Electrochemistry Girma M Chapter 1 Fundamental of Electrochemistry
















- Slides: 40

Electrochemistry Girma M.



Chapter 1 Fundamental of Electrochemistry

Electrochemistry involves two types of processes: 1. The production of an electric current from a chemical (oxidation reduction) reaction 2. The use of an electric current to produce a chemical change

Electrochemistry • the area of chemistry concerned with the interconversion of chemical and electrical energy, is enormously important in modern science and technology not only because of batteries but also because it makes possible the manufacture of essential industrial chemicals and materials. • Sodium hydroxide which is used in the manufacture of paper, textiles, soaps, and detergents, is produced by passing an electric current through an aqueous solution of sodium chloride. • Chlorine, essential to the manufacture of plastics such as poly(vinyl chloride) (PVC), is obtained in the same process. • Aluminum metal is also produced in an electrochemical process, as is pure copper for use in electrical wiring.

Electrochemistry applications Chapter 2 • Batteries Honda FCX fuel cell vehicle.

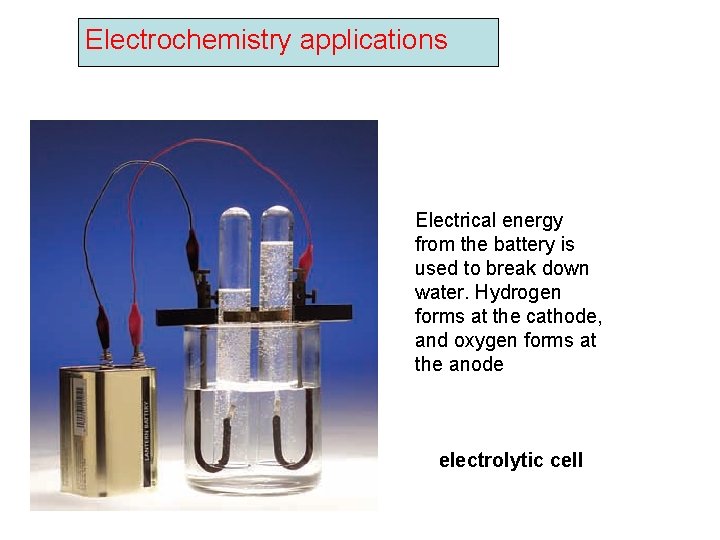
Electrochemistry applications Electrical energy from the battery is used to break down water. Hydrogen forms at the cathode, and oxygen forms at the anode electrolytic cell

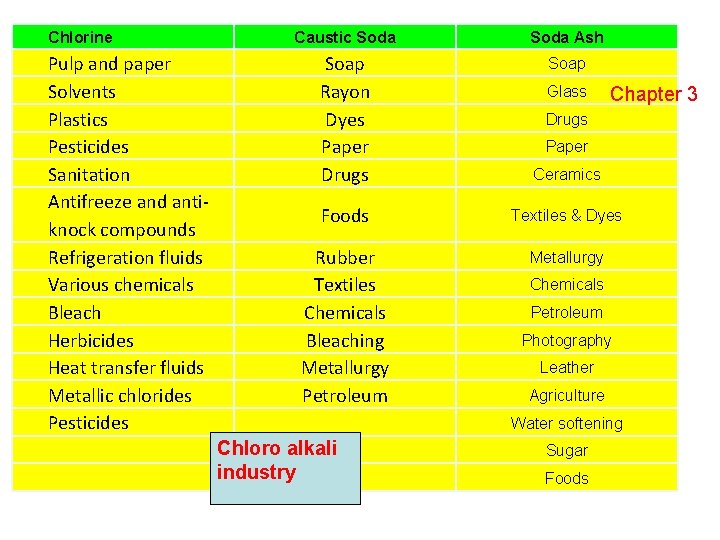
Chlorine Pulp and paper Solvents Plastics Pesticides Sanitation Antifreeze and antiknock compounds Refrigeration fluids Various chemicals Bleach Herbicides Heat transfer fluids Metallic chlorides Pesticides Caustic Soda Ash Soap Rayon Dyes Paper Drugs Soap Ceramics Foods Textiles & Dyes Rubber Textiles Chemicals Bleaching Metallurgy Petroleum Metallurgy Glass Chapter 3 Drugs Paper Chemicals Petroleum Photography Leather Agriculture Water softening Chloro alkali industry Sugar Foods

