Four types of AA deamination transamination oxidative deamination







- Slides: 42


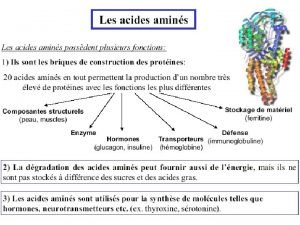
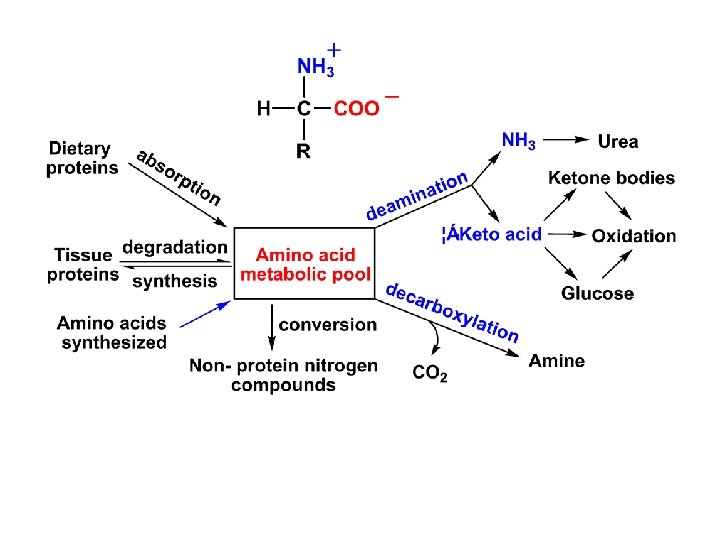
Four types of AA deamination: transamination oxidative deamination union deamination non-oxidative deamination


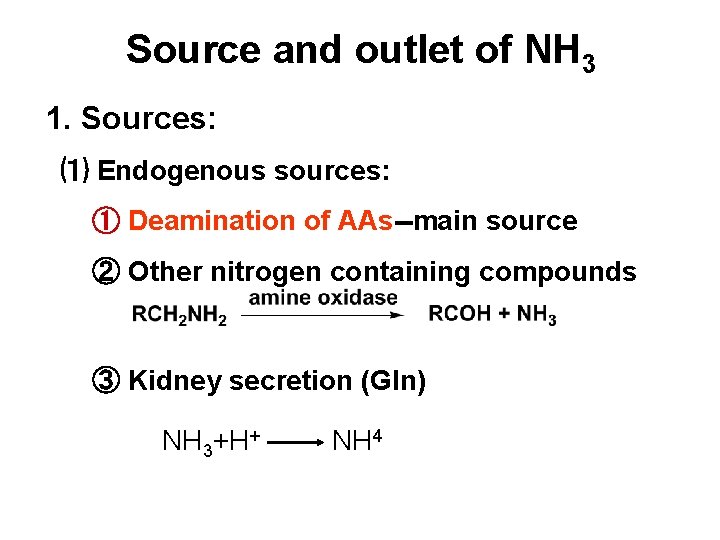
Source and outlet of NH 3 1. Sources: ⑴ Endogenous sources: ① Deamination of AAs--main source ② Other nitrogen containing compounds ③ Kidney secretion (Gln) NH 3+H+ NH 4

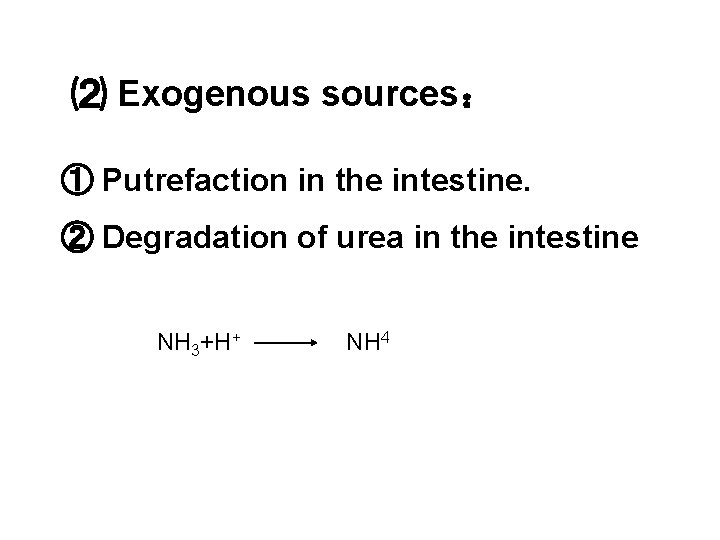
⑵ Exogenous sources: ① Putrefaction in the intestine. ② Degradation of urea in the intestine NH 3+H+ NH 4

