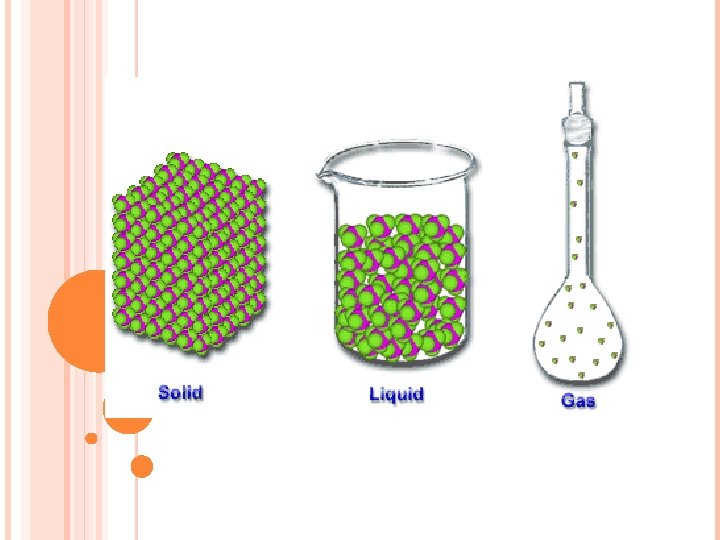
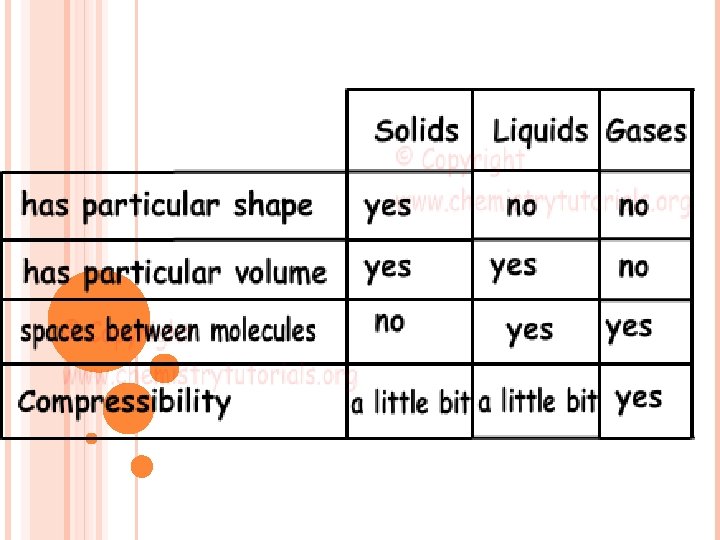
STATES OF MATTER PROPERTIES OF MATTER Solids Liquids













- Slides: 20

STATES OF MATTER & PROPERTIES OF MATTER Solids, Liquids & Gases Characteristic vs Non-characteristic properties


LET’S SEE WHAT OTHER PEOPLE THINK… https: //www. youtube. com/watch? v=KCL 8 zqj. Xb. ME



WORD BANK (SOME WORDS MAY BE USED MORE THAN ONCE) Particles Substances Motion Temperature Held Higher together Forces Spaces between Large Tiny Different Faster

PROPERTIES Properties are information about a substance that describes it and helps us identify it

NON-CHARACTERISTIC PROPERTIES Non-characteristic properties CANNOT help us identify a substance or the group it belongs to These are properties like: - Mass: tells you how much stuff is inside an object - Volume: tell you how much space an object occupies - Color - Temperature: tells you how much the particles of a substance are agitated. The higher the temperature, the higher the energy, the faster the particles move because they are more AGITATED

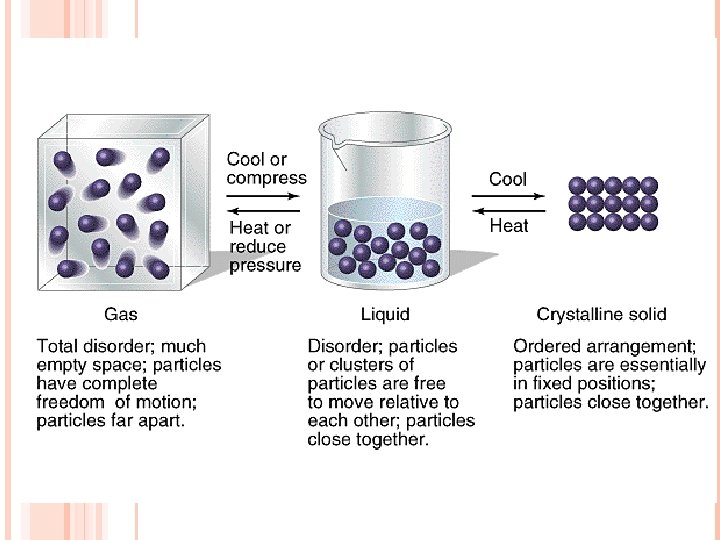
MORE ON TEMPERATURE… Like people, particles like to get close together when it is COLD, and far apart when it is HOT

