphysema Nitric Oxide – – Blood Pressure Regulation Blood Clotting Neurotransmission Immune Response • Metabolism • Wrinkles • Etc.

Importance in Biology & Medicine • Antioxidants - absorb free radicals – – – Vitamin E Vitamin C Beta-carotene Selenium Lycopene Melatonin (Link 2) Source: http: //www. biochem. wisc. edu/biochem 510/slides/20 antiox 1. pdf

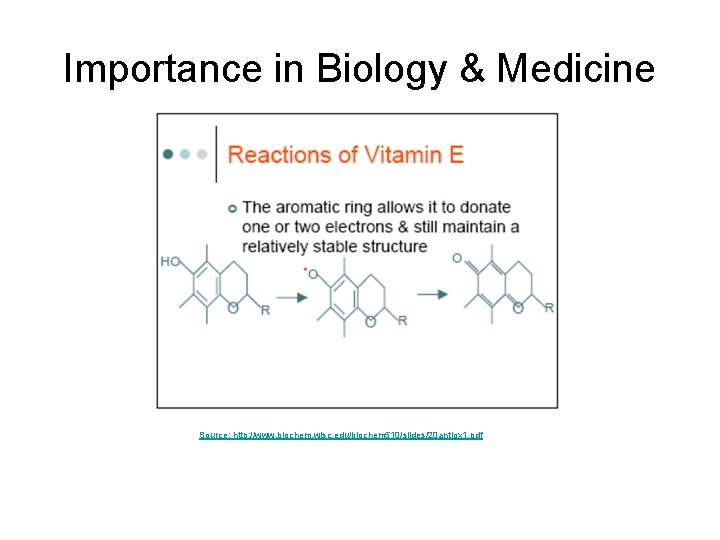
Importance in Biology & Medicine Source: http: //www. biochem. wisc. edu/biochem 510/slides/20 antiox 1. pdf

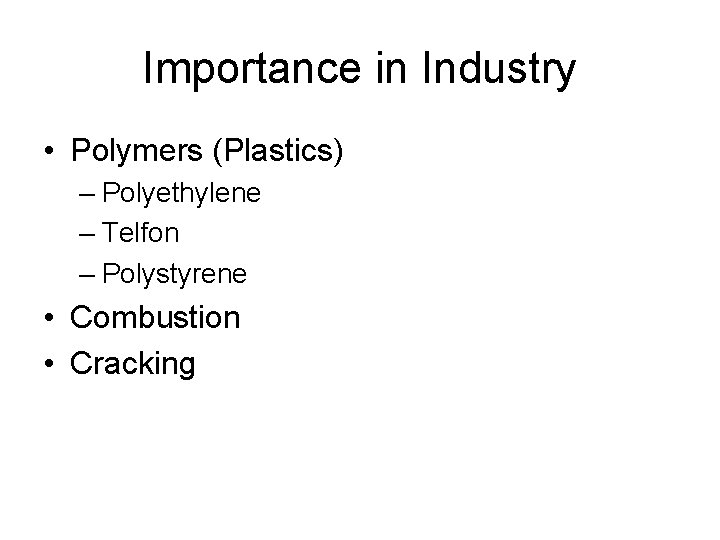
Importance in Industry • Polymers (Plastics) – Polyethylene – Telfon – Polystyrene • Combustion • Cracking