2. Outlets: (1) Formation of urea (2) Formation of Gln (3) Excrete in urine (4) Synthesis of AA

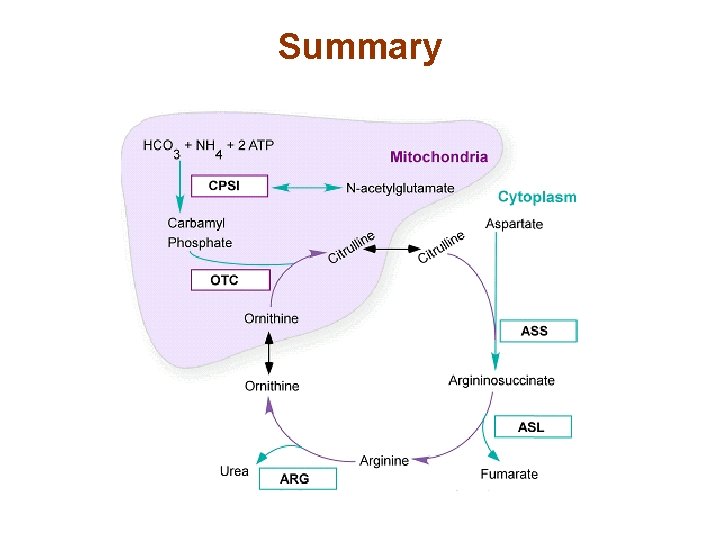
Summary

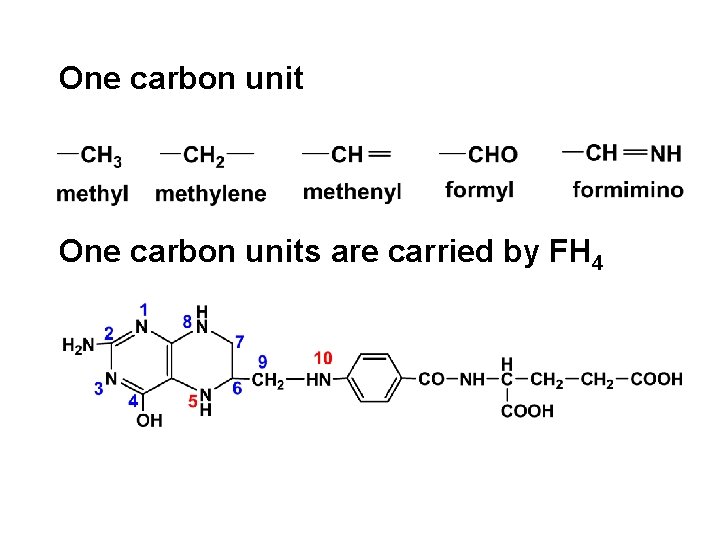
One carbon units are carried by FH 4

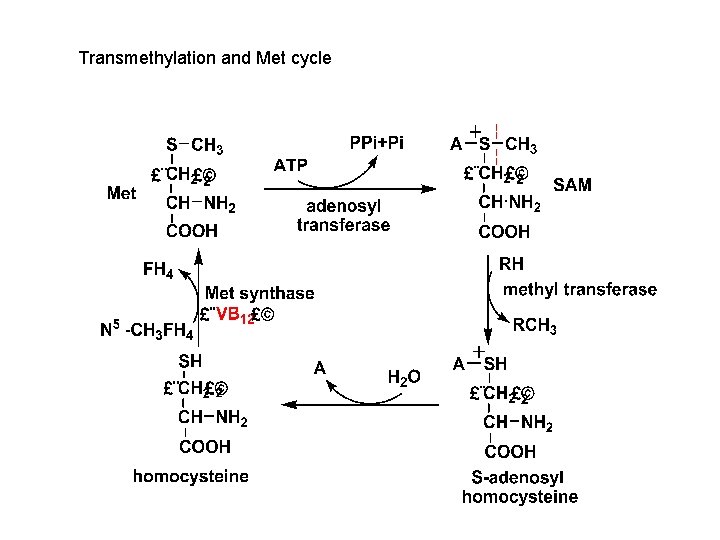
Transmethylation and Met cycle

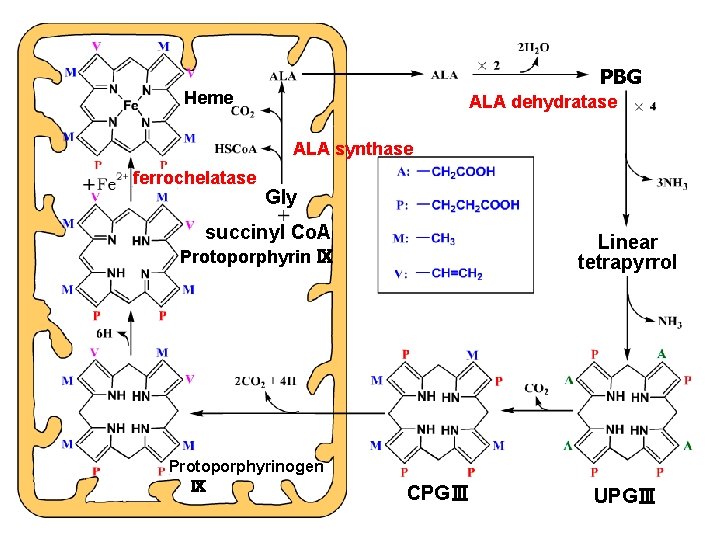
PBG Heme ALA dehydratase ALA synthase ferrochelatase Gly succinyl Co. A Linear tetrapyrrol Protoporphyrin Ⅸ Protoporphyrinogen Ⅸ CPGⅢ UPGⅢ

coenzyme -pyridoxal phosphate ① ALA synthase ② decarboxylase ③ transaminase

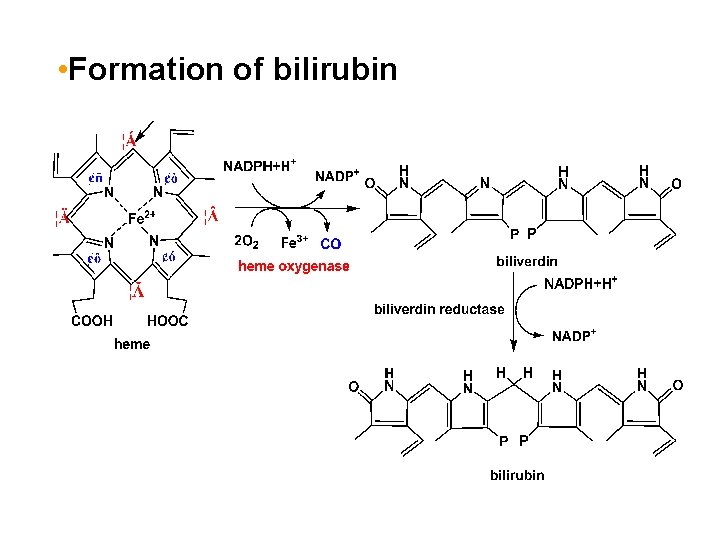
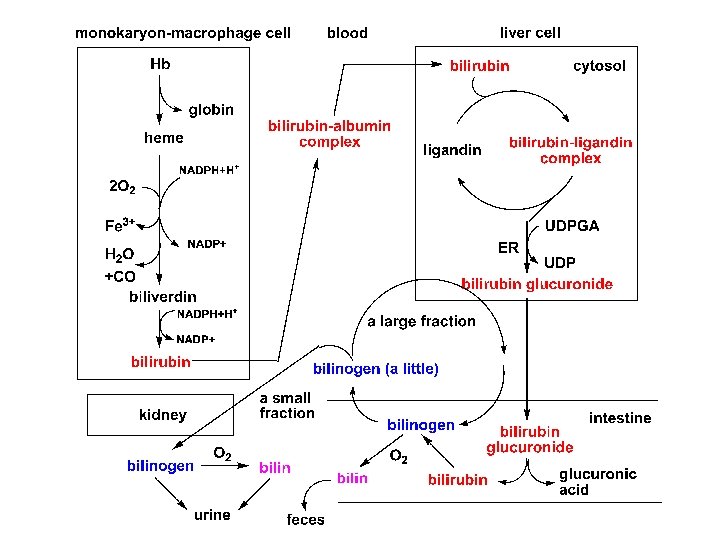
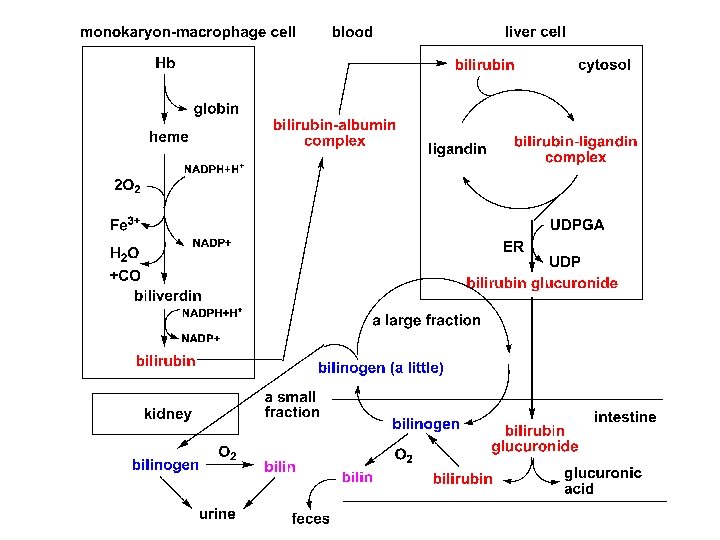
1. Formation and transport of bilirubin *The source of bilirubin The compounds involving iron prophyrin in the body are hemoglobin, myoglobin, cytochrome, peroxidase, and catalase, etc.

