A Reactions Review 1 2 3 FREE RADICAL




- Slides: 17

A Reactions Review 1. 2. 3. FREE RADICAL SUBSTITUTION POLYMERIZATION ELECTROPHILIC ADDITION ( types) A) Hydration (add water, HOH) B) Halogenation (add H 2) C) Bromination (add Br 2) D) Hydrohalogenation (add HCl, HBr, HI (acids) 4. ELIMINATION (Dehydrohalogenation) 5. NUCLEOPHILIC SUBSTITUTION (add : OH-, : CN-, H 2 O, 2: NH 3) 6. OXIDATION A) 10 ALCOHOLS TO ALDEHYDES THEN TO CARBOXYLIC ACIDS B) 20 ALCOHOLS TO KETONES

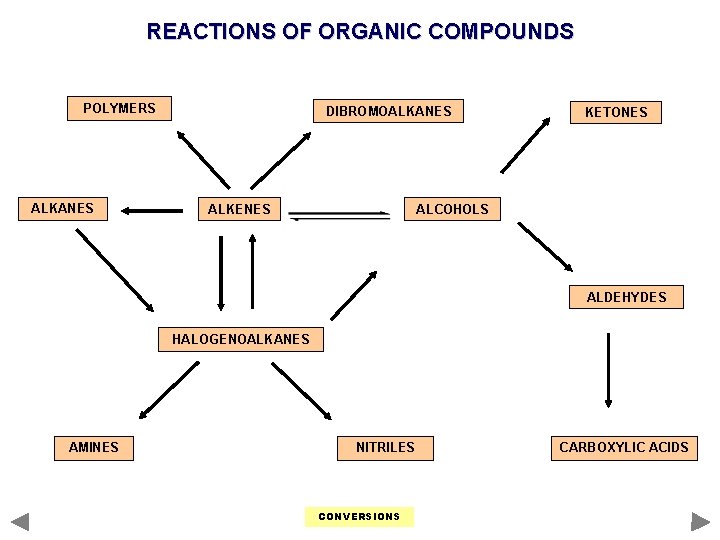
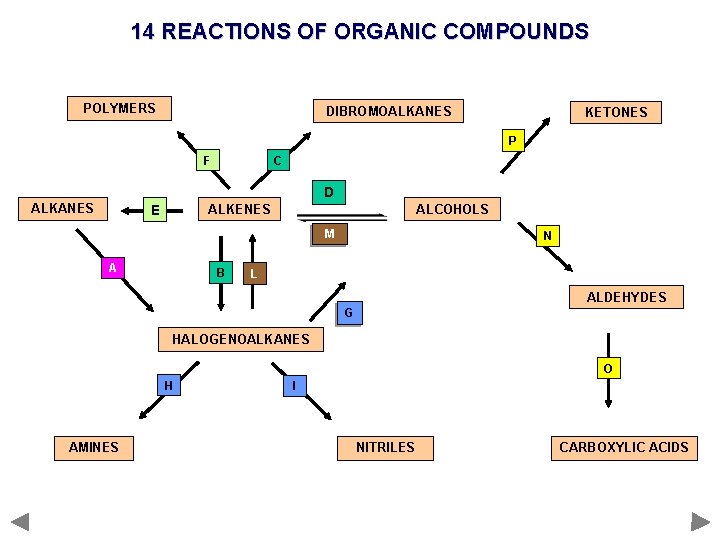
REACTIONS OF ORGANIC COMPOUNDS POLYMERS ALKANES DIBROMOALKANES ALKENES KETONES ALCOHOLS ALDEHYDES HALOGENOALKANES AMINES NITRILES CONVERSIONS CARBOXYLIC ACIDS

14 REACTIONS OF ORGANIC COMPOUNDS POLYMERS DIBROMOALKANES KETONES P F C D ALKANES ALKENES E ALCOHOLS M A B N L ALDEHYDES G HALOGENOALKANES O H AMINES I NITRILES CARBOXYLIC ACIDS