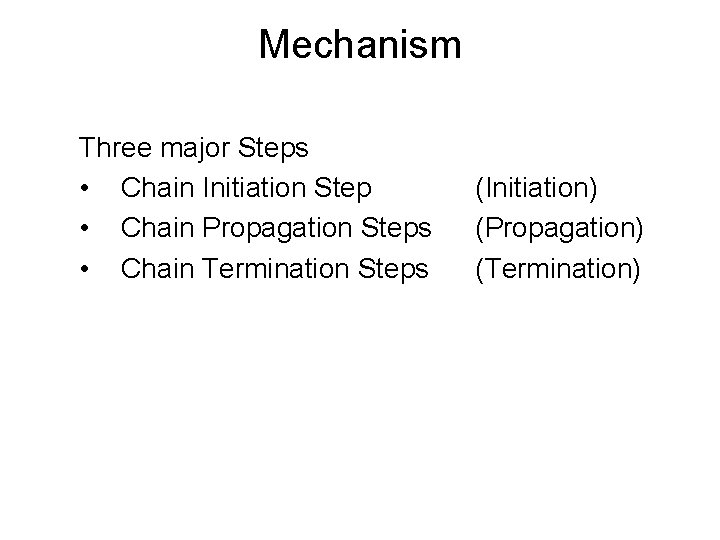
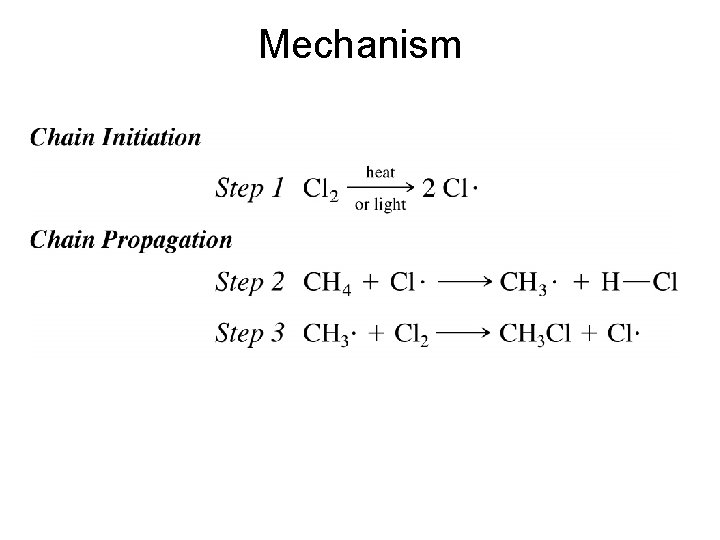
Mechanism Three major Steps • Chain Initiation Step • Chain Propagation Steps • Chain Termination Steps (Initiation) (Propagation) (Termination)

Mechanism

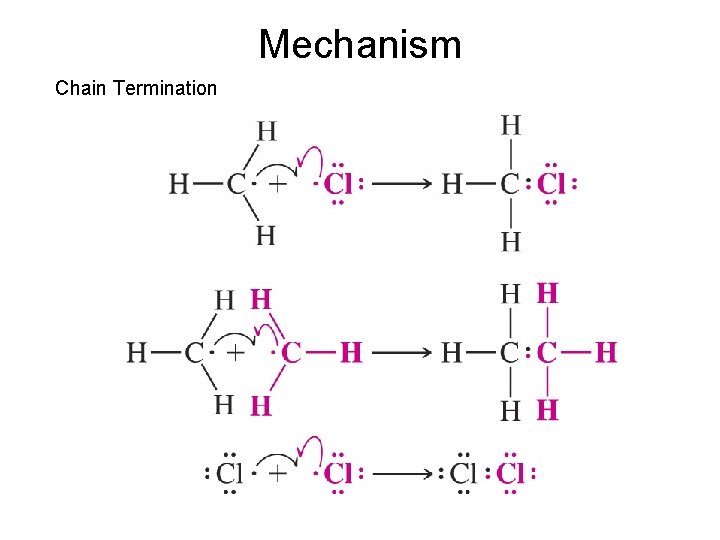
Mechanism Chain Termination

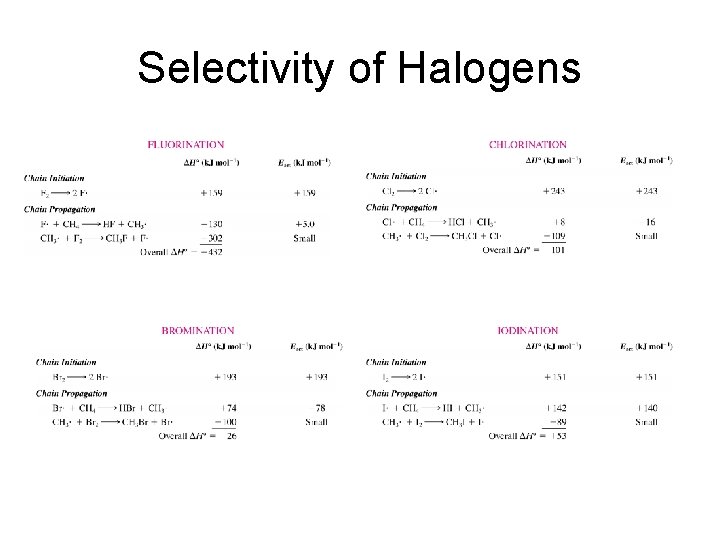
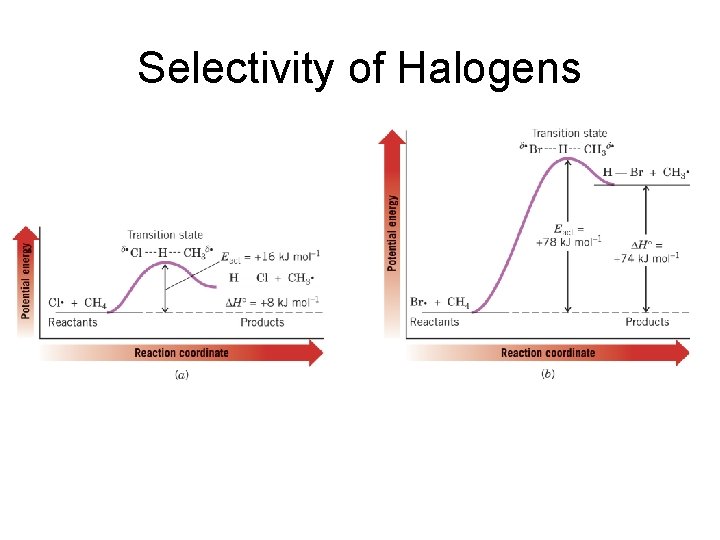
Selectivity of Halogens