• Formation of bilirubin

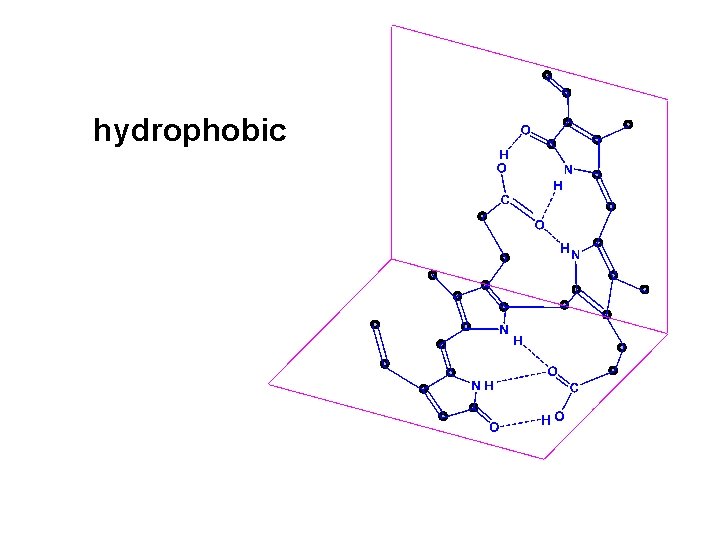
hydrophobic


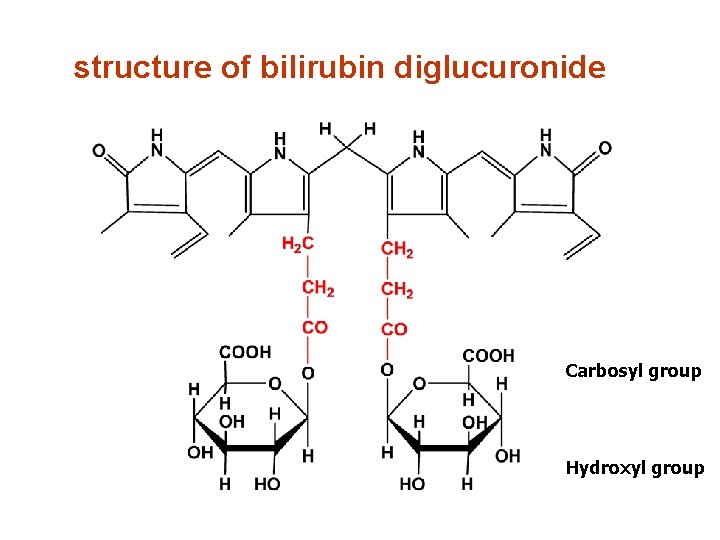
structure of bilirubin diglucuronide Carbosyl group Hydroxyl group

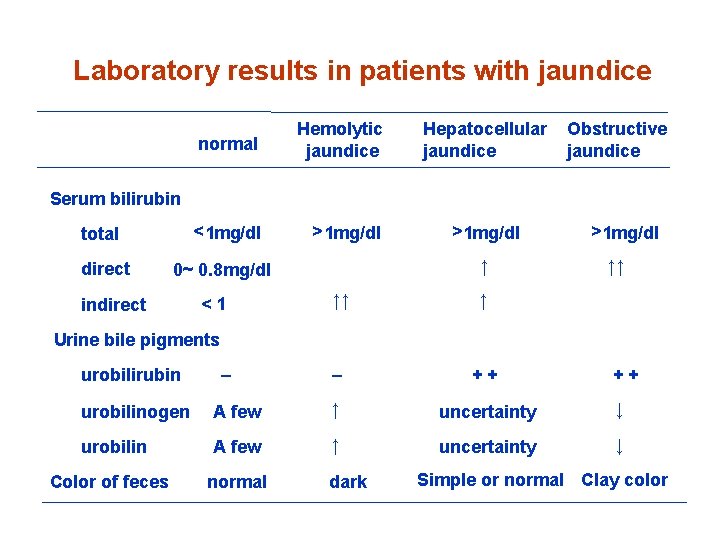
Jaundice • Hemolytic (prehepatic) jaundice • Hepatocellular (hepatic) jaundice • Obstructive (posthepatic) jaundice

Laboratory results in patients with jaundice normal Hemolytic jaundice < 1 mg/dl > 1 mg/dl Hepatocellular jaundice Obstructive jaundice Serum bilirubin total direct ↑ 0~ 0. 8 mg/dl indirect <1 > 1 mg/dl ↑↑ ↑ – ++ > 1 mg/dl ↑↑ Urine bile pigments urobilirubin – ++ urobilinogen A few ↑ uncertainty ↓ urobilin A few ↑ uncertainty ↓ Color of feces normal dark Simple or normal Clay color


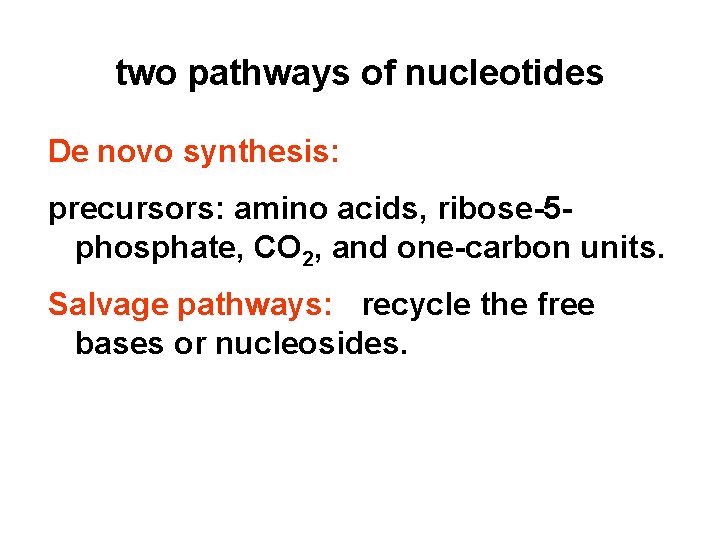
two pathways of nucleotides De novo synthesis: precursors: amino acids, ribose-5 phosphate, CO 2, and one-carbon units. Salvage pathways: recycle the free bases or nucleosides.