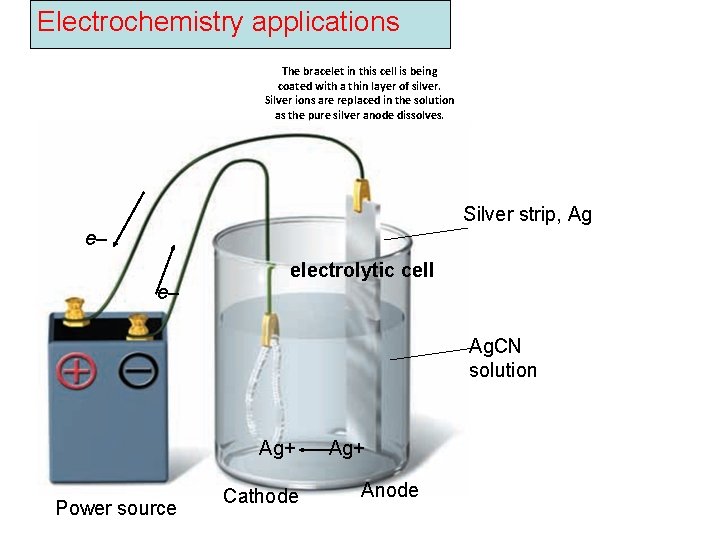
Electrochemistry applications The bracelet in this cell is being coated with a thin layer of silver. Silver ions are replaced in the solution as the pure silver anode dissolves. Silver strip, Ag e– e– electrolytic cell Ag. CN solution Ag+ Power source Cathode Ag+ Anode



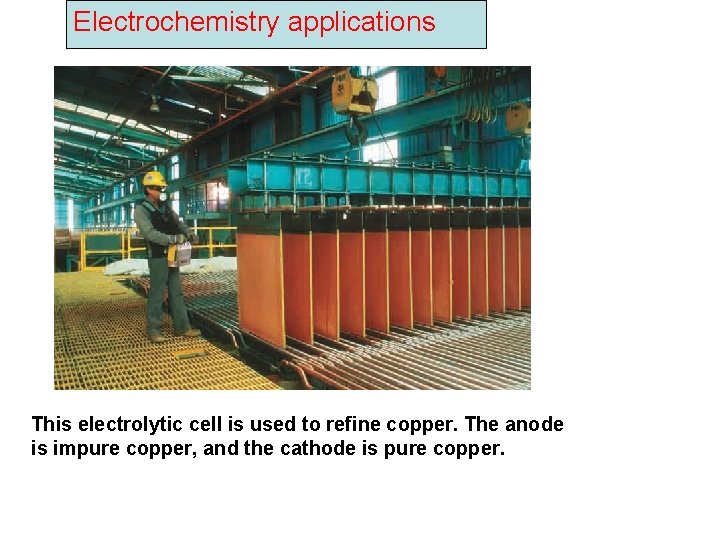
Electrochemistry applications This electrolytic cell is used to refine copper. The anode is impure copper, and the cathode is pure copper.

Chapter 4 Corrosion prevention

Chapter 5 Biosensors

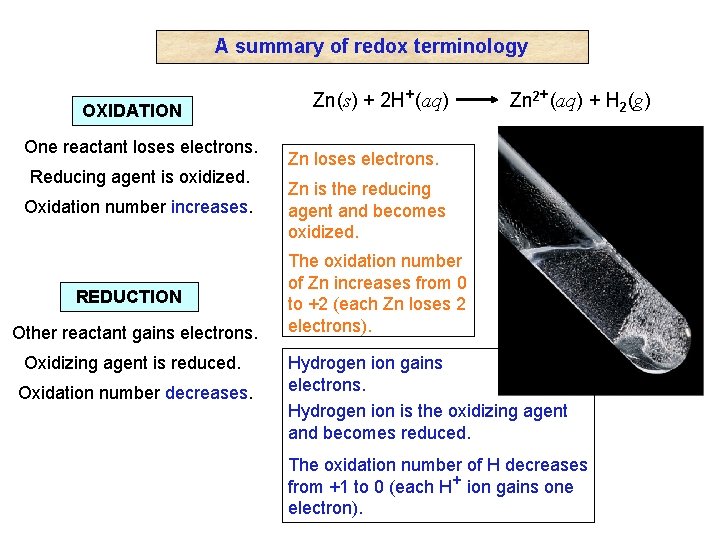
A summary of redox terminology OXIDATION One reactant loses electrons. Reducing agent is oxidized. Oxidation number increases. REDUCTION Other reactant gains electrons. Oxidizing agent is reduced. Oxidation number decreases. Zn(s) + 2 H+(aq) Zn 2+(aq) + H 2(g) Zn loses electrons. Zn is the reducing agent and becomes oxidized. The oxidation number of Zn increases from 0 to +2 (each Zn loses 2 electrons). Hydrogen ion gains electrons. Hydrogen ion is the oxidizing agent and becomes reduced. The oxidation number of H decreases from +1 to 0 (each H+ ion gains one electron).

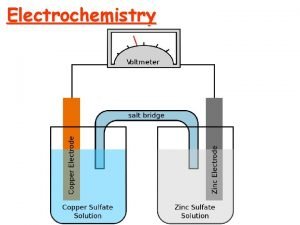
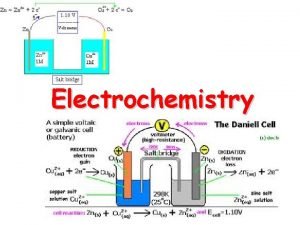
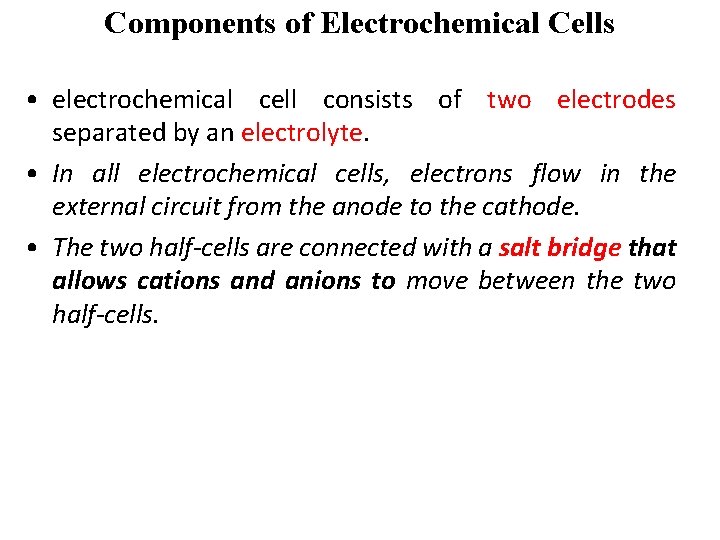
Components of Electrochemical Cells • electrochemical cell consists of two electrodes separated by an electrolyte. • In all electrochemical cells, electrons flow in the external circuit from the anode to the cathode. • The two half-cells are connected with a salt bridge that allows cations and anions to move between the two half-cells.

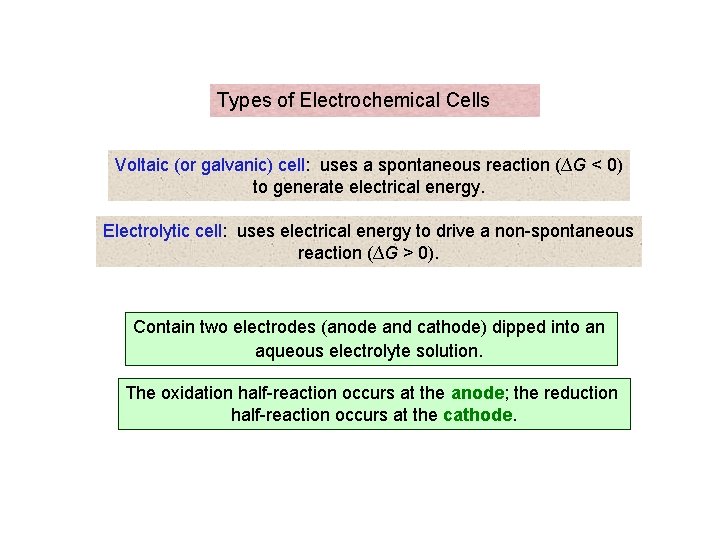
Types of Electrochemical Cells Voltaic (or galvanic) cell: uses a spontaneous reaction (∆G < 0) to generate electrical energy. Electrolytic cell: uses electrical energy to drive a non-spontaneous reaction (∆G > 0). Contain two electrodes (anode and cathode) dipped into an aqueous electrolyte solution. The oxidation half-reaction occurs at the anode; the reduction half-reaction occurs at the cathode.