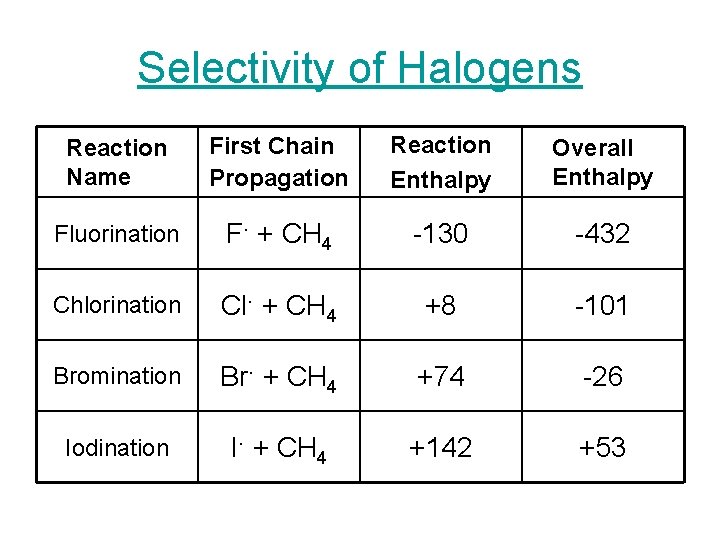
Selectivity of Halogens Reaction Name First Chain Propagation Reaction Enthalpy Overall Enthalpy Fluorination F. + CH 4 -130 -432 Chlorination Cl. + CH 4 +8 -101 Bromination Br. + CH 4 +74 -26 Iodination I. + CH 4 +142 +53

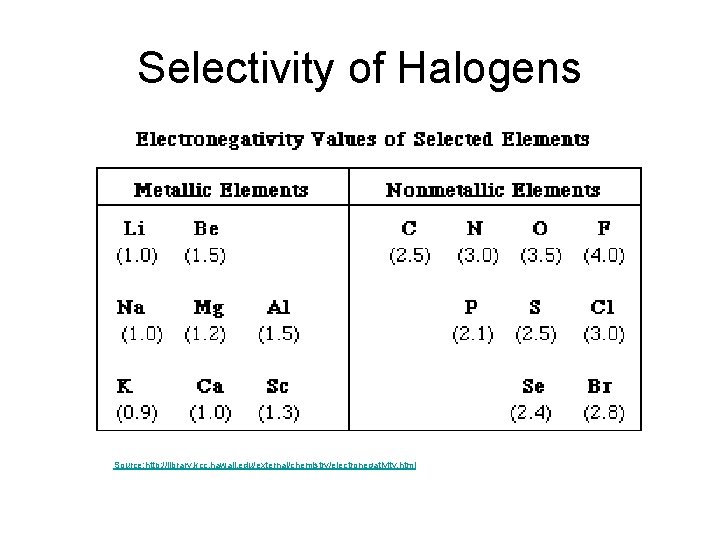
Selectivity of Halogens Source: http: //library. kcc. hawaii. edu/external/chemistry/electronegativity. html

Selectivity of Halogens • Reactivity – Fluorination > Chlorination > Bromination > Iodination • Higher the reactivity the lower the selectivity. • The decrease in electronegativity explains the decrease in reactivity and thus increase in selectivity.