Purine De novo synthesis a. Purines are synthesized using 5 phosphoribose as the starting material step by step. b. PRPP is active donor of R-5 -P. c. AMP and GMP are synthesized further at the base of IMP.

Pyrimidine De novo synthesis • The pyrimidine ring is first synthesized, then combines with PRPP. • UMP is first synthesized, then UMP is used for synthesizing other pyrimidine nucleotides.

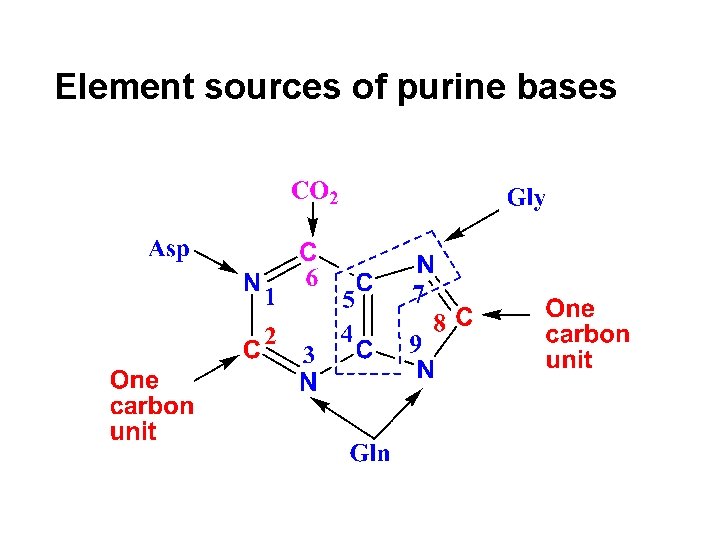
Element sources of purine bases

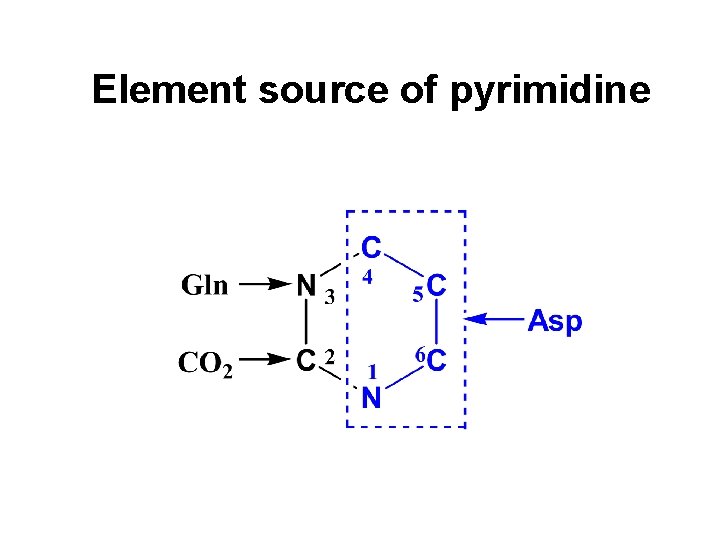
Element source of pyrimidine

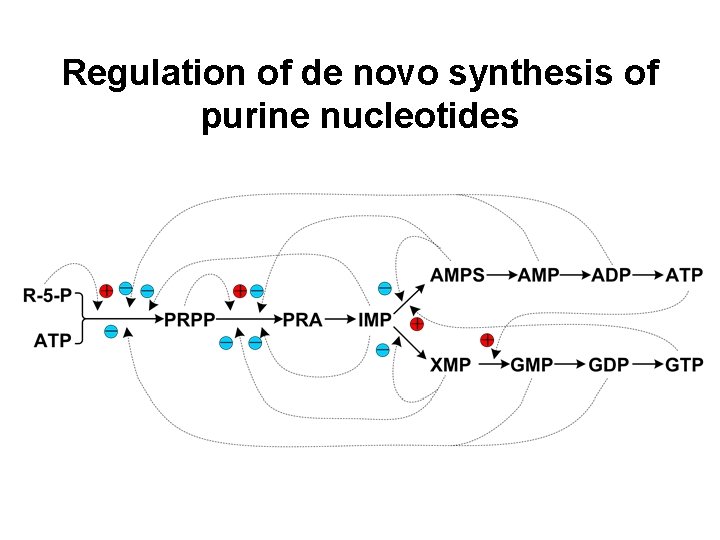
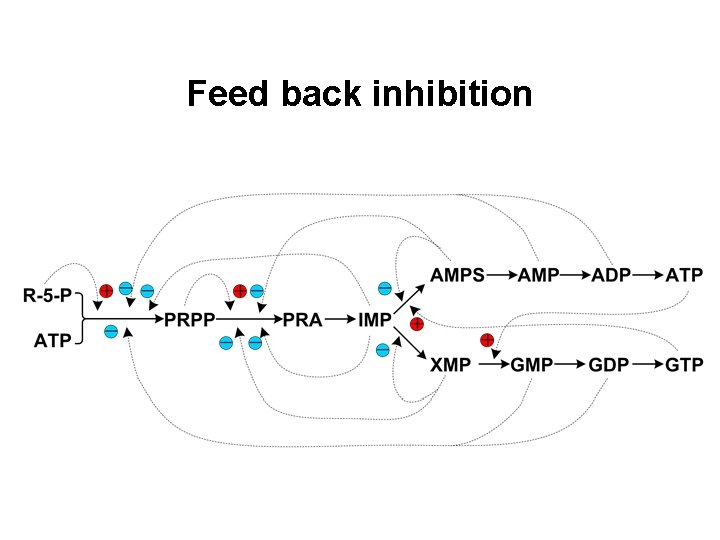
Regulation of de novo synthesis of purine nucleotides