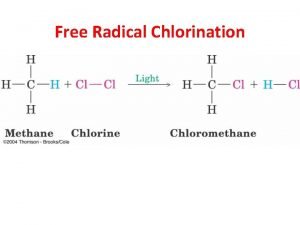
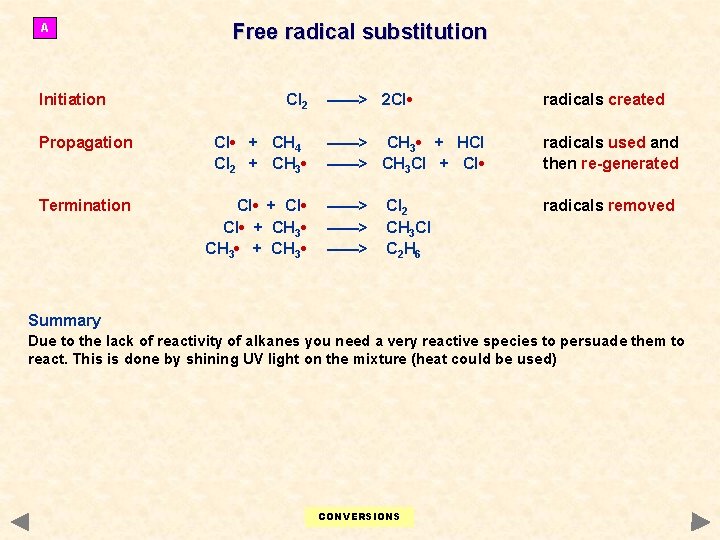
A Initiation Free radical substitution Cl 2 Propagation Cl • + CH 4 Cl 2 + CH 3 • Termination Cl • + CH 3 • + CH 3 • ——> 2 Cl • radicals created ——> CH 3 • + HCl ——> CH 3 Cl + Cl • radicals used and then re-generated ——> ——> radicals removed Cl 2 CH 3 Cl C 2 H 6 Summary Due to the lack of reactivity of alkanes you need a very reactive species to persuade them to react. This is done by shining UV light on the mixture (heat could be used) CONVERSIONS

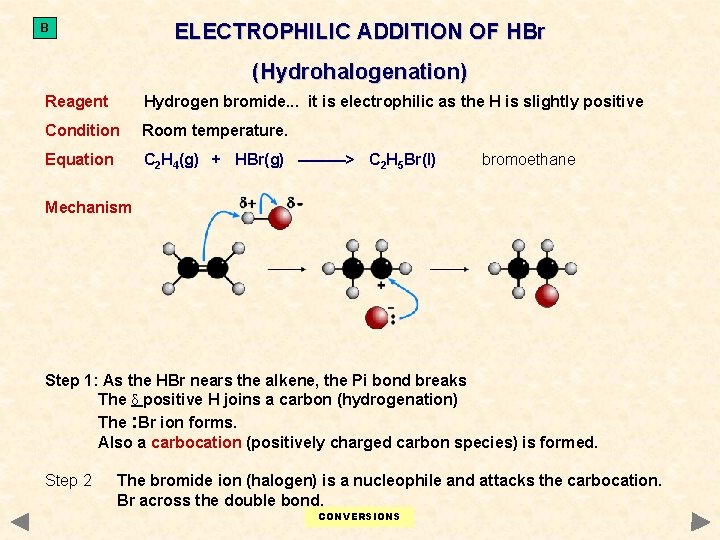
ELECTROPHILIC ADDITION OF HBr B (Hydrohalogenation) Reagent Hydrogen bromide. . . it is electrophilic as the H is slightly positive Condition Room temperature. Equation C 2 H 4(g) + HBr(g) ———> C 2 H 5 Br(l) bromoethane Mechanism Step 1: As the HBr nears the alkene, the Pi bond breaks The δ positive H joins a carbon (hydrogenation) The : Br ion forms. Also a carbocation (positively charged carbon species) is formed. Step 2 The bromide ion (halogen) is a nucleophile and attacks the carbocation. Br across the double bond. CONVERSIONS

