Oxidative Coupling Scott Dufour Oxidative coupling of aromatic




- Slides: 15

Oxidative Coupling Scott Dufour

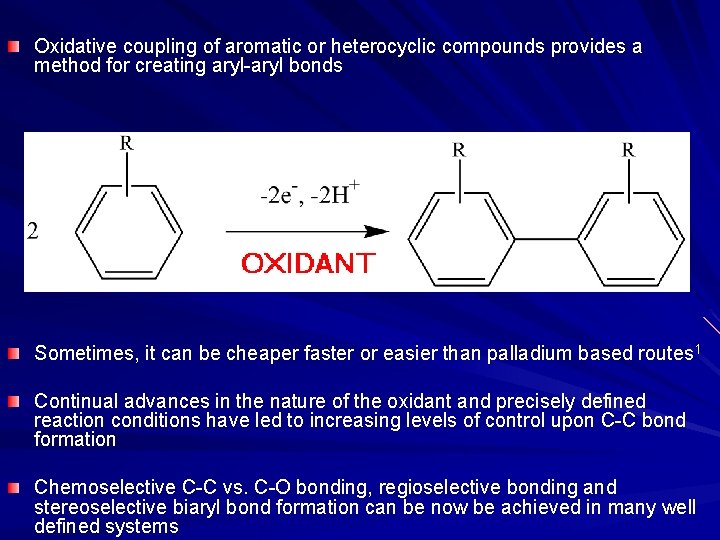
Oxidative coupling of aromatic or heterocyclic compounds provides a method for creating aryl-aryl bonds Sometimes, it can be cheaper faster or easier than palladium based routes 1 Continual advances in the nature of the oxidant and precisely defined reaction conditions have led to increasing levels of control upon C-C bond formation Chemoselective C-C vs. C-O bonding, regioselective bonding and stereoselective biaryl bond formation can be now be achieved in many well defined systems

Oxidants Most recently, the hypervalent iodine complexes: phenyliodine (III) diacetate (PIDA), and phenyliodine(III) bis(trifluoroacetate) (PIFA) have been applied leading to an efficient , highly regioselective, mild non toxic alternative to heavy metals Vanadium Complexes (VOCl 3, VOF 3), Thallium and Lead have been found to be reliable oxidative coupling agents; prevented overoxidations, and enhanced regio selectivety of C-C bond formation between aryl rings Iron (III) reagents were among the first tested and still have a wide following today; in the synthesis of triphenylenes it has been found the Fe. Cl 3 offers the best route of synthesis to these wide range of systems

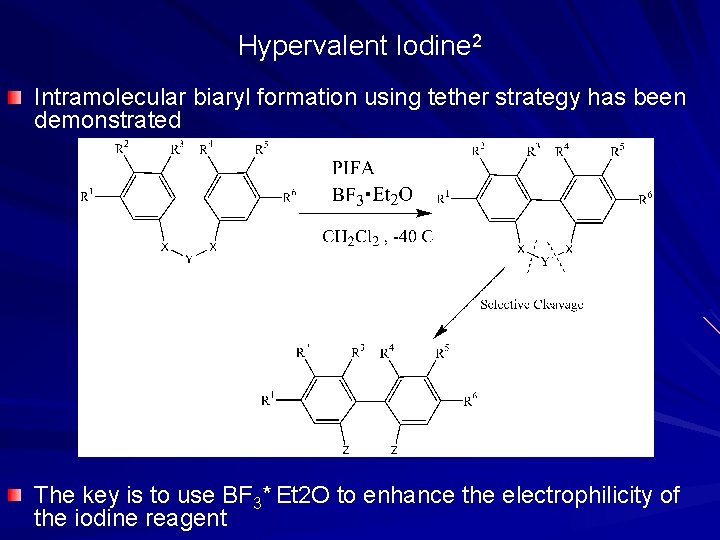
Hypervalent Iodine 2 Intramolecular biaryl formation using tether strategy has been demonstrated The key is to use BF 3* Et 2 O to enhance the electrophilicity of the iodine reagent