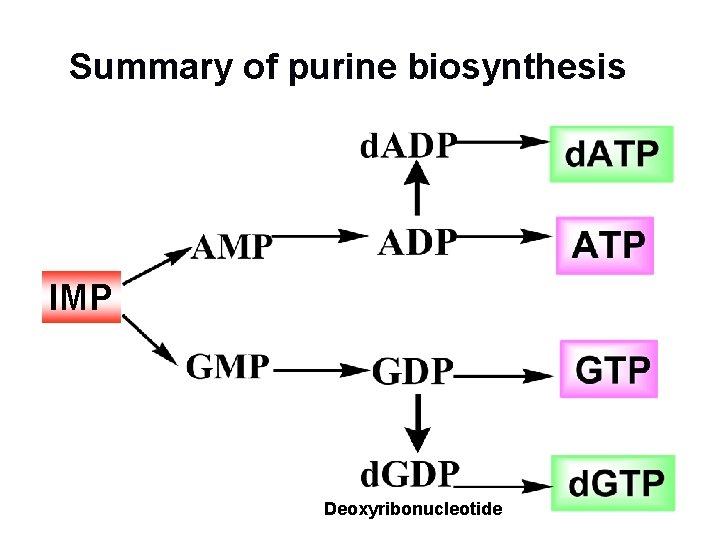
Summary of purine biosynthesis IMP Deoxyribonucleotide

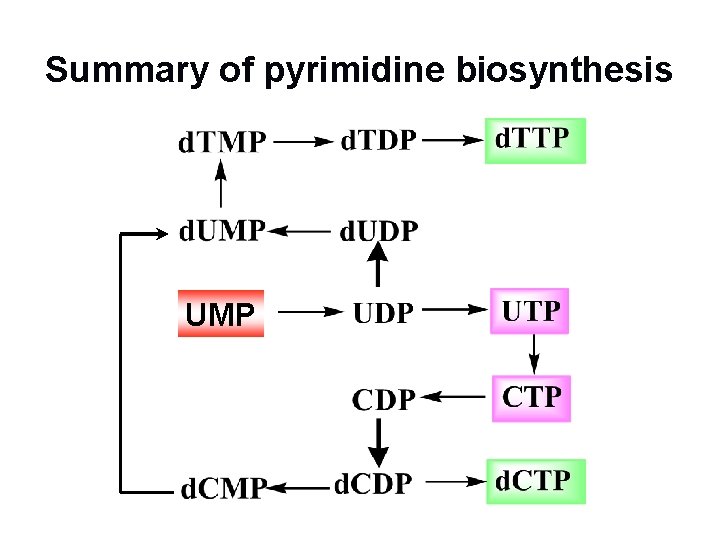
Summary of pyrimidine biosynthesis UMP

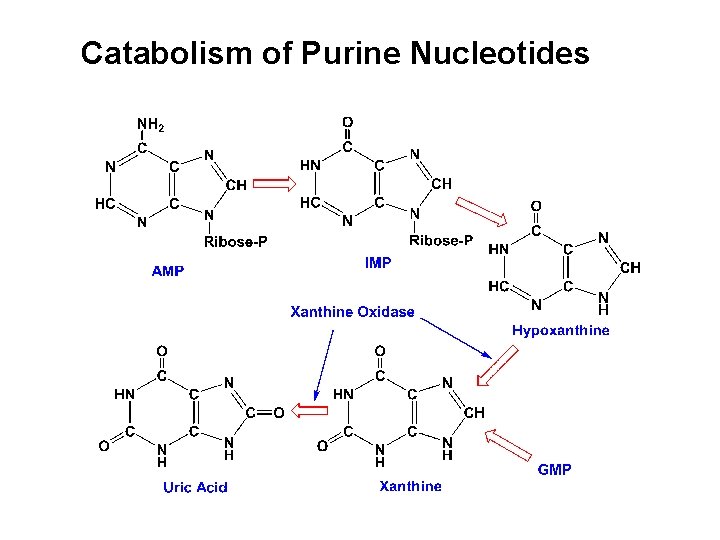
Catabolism of Purine Nucleotides

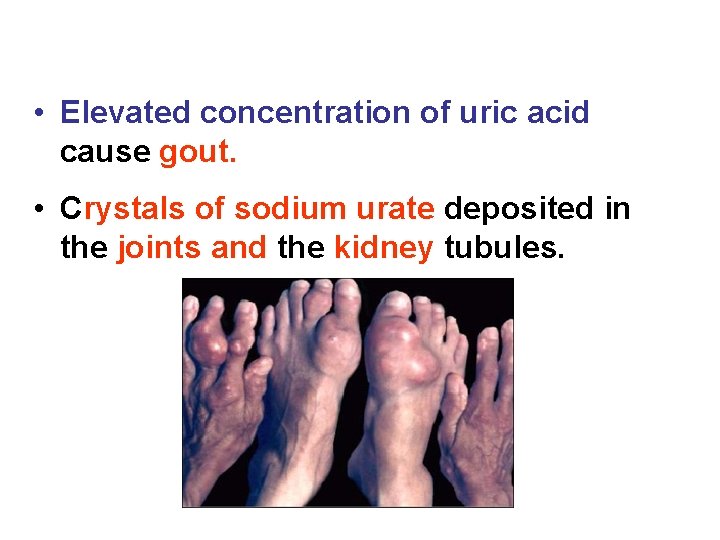
• Elevated concentration of uric acid cause gout. • Crystals of sodium urate deposited in the joints and the kidney tubules.

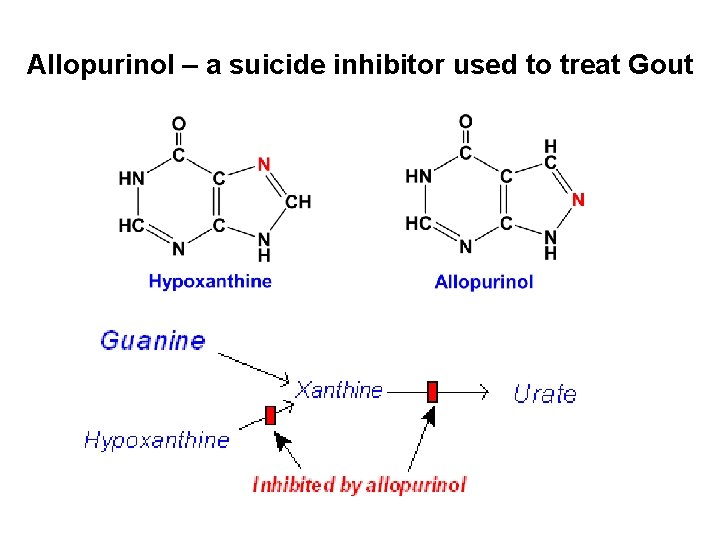
Allopurinol – a suicide inhibitor used to treat Gout