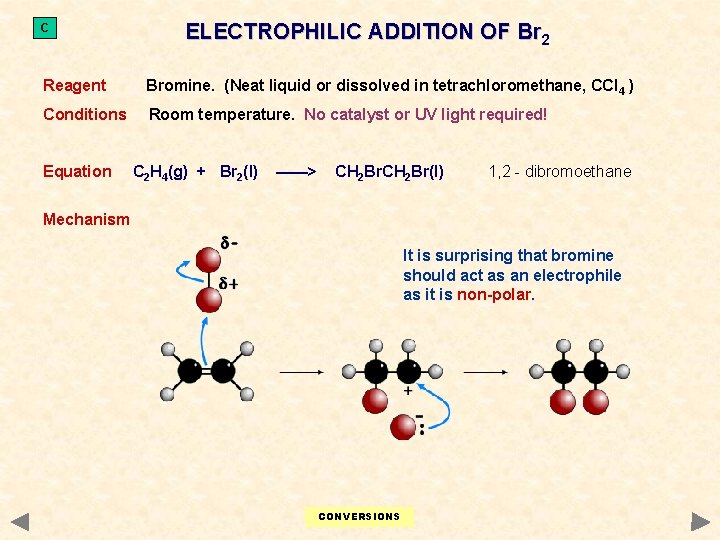
C ELECTROPHILIC ADDITION OF Br 2 Reagent Bromine. (Neat liquid or dissolved in tetrachloromethane, CCl 4 ) Conditions Room temperature. No catalyst or UV light required! Equation C 2 H 4(g) + Br 2(l) ——> CH 2 Br(l) 1, 2 - dibromoethane Mechanism It is surprising that bromine should act as an electrophile as it is non-polar. CONVERSIONS

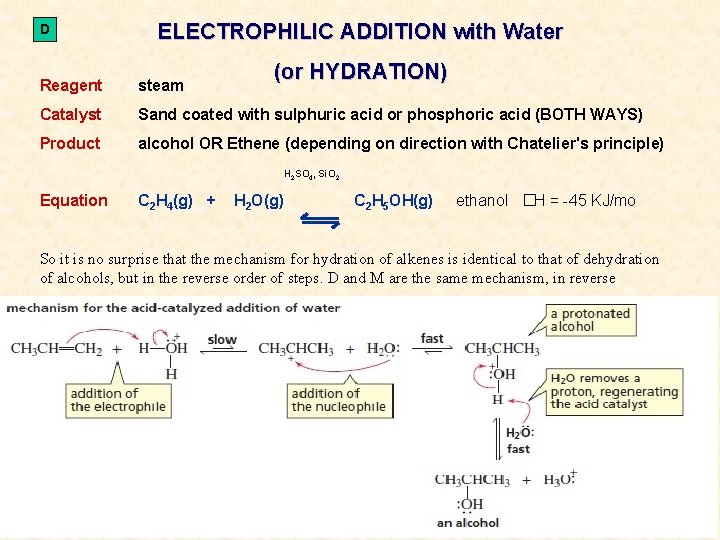
D ELECTROPHILIC ADDITION with Water (or HYDRATION) Reagent steam Catalyst Sand coated with sulphuric acid or phosphoric acid (BOTH WAYS) Product alcohol OR Ethene (depending on direction with Chatelier's principle) H 2 SO 4, Si. O 2 Equation C 2 H 4(g) + H 2 O(g) C 2 H 5 OH(g) ethanol �H = -45 KJ/mo So it is no surprise that the mechanism for hydration of alkenes is identical to that of dehydration of alcohols, but in the reverse order of steps. D and M are the same mechanism, in reverse CONVERSIONS