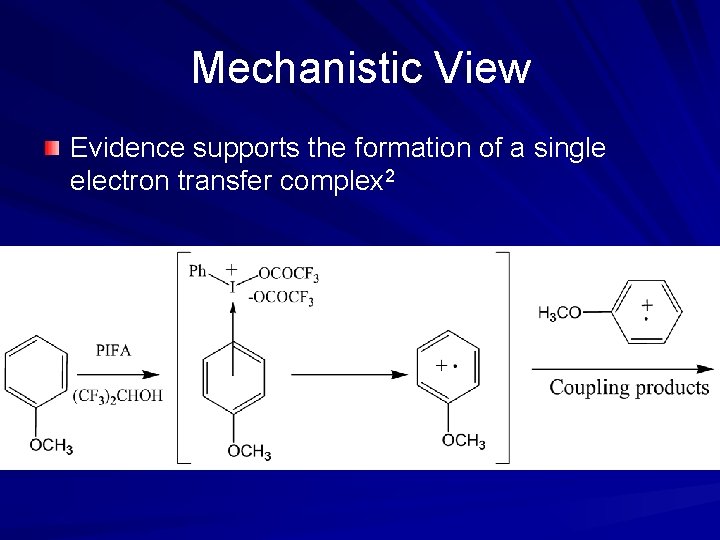
Mechanistic View Evidence supports the formation of a single electron transfer complex 2

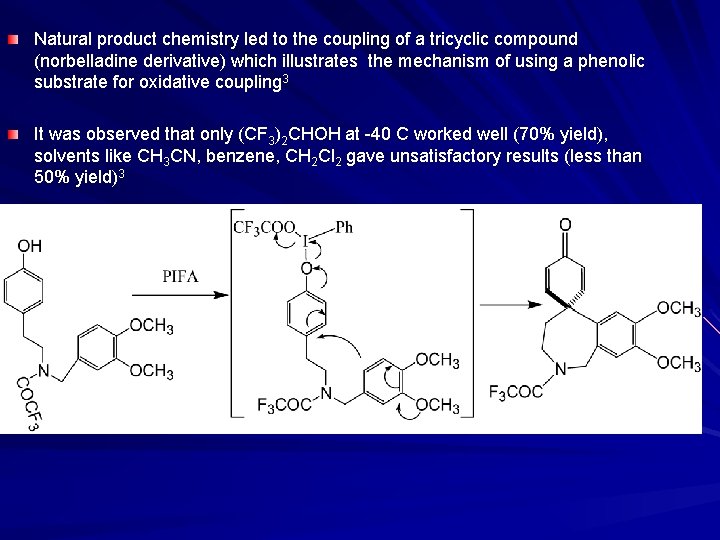
Natural product chemistry led to the coupling of a tricyclic compound (norbelladine derivative) which illustrates the mechanism of using a phenolic substrate for oxidative coupling 3 It was observed that only (CF 3)2 CHOH at -40 C worked well (70% yield), solvents like CH 3 CN, benzene, CH 2 Cl 2 gave unsatisfactory results (less than 50% yield)3