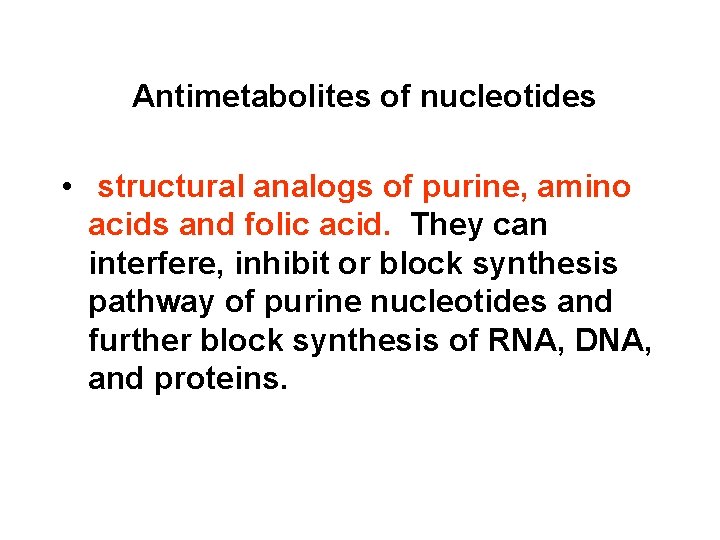
Antimetabolites of nucleotides • structural analogs of purine, amino acids and folic acid. They can interfere, inhibit or block synthesis pathway of purine nucleotides and further block synthesis of RNA, DNA, and proteins.

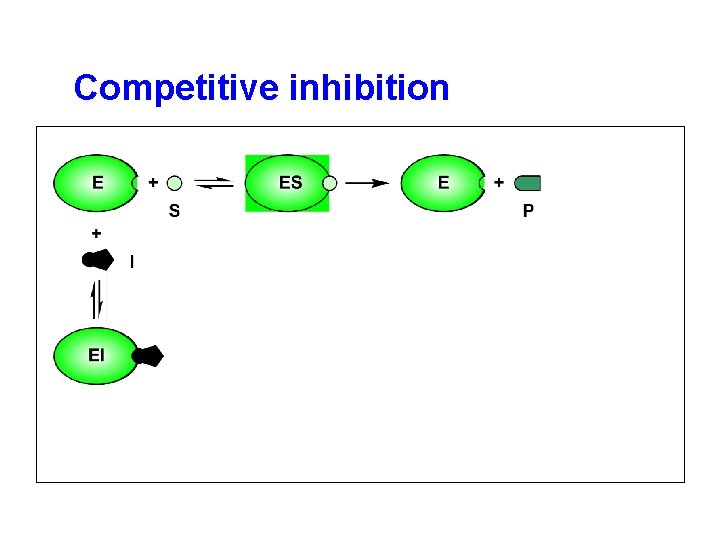
Competitive inhibition

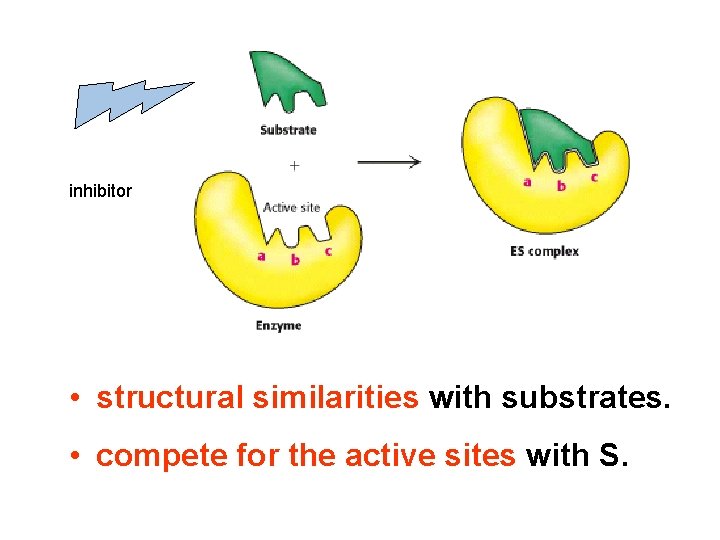
inhibitor • structural similarities with substrates. • compete for the active sites with S.

Feed back inhibition

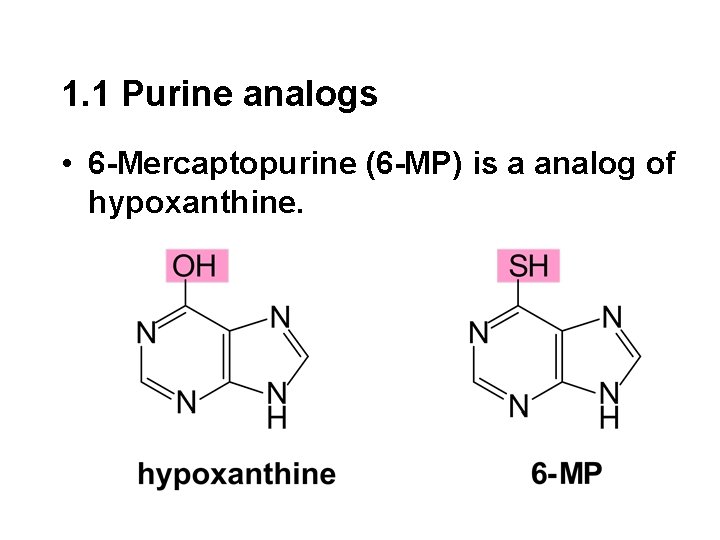
1. 1 Purine analogs • 6 -Mercaptopurine (6 -MP) is a analog of hypoxanthine.