M ELIMINATION OF WATER (DEHYDRATION) Reagent/catalyst sulphuric acid (H 2 SO 4) or conc. Conditions reflux at 180°C Equation e. g. C 2 H 5 OH(l) CH 2 = CH 2(g) + H 2 O(l) So it is no surprise that the mechanism for hydration of alkenes is identical (as it is a REVERSABLE reaction) to that of dehydration of alcohols, but in the reverse order of steps. D and M are the same mechanism, in reverse Mechanism CONVERSIONS

ELECTROPHILIC ADDITION OF H 2 E or HYDROGENATION Reagent Conditions Product Equation hydrogen nickel catalyst - finely divided alkanes C 2 H 4(g) + H 2(g) ———> C 2 H 6(g) ethane Ni Use margarine manufacture (Hydrogenated fats for spreading on bread) CONVERSIONS

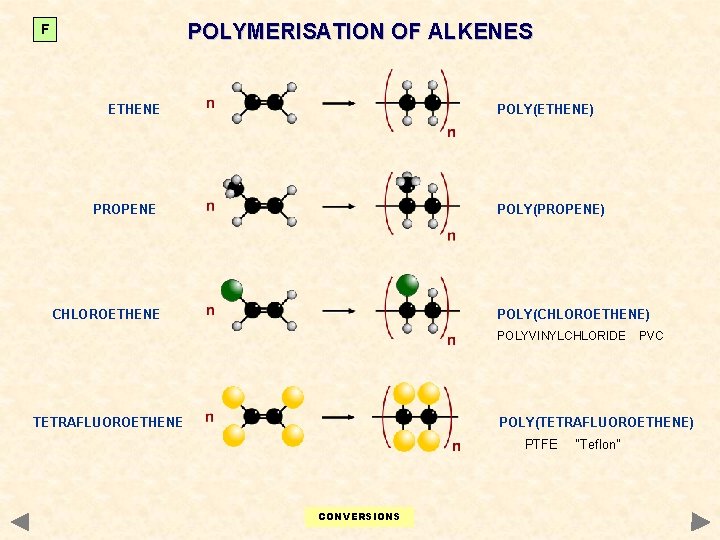
POLYMERISATION OF ALKENES F ETHENE POLY(ETHENE) PROPENE POLY(PROPENE) CHLOROETHENE POLY(CHLOROETHENE) POLYVINYLCHLORIDE TETRAFLUOROETHENE PVC POLY(TETRAFLUOROETHENE) PTFE CONVERSIONS “Teflon”

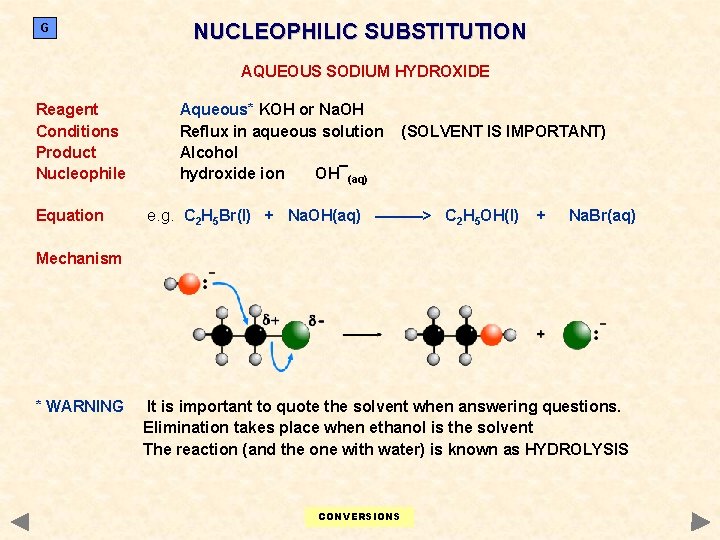
G NUCLEOPHILIC SUBSTITUTION AQUEOUS SODIUM HYDROXIDE Reagent Conditions Product Nucleophile Equation Aqueous* KOH or Na. OH Reflux in aqueous solution Alcohol hydroxide ion OH¯(aq) (SOLVENT IS IMPORTANT) e. g. C 2 H 5 Br(l) + Na. OH(aq) ———> C 2 H 5 OH(l) + Na. Br(aq) Mechanism * WARNING It is important to quote the solvent when answering questions. Elimination takes place when ethanol is the solvent The reaction (and the one with water) is known as HYDROLYSIS CONVERSIONS