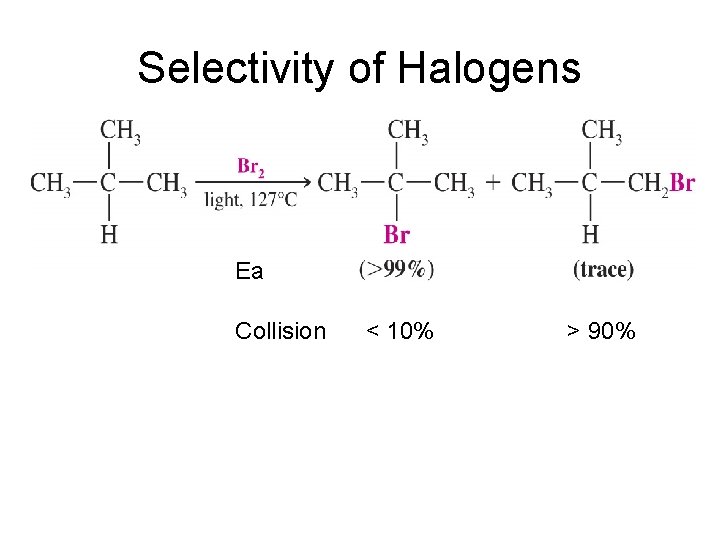
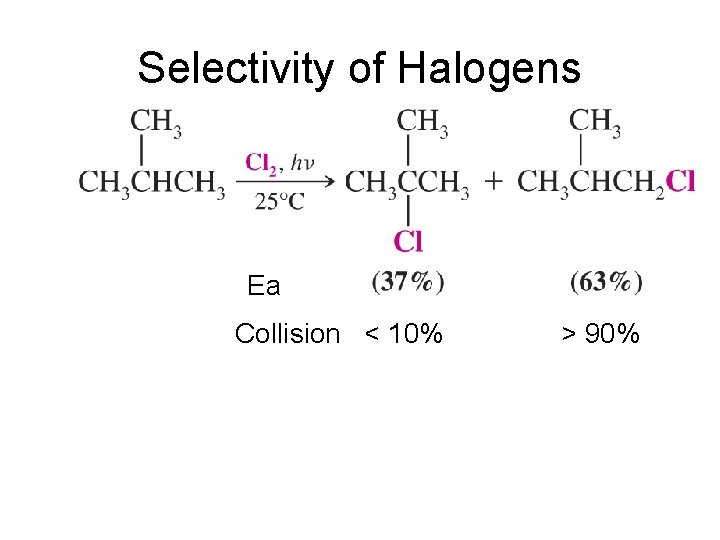
Selectivity of Halogens

Selectivity of Halogens Ea Collision < 10% > 90%

Selectivity of Halogens Ea Collision < 10% > 90%

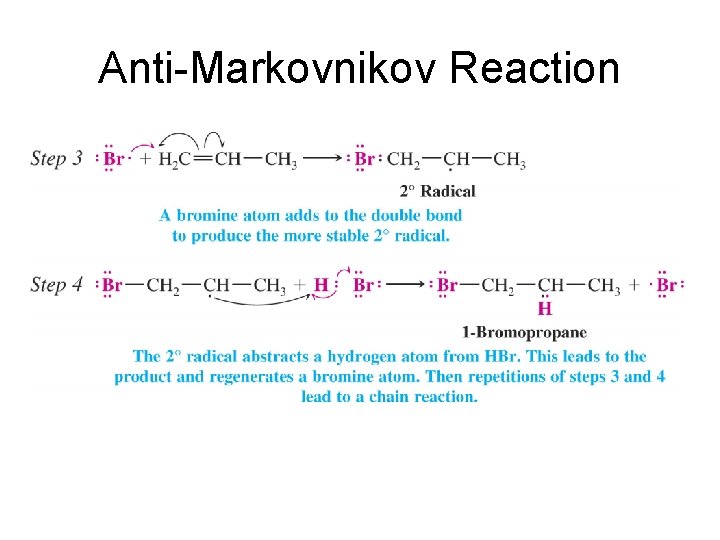
Anti-Markovnikov Reaction • Free Radical Reaction • Ea Driven Reaction
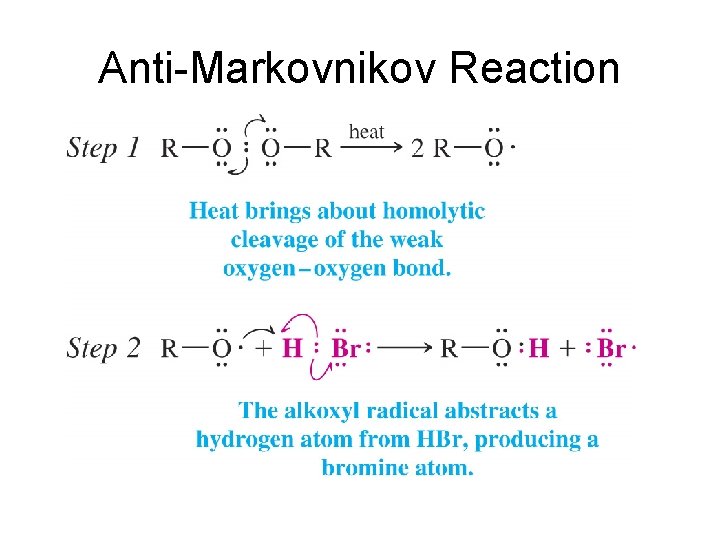
Anti-Markovnikov Reaction

