Oxidative Phosphorylation Making ATP i e phosphorylation of
- Slides: 8

Oxidative Phosphorylation Making ATP (i. e. phosphorylation of ADP) from energy released during oxidation of an e- donor. 4 main components: 1) Reduced compound donates high energy e- to ETC. 2) Oxidized compound accepts low energy e- from ETC. 3) Energy released in ETC does the work of pumping H+ across a membrane to establish the PMF. 4) PMF fuels ATP Synthase to phosphorylate ADP.

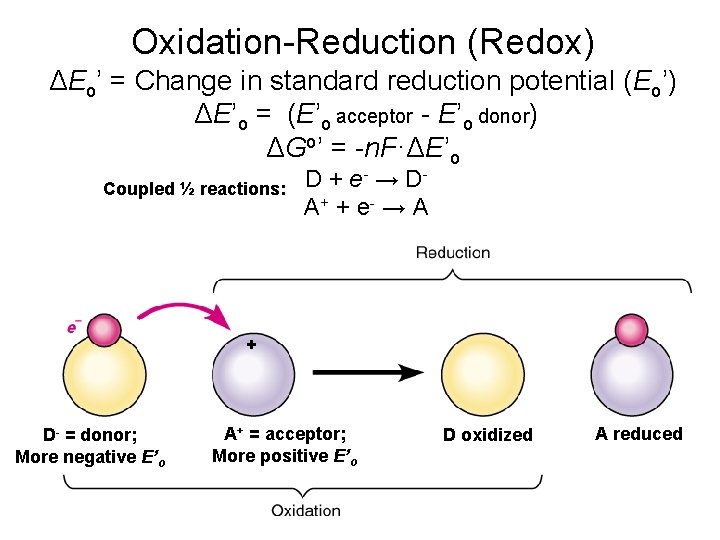
Oxidation-Reduction (Redox) ΔEo’ = Change in standard reduction potential (Eo’) ΔE’o = (E’o acceptor - E’o donor) ΔGo’ = -n. F·ΔE’o Coupled ½ reactions: D + e- → DA+ + e - → A + D- = donor; More negative E’o A+ = acceptor; More positive E’o D oxidized A reduced

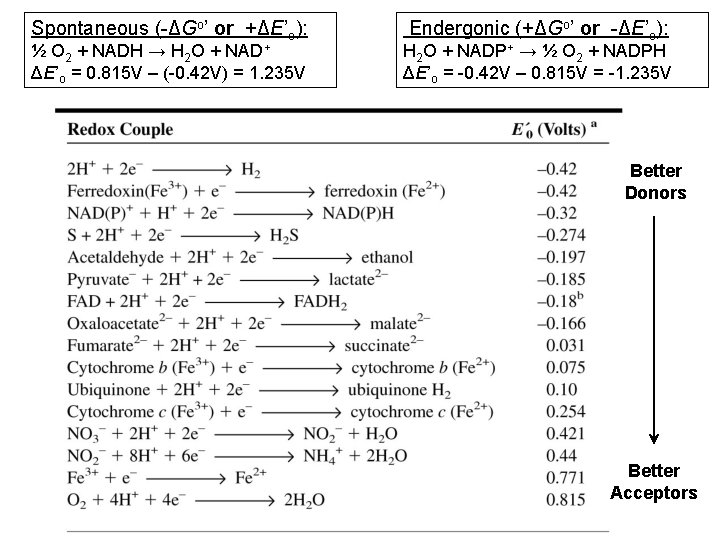
Spontaneous (-ΔGo’ or +ΔE’o): ½ O 2 + NADH → H 2 O + NAD+ ΔE’o = 0. 815 V – (-0. 42 V) = 1. 235 V Endergonic (+ΔGo’ or -ΔE’o): H 2 O + NADP+ → ½ O 2 + NADPH ΔE’o = -0. 42 V – 0. 815 V = -1. 235 V Better Donors Better Acceptors

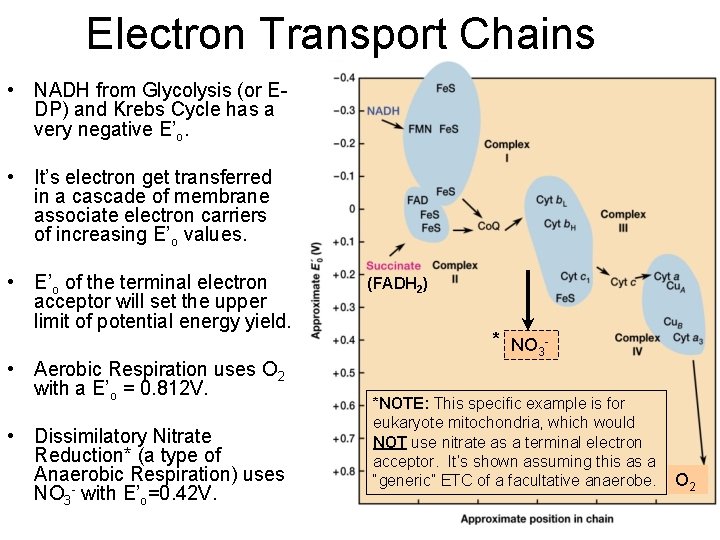
Electron Transport Chains • NADH from Glycolysis (or EDP) and Krebs Cycle has a very negative E’o. • It’s electron get transferred in a cascade of membrane associate electron carriers of increasing E’o values. • E’o of the terminal electron acceptor will set the upper limit of potential energy yield. • Aerobic Respiration uses O 2 with a E’o = 0. 812 V. • Dissimilatory Nitrate Reduction* (a type of Anaerobic Respiration) uses NO 3 - with E’o=0. 42 V. (FADH 2) * NO 3 *NOTE: This specific example is for eukaryote mitochondria, which would NOT use nitrate as a terminal electron acceptor. It’s shown assuming this as a “generic” ETC of a facultative anaerobe. O 2

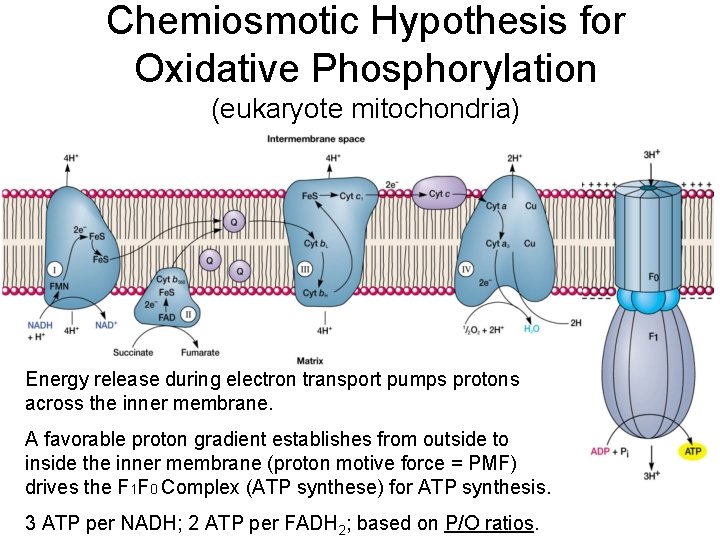
Chemiosmotic Hypothesis for Oxidative Phosphorylation (eukaryote mitochondria) Energy release during electron transport pumps protons across the inner membrane. A favorable proton gradient establishes from outside to inside the inner membrane (proton motive force = PMF) drives the F 1 F 0 Complex (ATP synthese) for ATP synthesis. 3 ATP per NADH; 2 ATP per FADH 2; based on P/O ratios.

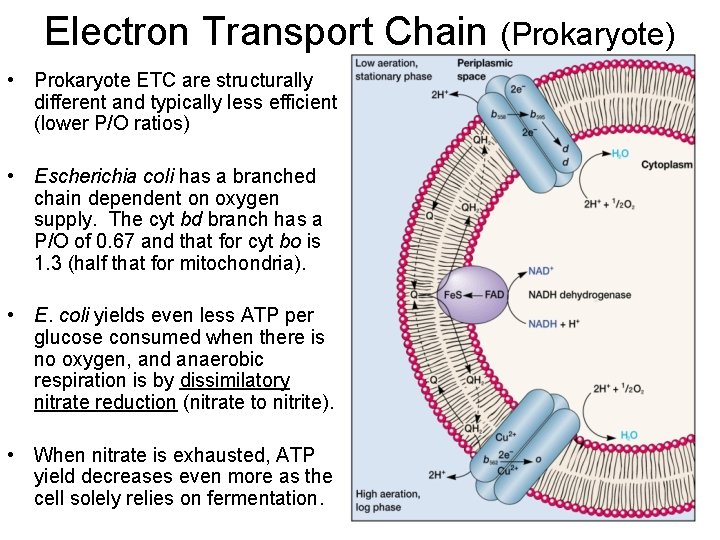
Electron Transport Chain (Prokaryote) • Prokaryote ETC are structurally different and typically less efficient (lower P/O ratios) • Escherichia coli has a branched chain dependent on oxygen supply. The cyt bd branch has a P/O of 0. 67 and that for cyt bo is 1. 3 (half that for mitochondria). • E. coli yields even less ATP per glucose consumed when there is no oxygen, and anaerobic respiration is by dissimilatory nitrate reduction (nitrate to nitrite). • When nitrate is exhausted, ATP yield decreases even more as the cell solely relies on fermentation.

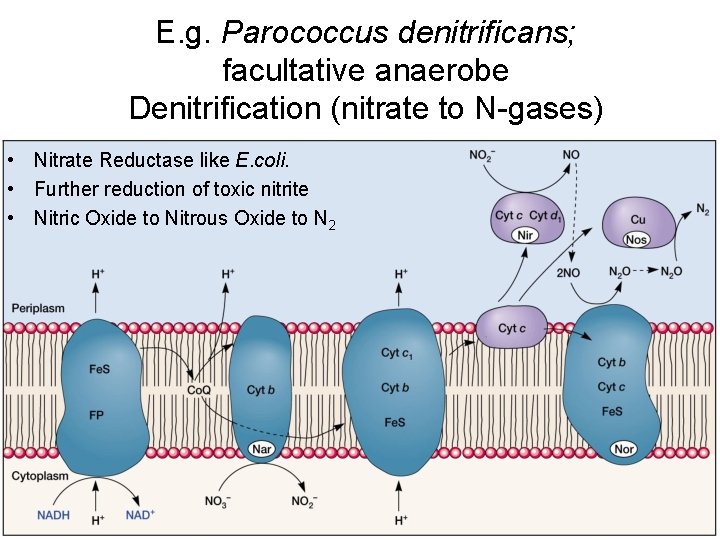
E. g. Parococcus denitrificans; facultative anaerobe Denitrification (nitrate to N-gases) • Nitrate Reductase like E. coli. • Further reduction of toxic nitrite • Nitric Oxide to Nitrous Oxide to N 2

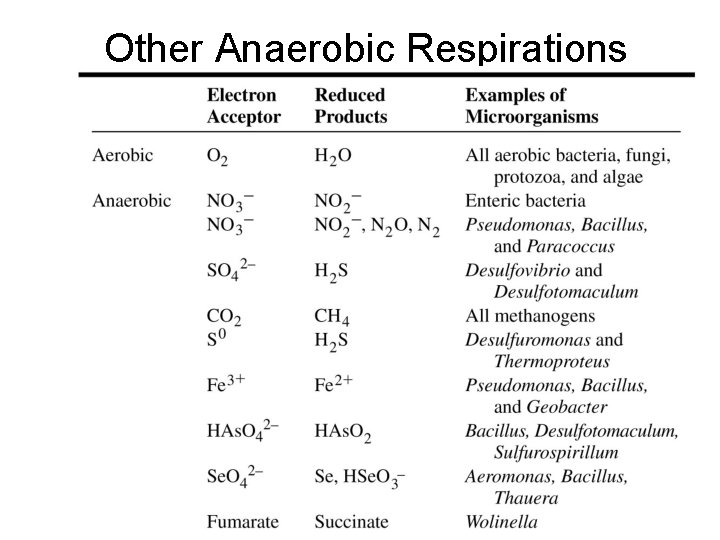
Other Anaerobic Respirations