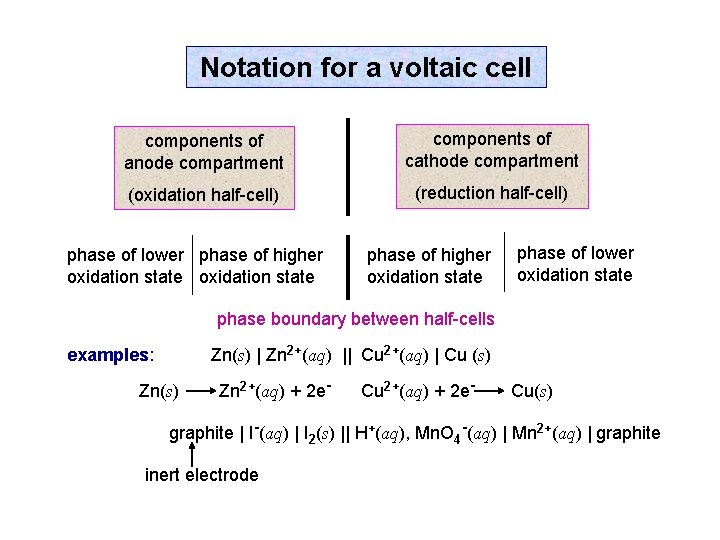
Notation for a voltaic cell components of anode compartment components of cathode compartment (oxidation half-cell) (reduction half-cell) phase of lower phase of higher oxidation state phase of lower oxidation state phase boundary between half-cells Zn(s) | Zn 2+(aq) || Cu 2+(aq) | Cu (s) examples: Zn(s) Zn 2+(aq) + 2 e- Cu(s) graphite | I-(aq) | I 2(s) || H+(aq), Mn. O 4 -(aq) | Mn 2+(aq) | graphite inert electrode

Calculating an unknown Eohalf-cell from Eocell Sample Problem PROBLEM: A voltaic cell houses the reaction between aqueous bromine and zinc metal: Br 2(aq) + Zn(s) Zn 2+(aq) + 2 Br-(aq) Eocell = 1. 83 V Calculate Eobromine given Eozinc = -0. 76 V PLAN: The reaction is spontaneous as written since the Eocell is (+). Zinc is being oxidized and is the anode. Therefore the Eobromine can be found using Eocell = Eocathode - Eoanode. SOLUTION: anode: Zn(s) - E = +0. 76 Zn 2+(aq) + 2 e� Eo. Zn as Zn 2+(aq) + 2 e- Zn(s) is -0. 76 V Eocell = Eocathode - Eoanode = 1. 83 = Eobromine - (-0. 76) Eobromine = 1. 86 - 0. 76 = 1. 07 V