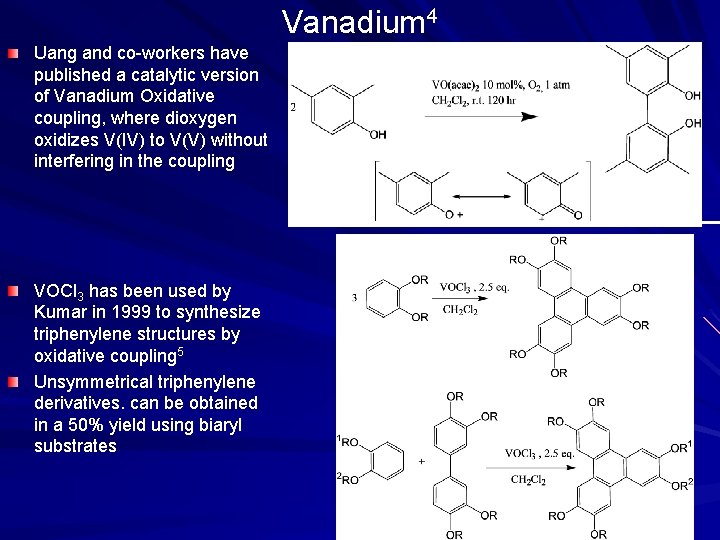
Vanadium 4 Uang and co-workers have published a catalytic version of Vanadium Oxidative coupling, where dioxygen oxidizes V(IV) to V(V) without interfering in the coupling VOCl 3 has been used by Kumar in 1999 to synthesize triphenylene structures by oxidative coupling 5 Unsymmetrical triphenylene derivatives. can be obtained in a 50% yield using biaryl substrates

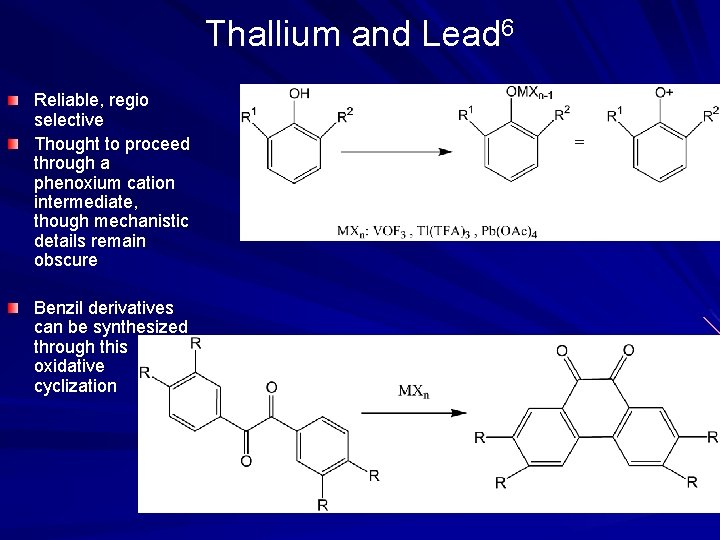
Thallium and Lead 6 Reliable, regio selective Thought to proceed through a phenoxium cation intermediate, though mechanistic details remain obscure Benzil derivatives can be synthesized through this oxidative cyclization

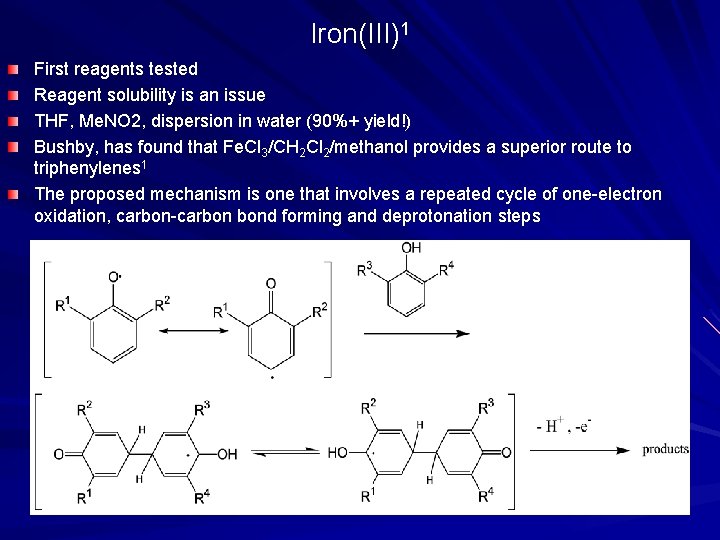
Iron(III)1 First reagents tested Reagent solubility is an issue THF, Me. NO 2, dispersion in water (90%+ yield!) Bushby, has found that Fe. Cl 3/CH 2 Cl 2/methanol provides a superior route to triphenylenes 1 The proposed mechanism is one that involves a repeated cycle of one-electron oxidation, carbon-carbon bond forming and deprotonation steps

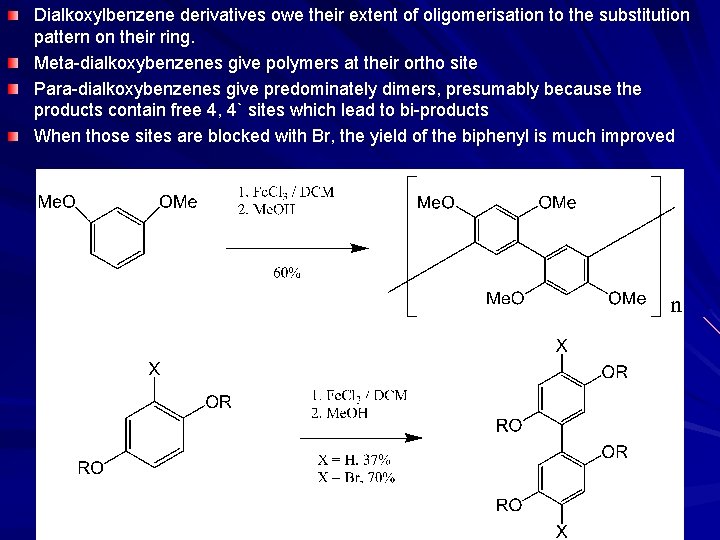
Dialkoxylbenzene derivatives owe their extent of oligomerisation to the substitution pattern on their ring. Meta-dialkoxybenzenes give polymers at their ortho site Para-dialkoxybenzenes give predominately dimers, presumably because the products contain free 4, 4` sites which lead to bi-products When those sites are blocked with Br, the yield of the biphenyl is much improved

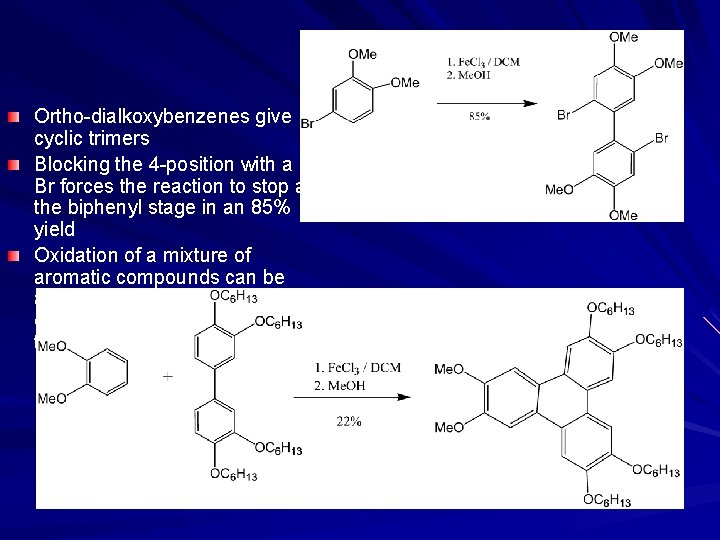
Ortho-dialkoxybenzenes give cyclic trimers Blocking the 4 -position with a Br forces the reaction to stop at the biphenyl stage in an 85% yield Oxidation of a mixture of aromatic compounds can be achieved where aromatic A can be mixed with aromatic B to form A-B and not B-B or A-A