Anti-Markovnikov Reaction

Markovnikov vs. Anti-Markovnikov • Both reactions are Ea driven • In Markovnikov addition the hydrogen adds first leading to the most stable carbocation. • In anti-Markovnikov addition the Bromine adds first leading to the most stable free radical.

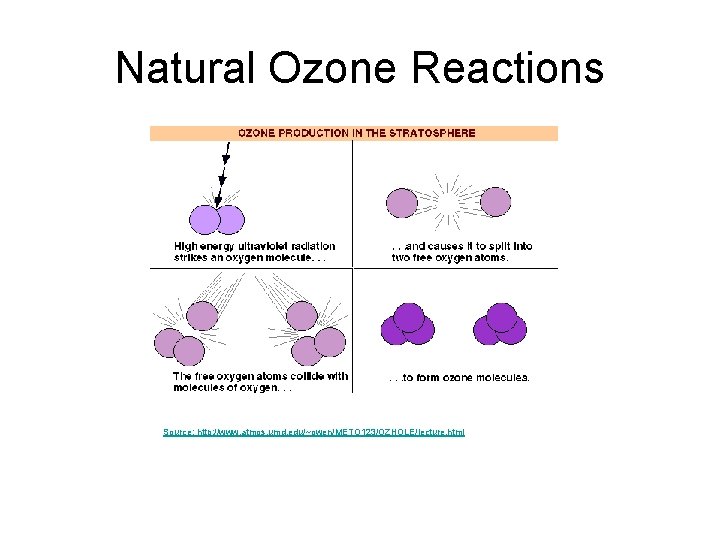
Natural Ozone Reactions Source: http: //www. atmos. umd. edu/~owen/METO 123/OZHOLE/lecture. html

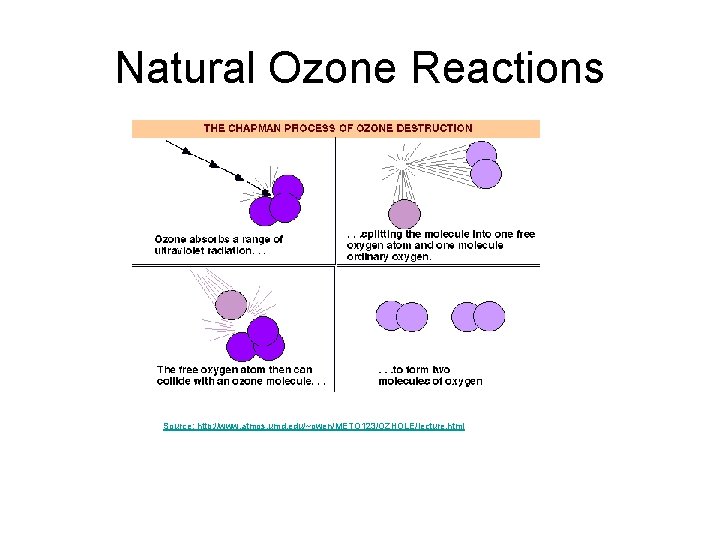
Natural Ozone Reactions Source: http: //www. atmos. umd. edu/~owen/METO 123/OZHOLE/lecture. html