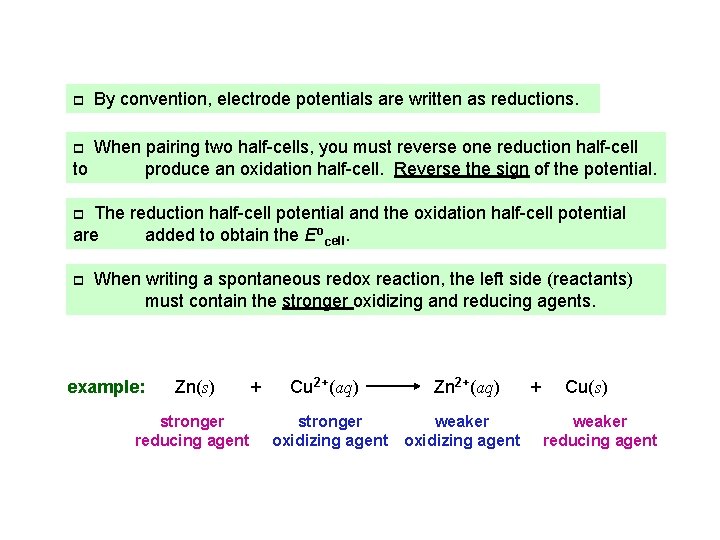
p By convention, electrode potentials are written as reductions. When pairing two half-cells, you must reverse one reduction half-cell to produce an oxidation half-cell. Reverse the sign of the potential. p The reduction half-cell potential and the oxidation half-cell potential are added to obtain the Eocell. p p When writing a spontaneous redox reaction, the left side (reactants) must contain the stronger oxidizing and reducing agents. example: Zn(s) stronger reducing agent + Cu 2+(aq) Zn 2+(aq) stronger weaker oxidizing agent + Cu(s) weaker reducing agent

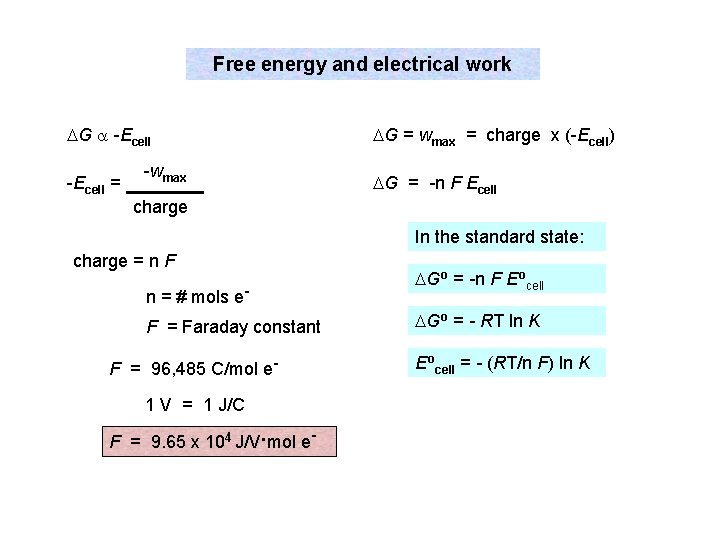
Free energy and electrical work DG a -Ecell = -wmax DG = wmax = charge x (-Ecell) DG = -n F Ecell charge In the standard state: charge = n F n = # mols e. F = Faraday constant F = 96, 485 C/mol e 1 V = 1 J/C F = 9. 65 x 104 J/V. mol e- DGo = -n F Eocell DGo = - RT ln K Eocell = - (RT/n F) ln K

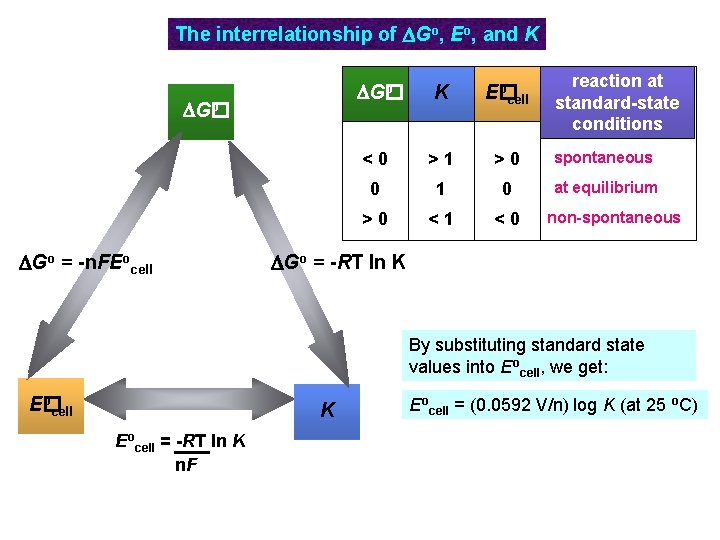
The interrelationship of DGo, Eo, and K o DG� DGo = -n. FEocell reaction at standard-state conditions K o E� cell <0 >1 >0 spontaneous 0 1 0 at equilibrium >0 <1 <0 non-spontaneous DGo = -RT ln K By substituting standard state values into Eocell, we get: o E� cell K Eocell = -RT ln K n. F Eocell = (0. 0592 V/n) log K (at 25 o. C)