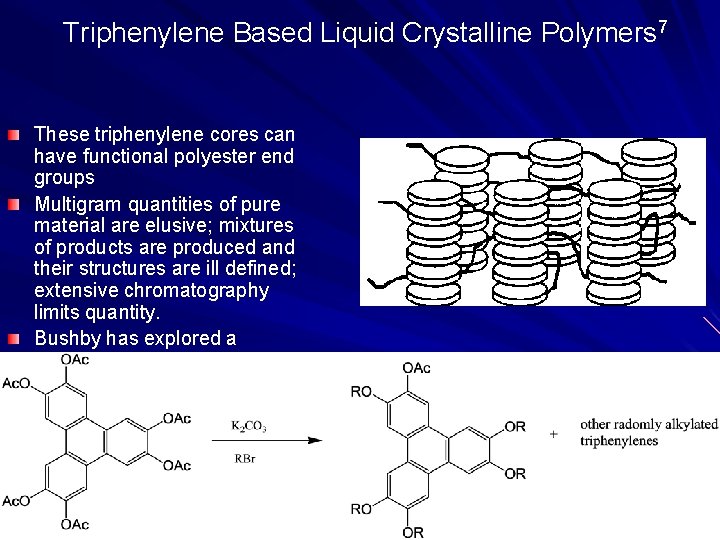
Triphenylene Based Liquid Crystalline Polymers 7 These triphenylene cores can have functional polyester end groups Multigram quantities of pure material are elusive; mixtures of products are produced and their structures are ill defined; extensive chromatography limits quantity. Bushby has explored a Universal rational synthesis to these problems

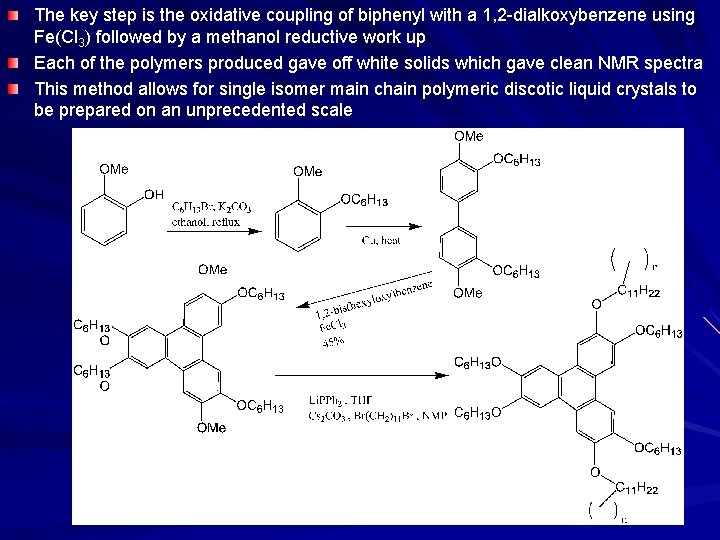
The key step is the oxidative coupling of biphenyl with a 1, 2 -dialkoxybenzene using Fe(Cl 3) followed by a methanol reductive work up Each of the polymers produced gave off white solids which gave clean NMR spectra This method allows for single isomer main chain polymeric discotic liquid crystals to be prepared on an unprecedented scale

Conclusion From the continuous effort in the advancement of oxidative aryl coupling, issues such as chemo -, regio- and setereoselectivity now have clear well defined solutions, that have been empirically proven By using inorganic combined with organic chemistry, researchers can approach oxidative coupling in an elegant way, rather than using “sledgehammer chemistry” Further research should provide even more selective and universal methods to fashion the link between two aromatic units

References 1. N. Boden, Bushby Oct 2000 Tet. Letters 41 2. Oxidative Aryl Coupling Reactions in Synthesis, pg 483 3. H. Tohma, Y. Kita, Tetrahedron, 2001, 57, 345 4. Oxidative Aryl Coupling Reactions in Synthesis, pg 499 5. Oxidative Aryl Coupling Reactions in Synthesis, pg 502 6. Oxidative Aryl Coupling Reactions in Synthesis, pg 506 7. A. N. Cammidge, R. Bushby, J. Am. Chem Soc. 1995, 117, 924 -927