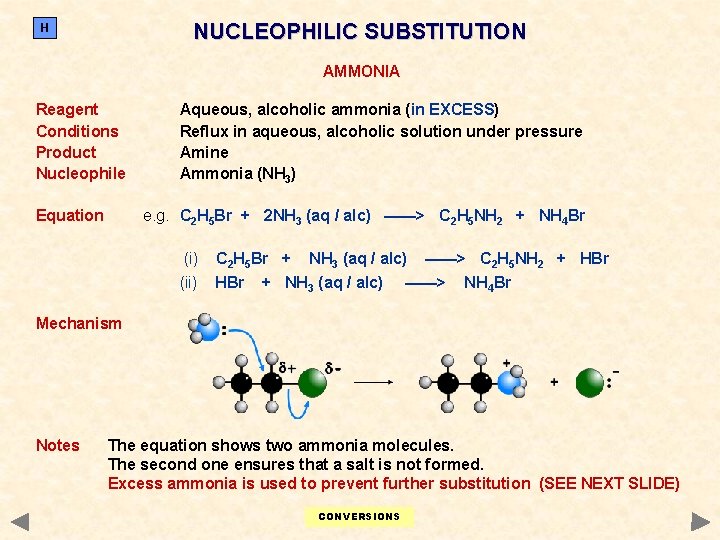
NUCLEOPHILIC SUBSTITUTION H AMMONIA Reagent Conditions Product Nucleophile Equation Aqueous, alcoholic ammonia (in EXCESS) Reflux in aqueous, alcoholic solution under pressure Amine Ammonia (NH 3) e. g. C 2 H 5 Br + 2 NH 3 (aq / alc) ——> C 2 H 5 NH 2 + NH 4 Br (i) C 2 H 5 Br + (ii) HBr NH 3 (aq / alc) + NH 3 (aq / alc) ——> C 2 H 5 NH 2 + HBr ——> NH 4 Br Mechanism Notes The equation shows two ammonia molecules. The second one ensures that a salt is not formed. Excess ammonia is used to prevent further substitution (SEE NEXT SLIDE) CONVERSIONS

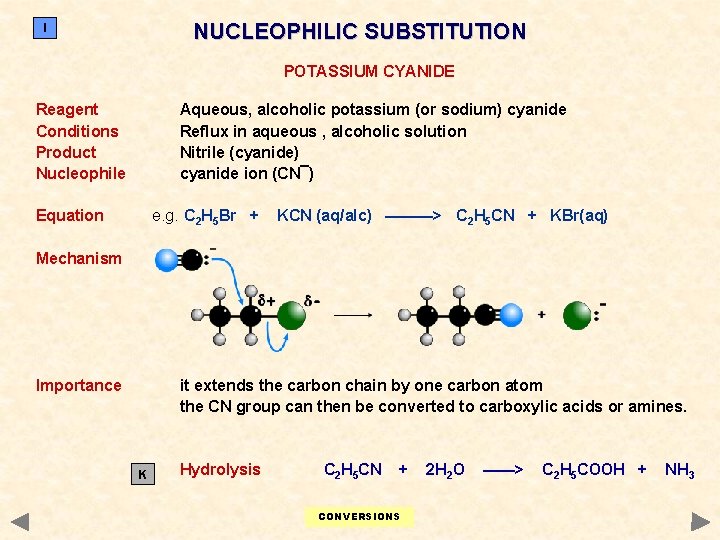
NUCLEOPHILIC SUBSTITUTION I POTASSIUM CYANIDE Reagent Conditions Product Nucleophile Aqueous, alcoholic potassium (or sodium) cyanide Reflux in aqueous , alcoholic solution Nitrile (cyanide) cyanide ion (CN¯) Equation e. g. C 2 H 5 Br + KCN (aq/alc) ———> C 2 H 5 CN + KBr(aq) Mechanism Importance it extends the carbon chain by one carbon atom the CN group can then be converted to carboxylic acids or amines. K Hydrolysis C 2 H 5 CN + CONVERSIONS 2 H 2 O ——> C 2 H 5 COOH + NH 3

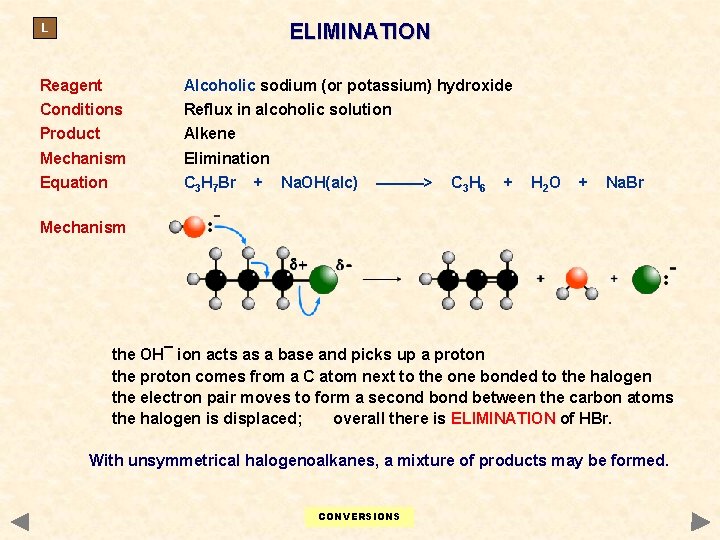
ELIMINATION L Reagent Alcoholic sodium (or potassium) hydroxide Conditions Reflux in alcoholic solution Product Alkene Mechanism Elimination Equation C 3 H 7 Br + Na. OH(alc) ———> C 3 H 6 + H 2 O + Na. Br Mechanism the OH¯ ion acts as a base and picks up a proton the proton comes from a C atom next to the one bonded to the halogen the electron pair moves to form a second between the carbon atoms the halogen is displaced; overall there is ELIMINATION of HBr. With unsymmetrical halogenoalkanes, a mixture of products may be formed. CONVERSIONS

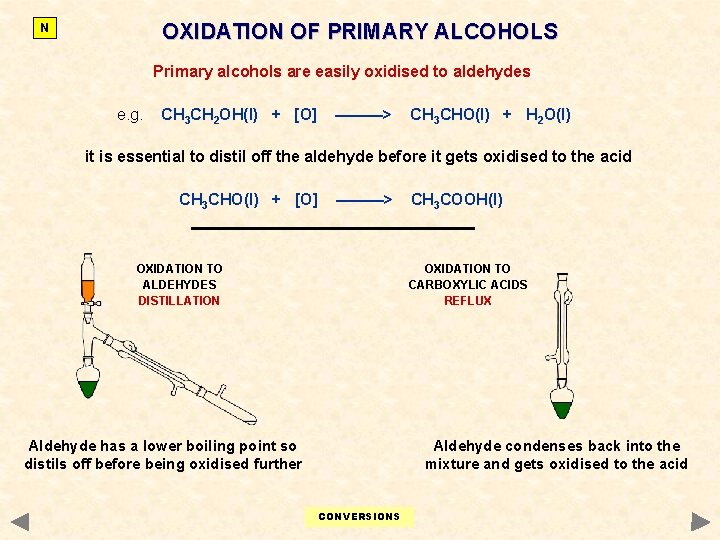
OXIDATION OF PRIMARY ALCOHOLS N Primary alcohols are easily oxidised to aldehydes e. g. CH 3 CH 2 OH(l) + [O] ———> CH 3 CHO(l) + H 2 O(l) it is essential to distil off the aldehyde before it gets oxidised to the acid CH 3 CHO(l) + [O] ———> OXIDATION TO ALDEHYDES DISTILLATION CH 3 COOH(l) OXIDATION TO CARBOXYLIC ACIDS REFLUX Aldehyde has a lower boiling point so distils off before being oxidised further Aldehyde condenses back into the mixture and gets oxidised to the acid CONVERSIONS