The Nernst equation • Working in nonstandard conditions



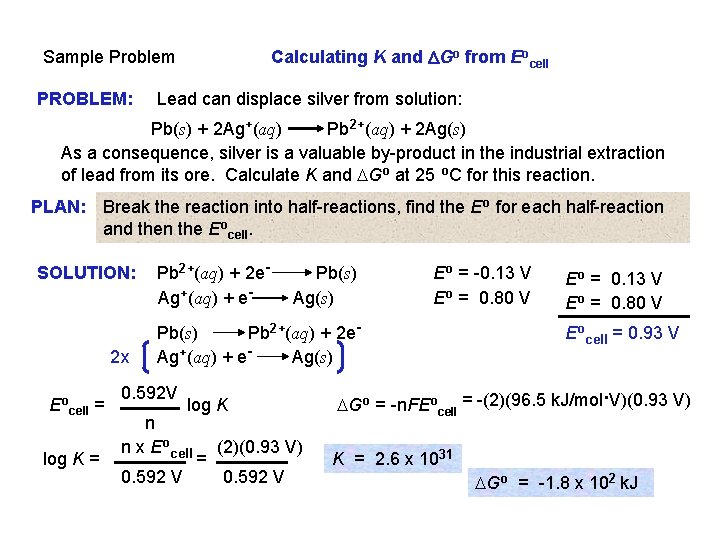
Sample Problem PROBLEM: Calculating K and DGo from Eocell Lead can displace silver from solution: Pb(s) + 2 Ag+(aq) Pb 2+(aq) + 2 Ag(s) As a consequence, silver is a valuable by-product in the industrial extraction of lead from its ore. Calculate K and DGo at 25 o. C for this reaction.


Calculating K and DGo from Eocell Sample Problem PROBLEM: Lead can displace silver from solution: Pb(s) + 2 Ag+(aq) Pb 2+(aq) + 2 Ag(s) As a consequence, silver is a valuable by-product in the industrial extraction of lead from its ore. Calculate K and DGo at 25 o. C for this reaction. PLAN: Break the reaction into half-reactions, find the Eo for each half-reaction and then the Eocell. SOLUTION: 2 x Eocell = log K = Pb 2+(aq) + 2 e. Ag+(aq) + e- Pb(s) Ag(s) Eo = -0. 13 V Eo = 0. 80 V Pb(s) Pb 2+(aq) + 2 e. Ag+(aq) + e. Ag(s) 0. 592 V log K n n x Eocell 0. 592 V = (2)(0. 93 V) 0. 592 V Eo = 0. 13 V Eo = 0. 80 V Eocell = 0. 93 V . DGo = -n. FEocell = -(2)(96. 5 k. J/mol V)(0. 93 V) K = 2. 6 x 1031 DGo = -1. 8 x 102 k. J

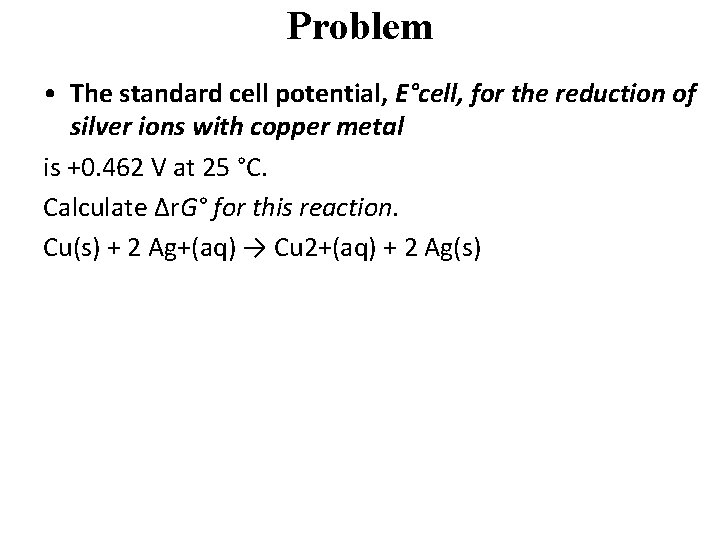
Problem • The standard cell potential, E°cell, for the reduction of silver ions with copper metal is +0. 462 V at 25 °C. Calculate Δr. G° for this reaction. Cu(s) + 2 Ag+(aq) → Cu 2+(aq) + 2 Ag(s)