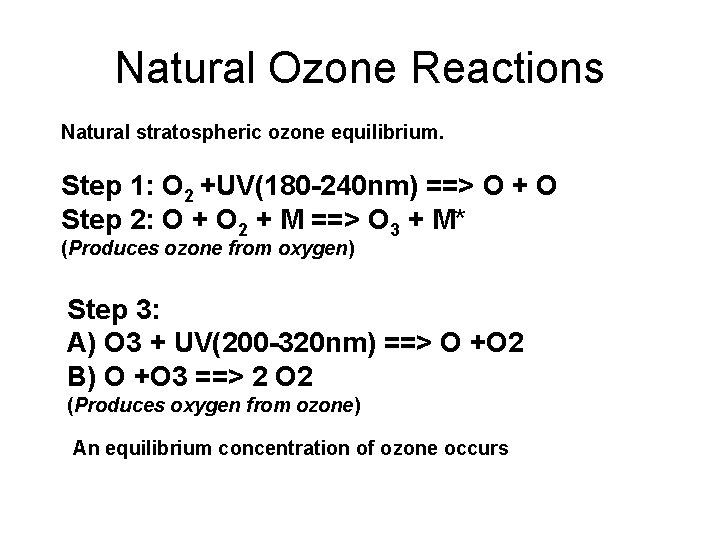
Natural Ozone Reactions Natural stratospheric ozone equilibrium. Step 1: O 2 +UV(180 -240 nm) ==> O + O Step 2: O + O 2 + M ==> O 3 + M* (Produces ozone from oxygen) Step 3: A) O 3 + UV(200 -320 nm) ==> O +O 2 B) O +O 3 ==> 2 O 2 (Produces oxygen from ozone) An equilibrium concentration of ozone occurs

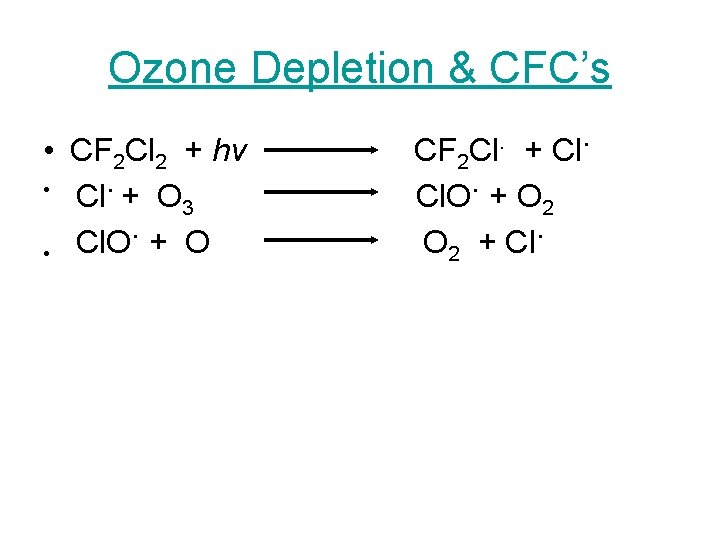
Ozone Depletion & CFC’s • CF 2 Cl 2 + hv • Cl. + O 3. • Cl. O + O Cl. . + Cl CF 2 Cl. O. + O 2 + Cl

Problems (p. 459, 460, 461) • • 10. 2 10. 4 10. 6 10. 8
 Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions How to write redox half reactions
How to write redox half reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers What is a entire radical
What is a entire radical Unit 6 test radical functions
Unit 6 test radical functions Entire radical
Entire radical Cl free radical
Cl free radical Radical substitution
Radical substitution Antioxidant free radical
Antioxidant free radical Reaction of propane with chlorine
Reaction of propane with chlorine Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key Chapter 9 chemical reactions test answers
Chapter 9 chemical reactions test answers Gibbs free energy and spontaneity
Gibbs free energy and spontaneity Gibbs free energy equation
Gibbs free energy equation Gibbs free energy unit
Gibbs free energy unit A free map determines which blocks are free, allocated.
A free map determines which blocks are free, allocated. Helmholtz free energy
Helmholtz free energy Free hearts free foreheads you and i are
Free hearts free foreheads you and i are That was then this is now summary
That was then this is now summary Free free absorption
Free free absorption Chapter 19 redox reactions answers
Chapter 19 redox reactions answers Chapter 9 chemical reactions
Chapter 9 chemical reactions Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 Chemical reactions chapter 9 study guide
Chemical reactions chapter 9 study guide Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Balancing equations chapter 8
Balancing equations chapter 8 Reducing agent strength table
Reducing agent strength table Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions.