Condensed States of Matter Solids and Liquids Chapter












- Slides: 26

Condensed States of Matter (Solids and Liquids) Chapter 13 (except 13 -1) Honors Chemistry Glencoe

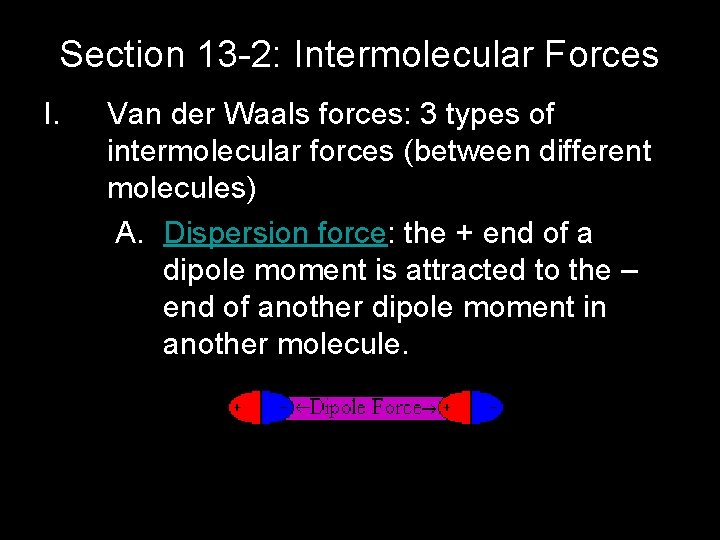
Section 13 -2: Intermolecular Forces I. Van der Waals forces: 3 types of intermolecular forces (between different molecules) A. Dispersion force: the + end of a dipole moment is attracted to the – end of another dipole moment in another molecule.

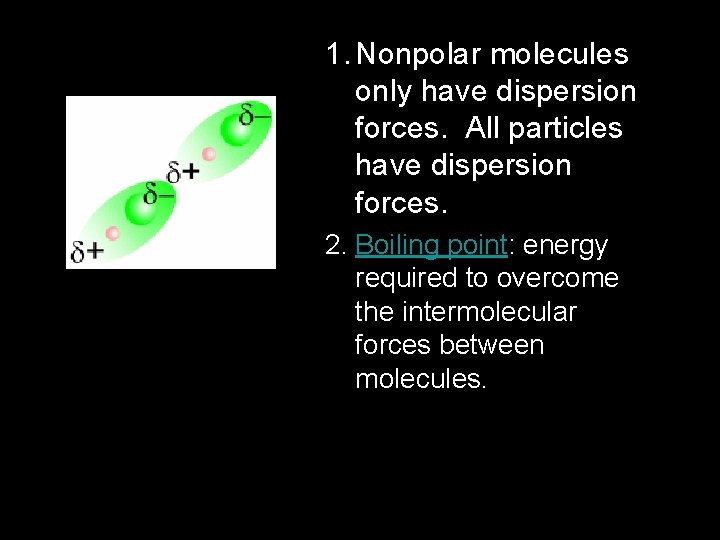
1. Nonpolar molecules only have dispersion forces. All particles have dispersion forces. 2. Boiling point: energy required to overcome the intermolecular forces between molecules.

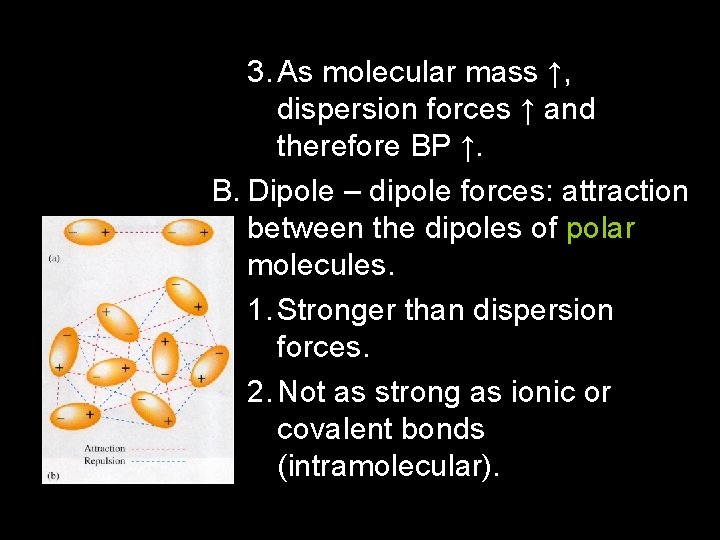
3. As molecular mass ↑, dispersion forces ↑ and therefore BP ↑. B. Dipole – dipole forces: attraction between the dipoles of polar molecules. 1. Stronger than dispersion forces. 2. Not as strong as ionic or covalent bonds (intramolecular).

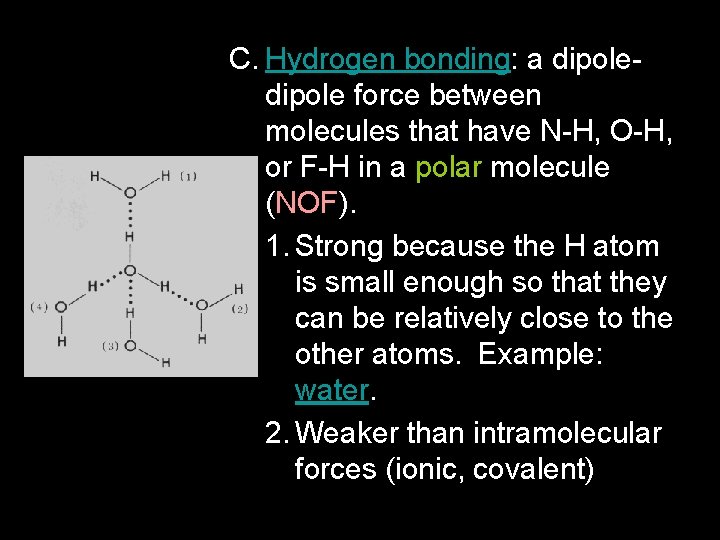
C. Hydrogen bonding: a dipole force between molecules that have N-H, O-H, or F-H in a polar molecule (NOF). 1. Strong because the H atom is small enough so that they can be relatively close to the other atoms. Example: water. 2. Weaker than intramolecular forces (ionic, covalent)

Determine the types of intermolecular forces in the following substances. 1. CO 2 nonpolar, therefore only dispersion 2. NH 3 polar and NOF, therefore all 3 3. CH 3 Cl polar and no NOF, therefore only dispersion and dipole

Determine the types of intermolecular forces in the following substances. 1. CO 2 nonpolar, therefore only dispersion 2. NH 3 polar and NOF, therefore all 3 3. CH 3 Cl polar and no NOF, therefore only dispersion and dipole You’ll need to determine the Lewis structure of the molecule, the VSEPR shape and polarity

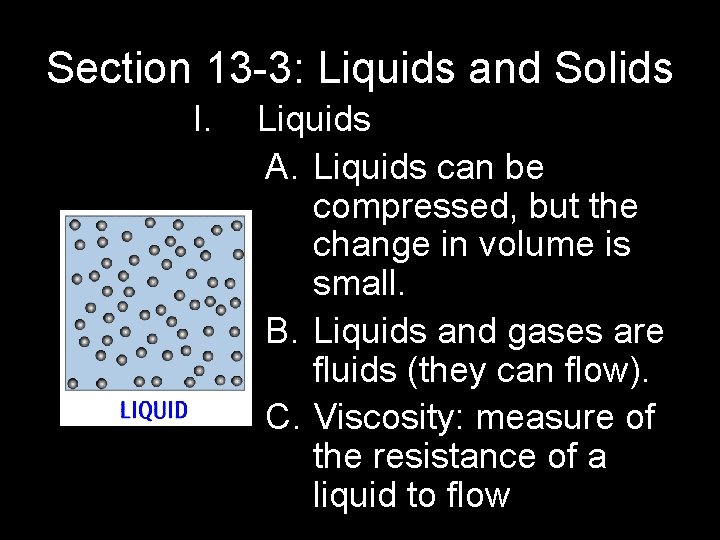
Section 13 -3: Liquids and Solids I. Liquids A. Liquids can be compressed, but the change in volume is small. B. Liquids and gases are fluids (they can flow). C. Viscosity: measure of the resistance of a liquid to flow

1. Substances with hydrogen bonds tend to have high viscosity because of the strong H-bonds. Alcohol and gas have lower viscosity than water Molasses has VERY (dispersion forces). high viscosity! What 2. Viscosity decreases at does this say about its intermolecular forces higher temperatures when compared with (breaking of H-bonds). water?

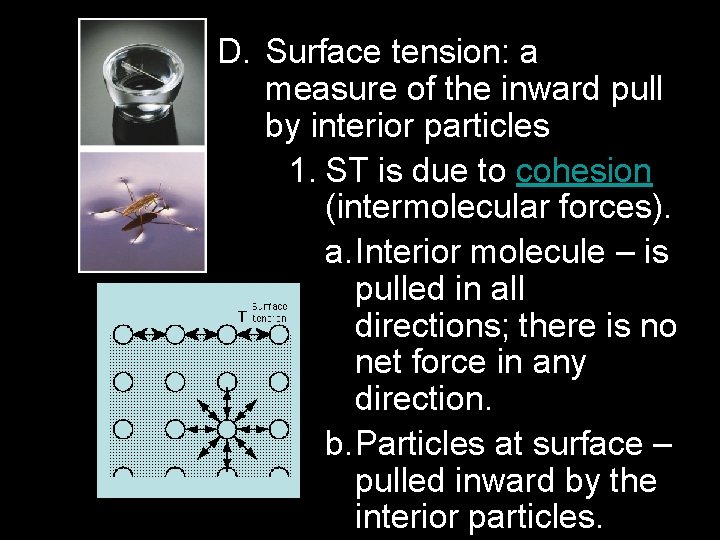
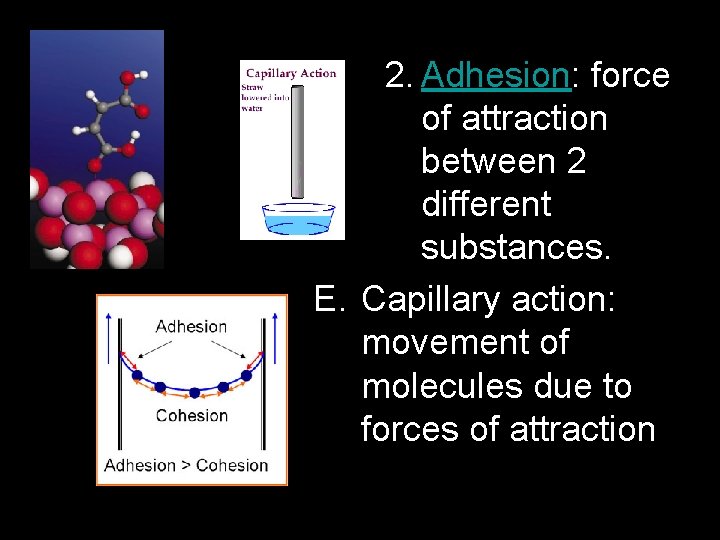
D. Surface tension: a measure of the inward pull by interior particles 1. ST is due to cohesion (intermolecular forces). a. Interior molecule – is pulled in all directions; there is no net force in any direction. b. Particles at surface – pulled inward by the interior particles.

2. Adhesion: force of attraction between 2 different substances. E. Capillary action: movement of molecules due to forces of attraction

II. Solids A. Density – for most substances, the solid is more dense than the liquid; different for water. When water freezes, it creates pockets of air that make the ice less dense.

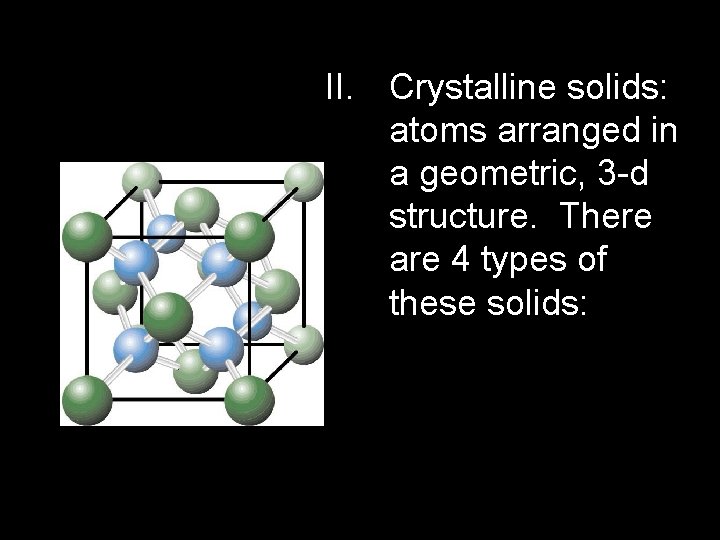
II. Crystalline solids: atoms arranged in a geometric, 3 -d structure. There are 4 types of these solids:

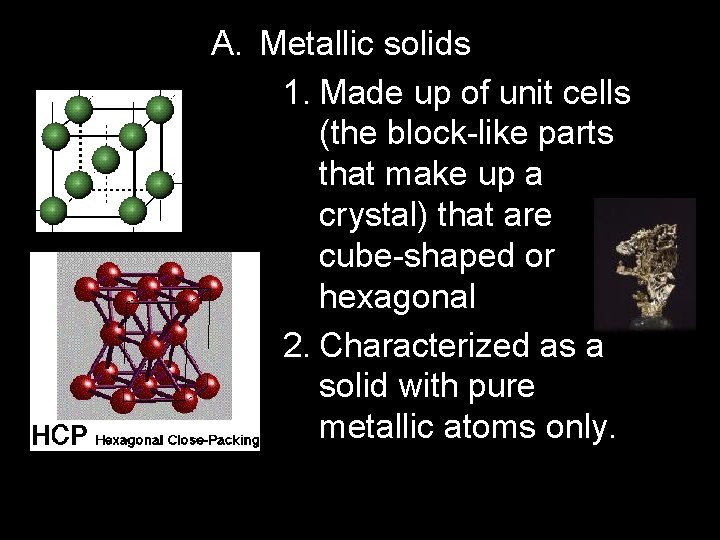
A. Metallic solids 1. Made up of unit cells (the block-like parts that make up a crystal) that are cube-shaped or hexagonal 2. Characterized as a solid with pure metallic atoms only.

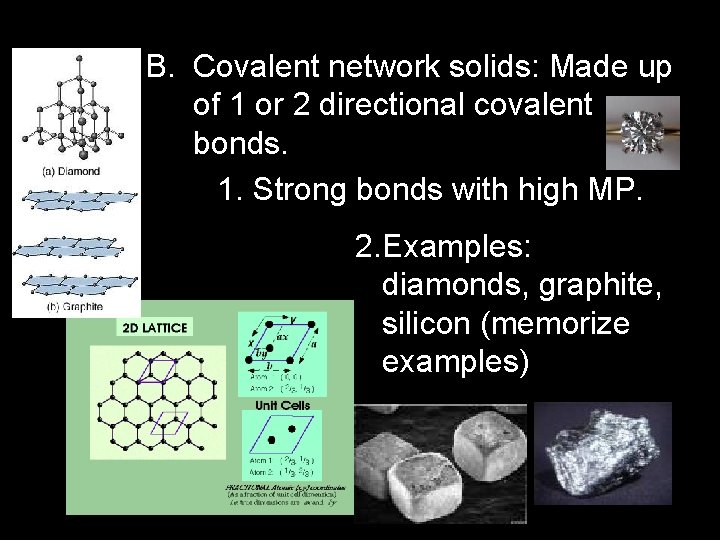
B. Covalent network solids: Made up of 1 or 2 directional covalent bonds. 1. Strong bonds with high MP. 2. Examples: diamonds, graphite, silicon (memorize examples)

C. Molecular Solids 1. Weak intermolecular forces; therefore low MP. 2. Examples: solids with nonmetals only; Ice (H 2 O), Sugar (C 12 H 22 O 11) and CO 2 (dry ice). D. Ionic Solids 1. Metal with a nonmetal. 2. + or – ions with a crystalline shape. 3. Ionic bonds are strong, therefore MP is high.

4. Arrangement of ions determined by: a. Size of ions b. Ratio of + and – ions 5. The higher the # of the charge, the greater the attraction (Ca. Cl 2 has greater attraction than Na. Cl) 6. Examples: table salt, Ca. Cl 2, etc. What are the following types of solids? 1. Tungsten 2. Copper (I) chloride 3. Sulfur dioxide

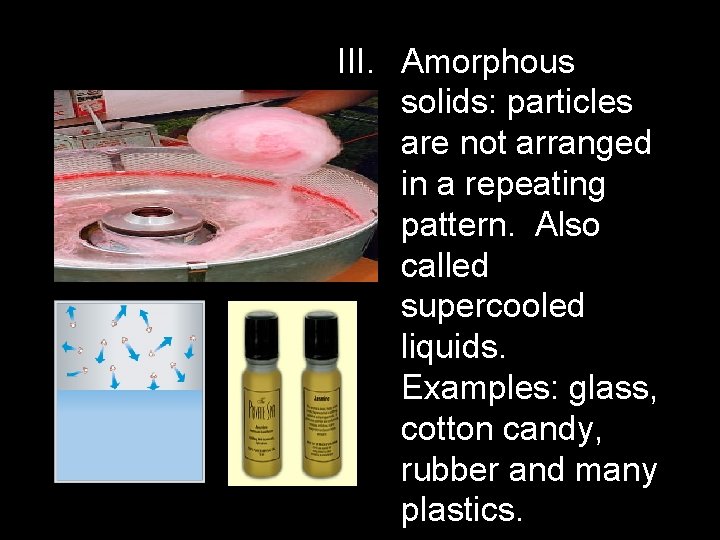
III. Amorphous solids: particles are not arranged in a repeating pattern. Also called supercooled liquids. Examples: glass, cotton candy, rubber and many plastics.

Section 13 -4: Phase Changes I. Phase Changes that require energy A. Melting (fusion) : solid into a liquid B. Vaporization: liquid becomes gas. Evaporation occurs at the surface of the liquid. 1. Boiling point: temperature at which the vapor pressure equals the external/atmospheric pressure

2. Vapor pressure: partial pressure of the vapor above the liquid. a. Increasing the # of particles will increase VP. b. VP depends on intermolecular forces of the liquid. If there is strong intermolecular forces in liquid, then the lower the VP. (Harder to become a vapor. )

