Chemistry of the Stratosphere Introduction Chemical Composition A












![• NO 2 -NO Interconversion z (km) T (K) [NO]/[NO 2] 0 288 • NO 2 -NO Interconversion z (km) T (K) [NO]/[NO 2] 0 288](https://slidetodoc.com/presentation_image_h2/19b257c482cf5c3c9ff1ea07a04e579e/image-36.jpg)






- Slides: 49

Chemistry of the Stratosphere

Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle Stratospheric Ozone Loss Halides Stratospheric O 3 Production and Loss NOx Contribution to O 3 Destruction Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Why is there “no” Arctic Ozone Hole? Policy Solution to ozone hole problem Recovery of stratospheric ozone Summary 2 12/19/2021 Add a footer

Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Stratospheric O 3 Production and Loss NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone 3 12/19/2021 Introduction • 10 -50 km above the surface • Temperature constant or INCREASING with height • Stable (not a lot of vertical mixing) and dry • Only occasionally get overshooting cloud tops from convection pushing into this layer • “Oxygen-only chemistry…” The Stratosphere CHEM 196 Petrucci

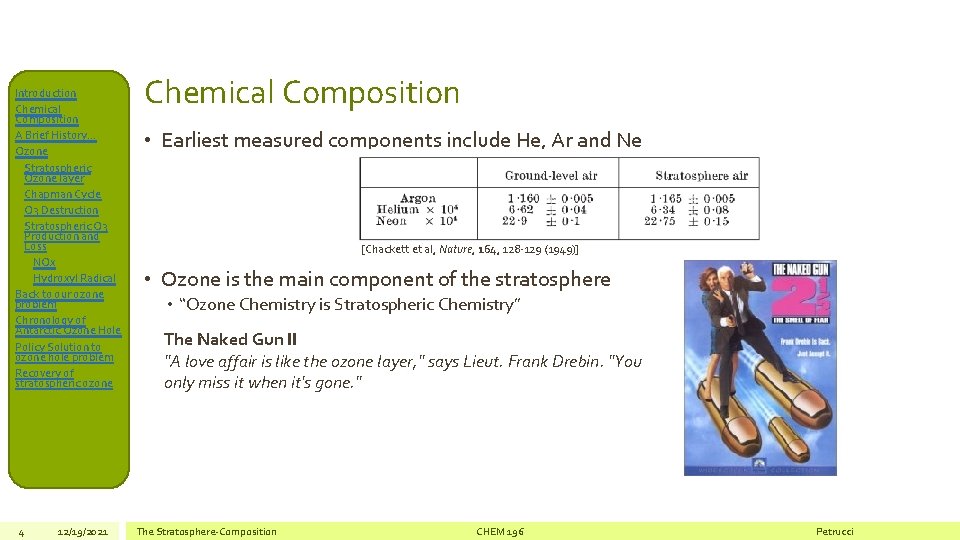
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Stratospheric O 3 Production and Loss NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone 4 12/19/2021 Chemical Composition • Earliest measured components include He, Ar and Ne [Chackett et al, Nature, 164, 128 -129 (1949)] • Ozone is the main component of the stratosphere • “Ozone Chemistry is Stratospheric Chemistry” The Naked Gun II "A love affair is like the ozone layer, " says Lieut. Frank Drebin. "You only miss it when it's gone. " The Stratosphere-Composition CHEM 196 Petrucci

Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Stratospheric O 3 Production and Loss NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone A Brief History… • 1840’s Ozone first discovered and measured in air by Schonbein (from Greek ozein = smell) • 1880 -1900’s: Hartley postulates the existence of a layer above the troposphere, where ozone is responsible for the absorption of solar UV between 200 and 300 nm • 1913: Fabry and Buisson used UV measurements to estimate that if brought down to the surface at STP, O 3 would form a layer ~ 3 mm thick • 1920 -25: Dobson first shows that T(z) in stratosphere not constant but increases with height (z) and implicates O 3 absorption. Makes first extensive set of O 3 column measurements with his spectrophotometer • 1930: Chapman proposed that O 3 is produced continually in a cycle initiated by O 2 photolysis 5 12/19/2021 The Stratosphere-Composition CHEM 196 Petrucci