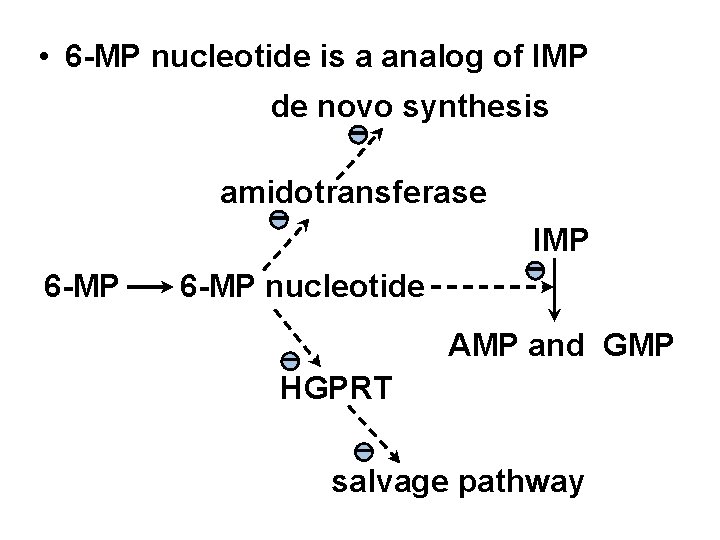
• 6 -MP nucleotide is a analog of IMP de novo synthesis - amidotransferase - 6 -MP IMP 6 -MP nucleotide - AMP and GMP - HGPRT - salvage pathway

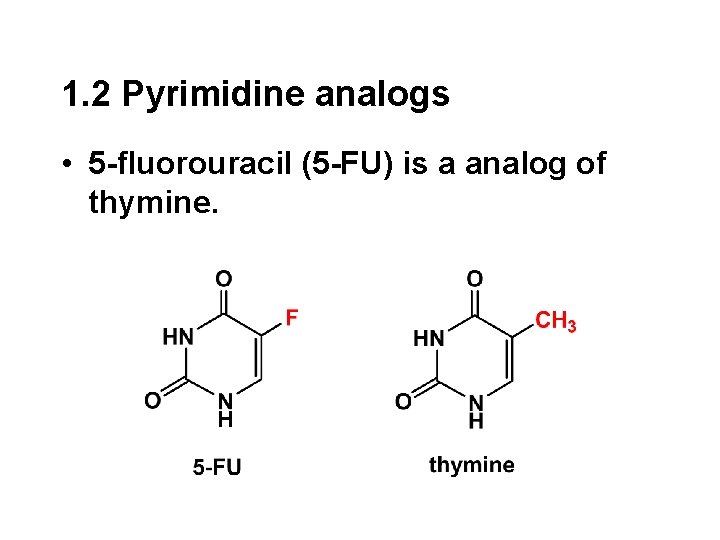
1. 2 Pyrimidine analogs • 5 -fluorouracil (5 -FU) is a analog of thymine.

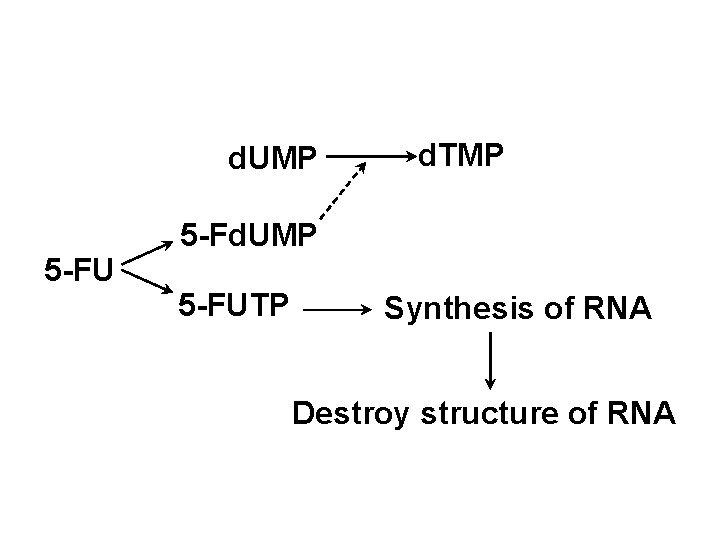
d. UMP 5 -FU d. TMP 5 -Fd. UMP 5 -FUTP Synthesis of RNA Destroy structure of RNA

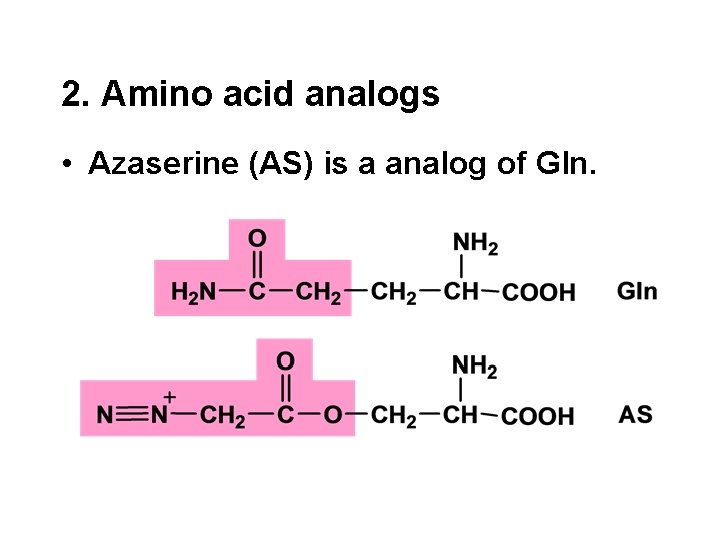
2. Amino acid analogs • Azaserine (AS) is a analog of Gln.

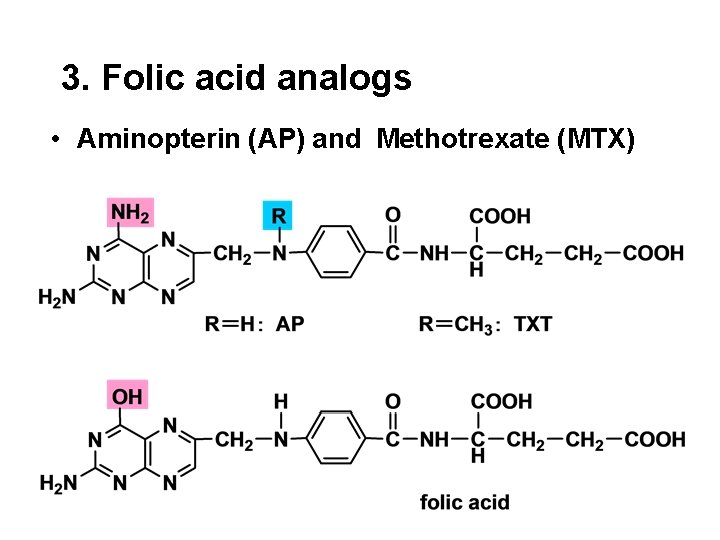
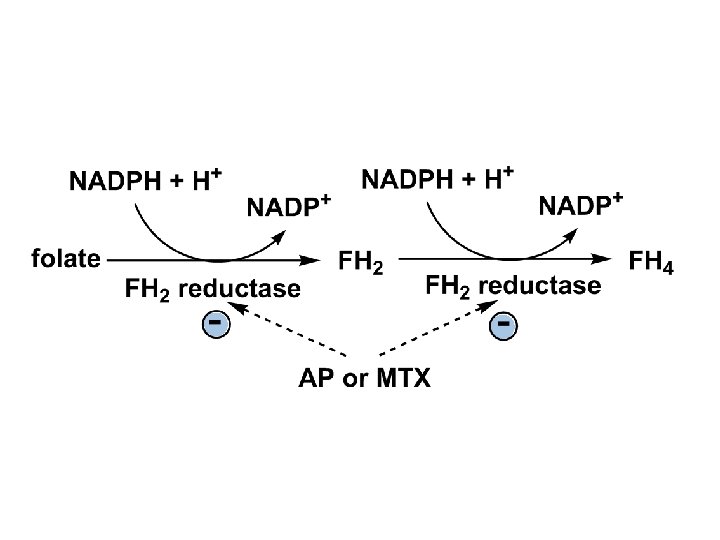
3. Folic acid analogs • Aminopterin (AP) and Methotrexate (MTX)


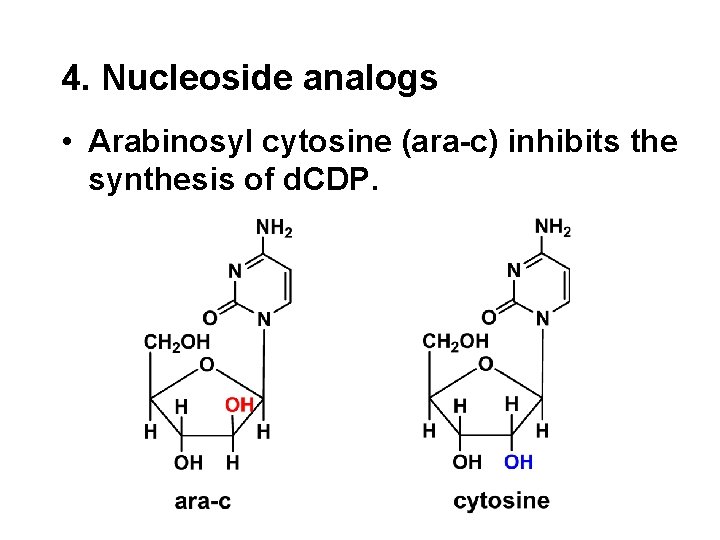
4. Nucleoside analogs • Arabinosyl cytosine (ara-c) inhibits the synthesis of d. CDP.
 Regulation of urea
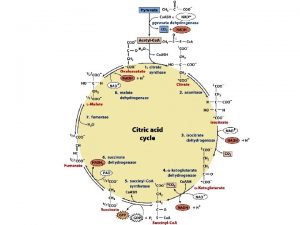
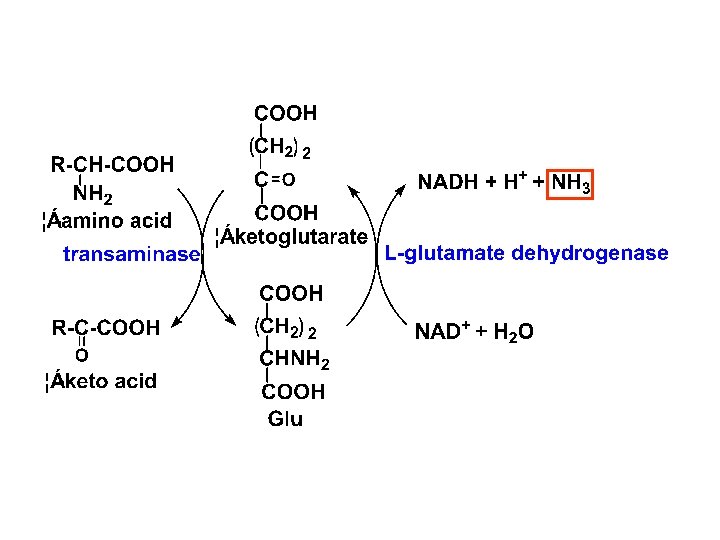
Regulation of urea Transamination and oxidative deamination
Transamination and oxidative deamination Transamination and oxidative deamination
Transamination and oxidative deamination Transamination vs deamination
Transamination vs deamination Transamination reaction
Transamination reaction Protein metabolism notes
Protein metabolism notes Deamination of glutamine
Deamination of glutamine Plp mechanism transamination
Plp mechanism transamination Jana novotn
Jana novotn Transamination
Transamination Transamination
Transamination Transamination
Transamination Transamination
Transamination Transamination
Transamination Transamination
Transamination Deamination of amino acids
Deamination of amino acids Bv of protein
Bv of protein Adenine imino form
Adenine imino form Deamination of glutamine
Deamination of glutamine Carbamoyl phosphate synthetase reaction
Carbamoyl phosphate synthetase reaction Glutamine deamination
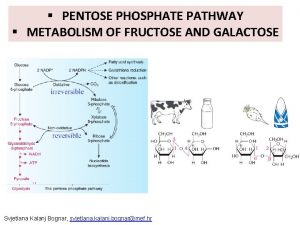
Glutamine deamination Pentose phosphate pathway
Pentose phosphate pathway Uncouple oxidative phosphorylation
Uncouple oxidative phosphorylation Uncouplers of oxidative phosphorylation
Uncouplers of oxidative phosphorylation Hmp pathway
Hmp pathway Anionation
Anionation Chat
Chat Thermogin
Thermogin Principle of corrosion
Principle of corrosion Substrate level phosphorylation vs oxidative
Substrate level phosphorylation vs oxidative Uncouplers of oxidative phosphorylation
Uncouplers of oxidative phosphorylation Concept map of oxidative phosphorylation
Concept map of oxidative phosphorylation Cellular respiration harvesting chemical energy
Cellular respiration harvesting chemical energy Oxidative phosphorylation energy yield
Oxidative phosphorylation energy yield Non-oxidative phase of pentose phosphate pathway
Non-oxidative phase of pentose phosphate pathway Oxidative phosphorylation
Oxidative phosphorylation Azide electron transport chain
Azide electron transport chain Acid desizing
Acid desizing Inhibitor of oxidative phosphorylation
Inhibitor of oxidative phosphorylation Inhibitors of oxidative phosphorylation
Inhibitors of oxidative phosphorylation Oxidative decarboxylation
Oxidative decarboxylation Uncouplers of oxidative phosphorylation
Uncouplers of oxidative phosphorylation Oxidative phosphorylation
Oxidative phosphorylation