3. Volatile liquids: can easily evaporate at room temperature.

C. Sublimation: solid to gas 1. Solids also have vapor pressure. 2. Ex: dry ice and mothballs.

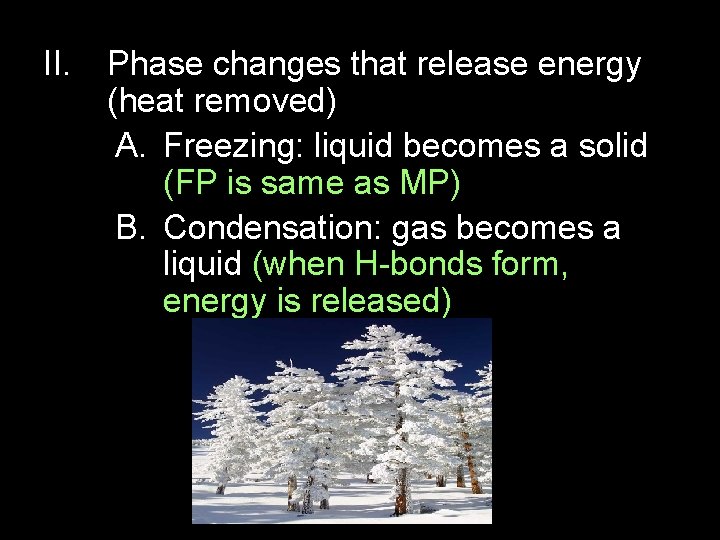
II. Phase changes that release energy (heat removed) A. Freezing: liquid becomes a solid (FP is same as MP) B. Condensation: gas becomes a liquid (when H-bonds form, energy is released)

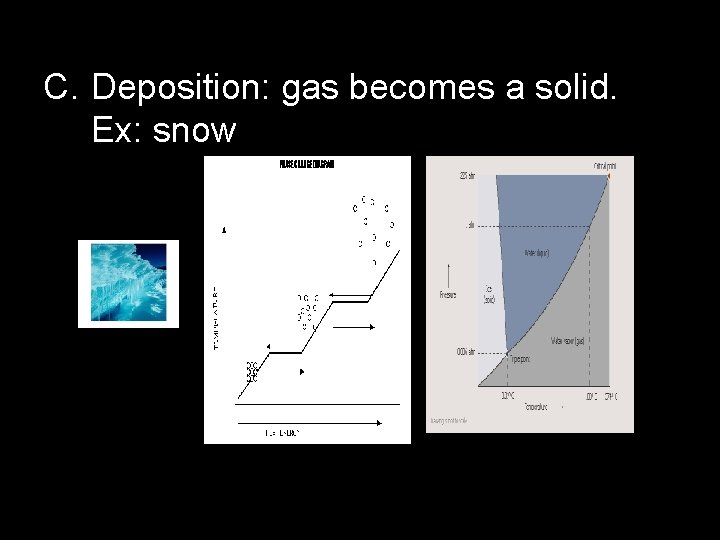
C. Deposition: gas becomes a solid. Ex: snow

III. Phase Diagrams: Relate the phases to temperature & pressure

 Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kinetic molecular theory of solid
Kinetic molecular theory of solid Thermal expansion and contraction examples
Thermal expansion and contraction examples Buoyancyability
Buoyancyability Properties of solids and liquids
Properties of solids and liquids Venn diagram states of matter
Venn diagram states of matter Properties of a solid
Properties of a solid Example of solid liquid and gas
Example of solid liquid and gas Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Liquids and solids menu
Liquids and solids menu Lesson 1 thermal energy and the behavior of matter
Lesson 1 thermal energy and the behavior of matter Liquids and solids
Liquids and solids Particle movement in solids liquids and gases
Particle movement in solids liquids and gases How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Properties of solids liquids and gases
Properties of solids liquids and gases Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Why are gases easy to compress
Why are gases easy to compress Constant rate filtration example
Constant rate filtration example Umd cmtc
Umd cmtc Chapter 12 states of matter study guide
Chapter 12 states of matter study guide Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Plasma particles arrangement
Plasma particles arrangement Gray matter in the brain
Gray matter in the brain Cerebral aqueduct
Cerebral aqueduct Gray matter and white matter
Gray matter and white matter