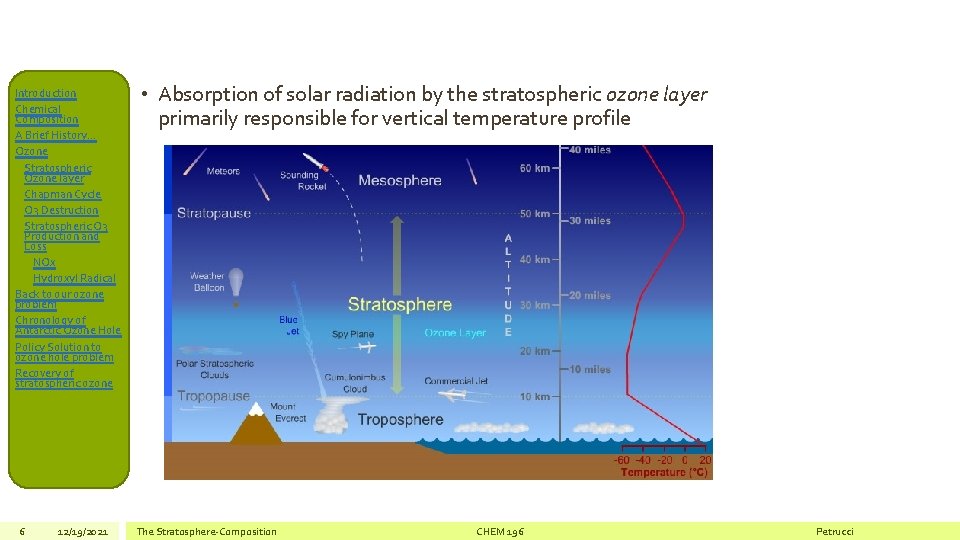
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Stratospheric O 3 Production and Loss NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone 6 12/19/2021 • Absorption of solar radiation by the stratospheric ozone layer primarily responsible for vertical temperature profile The Stratosphere-Composition CHEM 196 Petrucci

Ozone

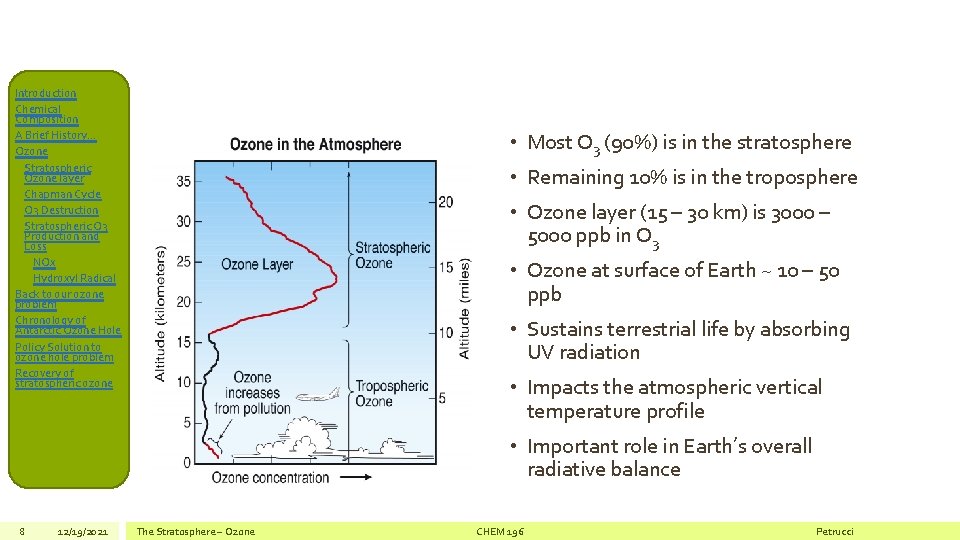
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Stratospheric O 3 Production and Loss NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone • Most O 3 (90%) is in the stratosphere • Remaining 10% is in the troposphere • Ozone layer (15 – 30 km) is 3000 – 5000 ppb in O 3 • Ozone at surface of Earth ~ 10 – 50 ppb • Sustains terrestrial life by absorbing UV radiation • Impacts the atmospheric vertical temperature profile • Important role in Earth’s overall radiative balance 8 12/19/2021 The Stratosphere – Ozone CHEM 196 Petrucci