Solution • In this cell, copper is the anode, and silver is the cathode. The overall cell reaction is Cu(s) + 2 Ag+(aq) → Cu 2+(aq) + 2 Ag(s) • each mole of copper transfers 2 mol of electrons to 2 mol of Ag+ ions. That is, n = 2. Now use • Δr. G° = −n. FE° = −(2 mol e−)(96, 485 C/mol e−)(0. 462 V) = − 89, 200 C ∙ V Because 1 C ∙ V = 1 J, we have Δr. G° = − 89, 200 J or − 89. 2 k. J

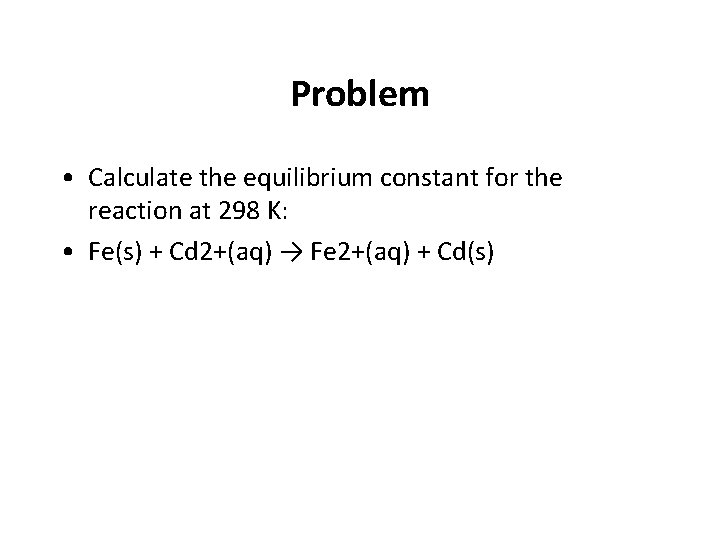
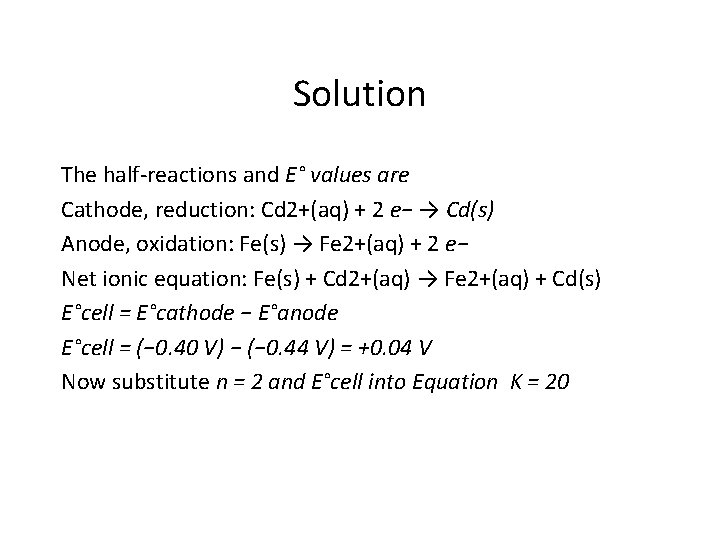
Problem • Calculate the equilibrium constant for the reaction at 298 K: • Fe(s) + Cd 2+(aq) → Fe 2+(aq) + Cd(s)

Solution The half-reactions and E° values are Cathode, reduction: Cd 2+(aq) + 2 e− → Cd(s) Anode, oxidation: Fe(s) → Fe 2+(aq) + 2 e− Net ionic equation: Fe(s) + Cd 2+(aq) → Fe 2+(aq) + Cd(s) E°cell = E°cathode − E°anode E°cell = (− 0. 40 V) − (− 0. 44 V) = +0. 04 V Now substitute n = 2 and E°cell into Equation K = 20