When an object is heated, it will expand because the particles do not want to be near each other The particles will become more and more agitated until it melts or vaporizes

CHARACTERISTIC PROPERTIES Characteristic properties EASILY help you IDENTIFY a substance or the group it belongs to because they never change These properties can either be: - Characteristic physical properties, which can be measured - Characteristic chemical properties, which can be observed when chemical reactions take place

CHARACTERISTIC PHYSICAL PROPERTIES Density: the density of a substance refers to how much stuff (matter) is inside a certain volume. To - find density, you must: Weigh an object Determine its volume Divide it mass by its volume Density = mass volume

Gases The have a lower density that solids. density of a substance at a specific temperature always remains the same. For example, Pure water at room temperature always has a density of 1. 00 g/m. L.

CHARACTERISTIC PHYSICAL PROPERTIES Other physical characteristic properties that can be measured are: - Melting point Boiling point Freezing point Electrical conductivity

CHARACTERISTIC CHEMICAL PROPERTIES Can - - be observed if a chemical reaction happens Reaction to a flame (hydrogen explodes when exposed to a flame) Reaction to limewater (carbon dioxide makes limewater become cloudy) Reaction to cobalt chloride paper (water makes this paper turn pink) Reaction to litmus paper (acids turn litmus paper red and bases turn litmus paper blue)

USES OF MATERIALS How we use different materials depends on their characteristic properties Examples: - Metal is used to make pots because it is a good conductor of heat

ACIDS, BASES & SALTS Acids, bases and salts are CHEMICALS Most compounds can be classified as acids, bases or salts Acids taste SOUR (citrus fruits have acids in them) Bases taste BITTER (most cleaning products are bases) Bases that dissolve in water are called ALKALIS The amount of acidity or alkalinity of a substance can be classified by understanding the p. H scale

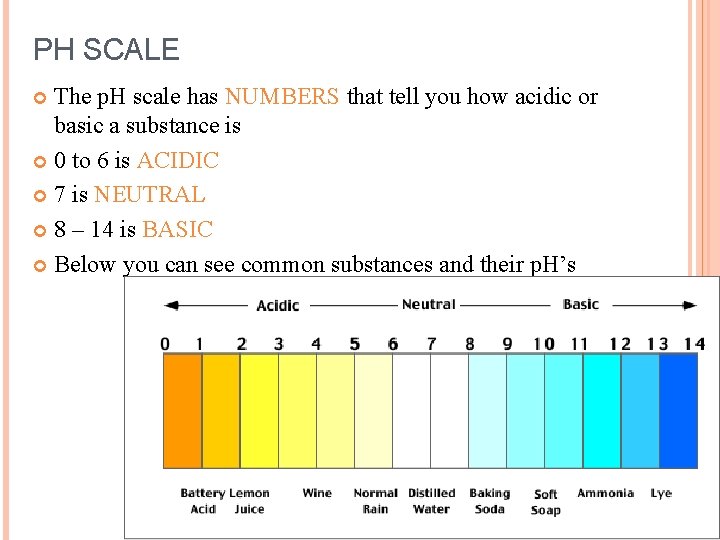
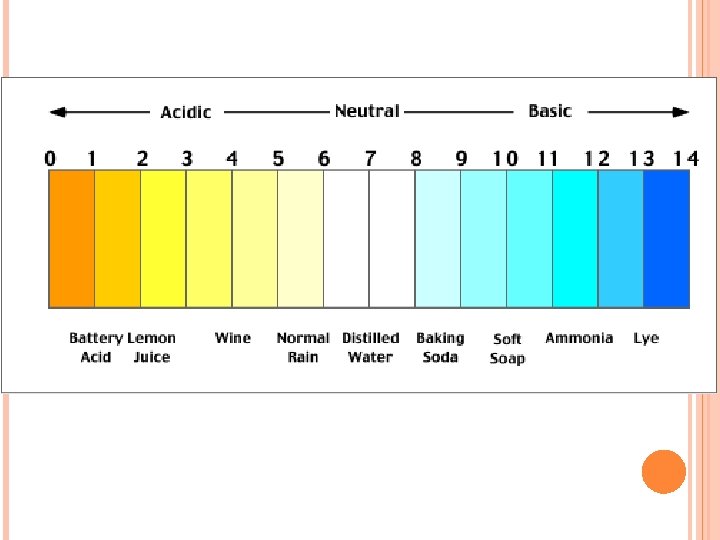
PH SCALE The p. H scale has NUMBERS that tell you how acidic or basic a substance is 0 to 6 is ACIDIC 7 is NEUTRAL 8 – 14 is BASIC Below you can see common substances and their p. H’s


ACIDS, BASES & SALTS When equal amounts of an acid and base are mixed, they neutralize each other New substances are formed: a salt and water The p. H of these new substance changes to 7 (neutral)