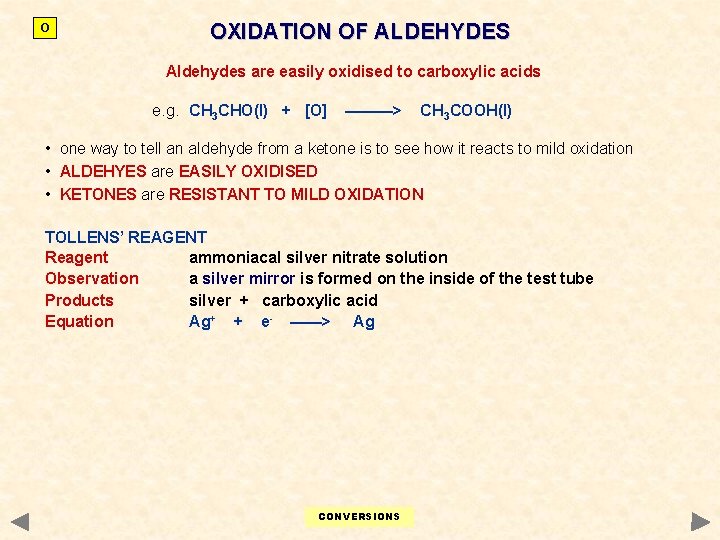
O OXIDATION OF ALDEHYDES Aldehydes are easily oxidised to carboxylic acids e. g. CH 3 CHO(l) + [O] ———> CH 3 COOH(l) • one way to tell an aldehyde from a ketone is to see how it reacts to mild oxidation • ALDEHYES are EASILY OXIDISED • KETONES are RESISTANT TO MILD OXIDATION TOLLENS’ REAGENT Reagent ammoniacal silver nitrate solution Observation a silver mirror is formed on the inside of the test tube Products silver + carboxylic acid Equation Ag+ + e- ——> Ag CONVERSIONS

P OXIDATION OF SECONDARY ALCOHOLS Secondary alcohols are easily oxidised to ketones e. g. CH 3 CHOHCH 3(l) + [O] ———> CH 3 COCH 3(l) + H 2 O(l) The alcohol is refluxed with acidified K 2 Cr 2 O 7. However, on prolonged treatment with a powerful oxidising agent they can be further oxidised to a mixture of acids with fewer carbon atoms than the original alcohol. CONVERSIONS
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Basic redox reactions
Basic redox reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Types of reactions
Types of reactions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Entire radical to mixed radical
Entire radical to mixed radical Unit 6 test radical functions
Unit 6 test radical functions Entire radical examples
Entire radical examples Free radical substitution propagation
Free radical substitution propagation Radical substitution
Radical substitution Antioxidant free radical
Antioxidant free radical Free radical halogenation
Free radical halogenation Chapter 19 redox reactions answers
Chapter 19 redox reactions answers Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chemical equations and reactions chapter 8
Chemical equations and reactions chapter 8 Chapter 19 review oxidation reduction reactions answers
Chapter 19 review oxidation reduction reactions answers Gibbs free energy vs standard free energy
Gibbs free energy vs standard free energy Gibbs energy and equilibrium
Gibbs energy and equilibrium