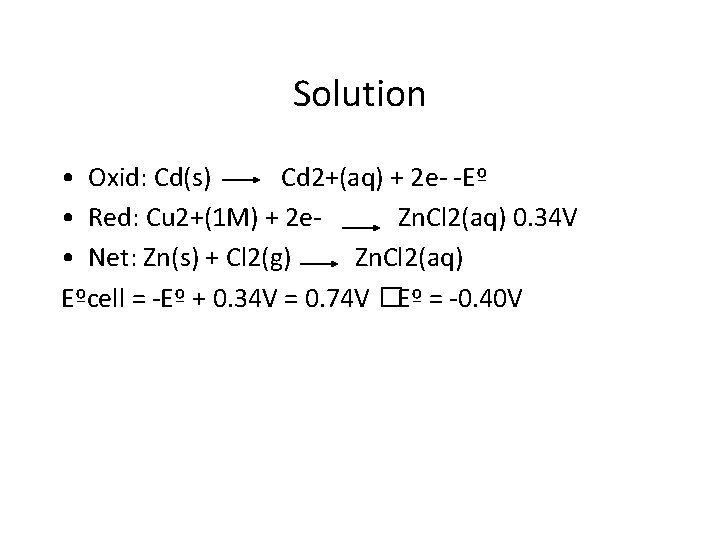
Exercise Cadmium is usually found in small quantities wherever zinc is found. Unlike zinc, which is an essential element for life, Cd is a poison. To measure Cd 2+ we need to know the Eº for the Cd 2+/Cd couple. The following cell is constructed and its voltage is measured. Cd(s)|Cd 2+(1 M)||Cu 2+(1 M)|Cu(s) E°cell = 0. 74 V What is the standard potential for the Cd 2+/Cd electrode?

Solution • Oxid: Cd(s) Cd 2+(aq) + 2 e- -Eº • Red: Cu 2+(1 M) + 2 e. Zn. Cl 2(aq) 0. 34 V • Net: Zn(s) + Cl 2(g) Zn. Cl 2(aq) Eºcell = -Eº + 0. 34 V = 0. 74 V �Eº = -0. 40 V
 Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Introduction of electrochemistry
Introduction of electrochemistry Ap chem electrochemistry review
Ap chem electrochemistry review Chapter 21 electrochemistry
Chapter 21 electrochemistry Electrolytic cell
Electrolytic cell Chapter 20 electrochemistry
Chapter 20 electrochemistry Basic chemistry tutorial
Basic chemistry tutorial State hittorf s rule
State hittorf s rule Junction potential
Junction potential Concentration cell
Concentration cell What is electrochemistry?
What is electrochemistry? Electrochemistry balancing equations
Electrochemistry balancing equations Electrochemistry stoichiometry
Electrochemistry stoichiometry Balancing redox reactions khan academy
Balancing redox reactions khan academy Ir drop
Ir drop Balance redox reactions
Balance redox reactions Ap chemistry electrochemistry
Ap chemistry electrochemistry Balancing redox reactions
Balancing redox reactions E cell formula
E cell formula Electroanalytical methods
Electroanalytical methods Electrochemistry eds
Electrochemistry eds Fundamentals of electrochemistry
Fundamentals of electrochemistry What is electrochemistry
What is electrochemistry What is electrochemistry in chemistry
What is electrochemistry in chemistry Kno3 salt bridge
Kno3 salt bridge Diagonal rule electrochemistry
Diagonal rule electrochemistry Polarization in electrochemistry
Polarization in electrochemistry Electrochemistry lesson
Electrochemistry lesson Branches of electrochemistry
Branches of electrochemistry Chemistry
Chemistry Mass transport electrochemistry
Mass transport electrochemistry Electroanalytical techniques
Electroanalytical techniques Fundamentals of electrochemistry
Fundamentals of electrochemistry Electrochemistry
Electrochemistry Diagonal rule electrochemistry
Diagonal rule electrochemistry Ir drop in electrochemistry
Ir drop in electrochemistry Electrochemistry balancing equations
Electrochemistry balancing equations Whats electrochemistry
Whats electrochemistry Red cat electrochemistry
Red cat electrochemistry Concentration cell
Concentration cell Concentration cell
Concentration cell