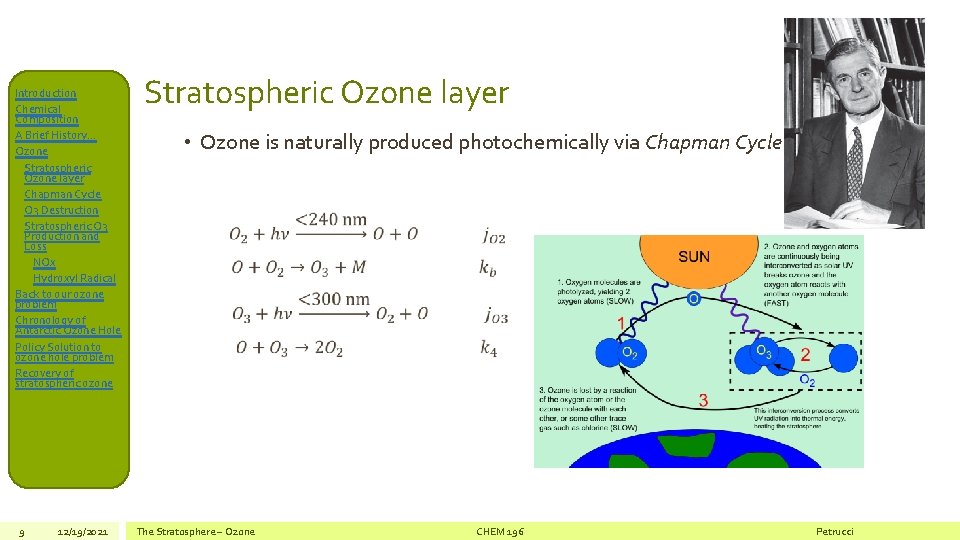
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Stratospheric O 3 Production and Loss NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone 9 12/19/2021 Stratospheric Ozone layer • Ozone is naturally produced photochemically via Chapman Cycle The Stratosphere – Ozone CHEM 196 Petrucci

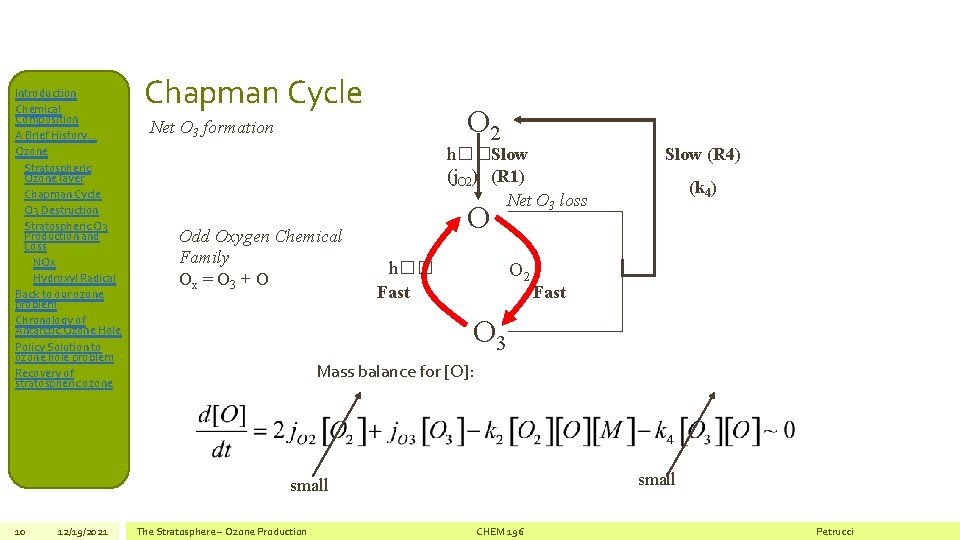
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Stratospheric O 3 Production and Loss NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone Chapman Cycle O 2 Net O 3 formation h��Slow (j. O 2) (R 1) Net O 3 loss Odd Oxygen Chemical Family Ox = O 3 + O 12/19/2021 (k 4) O h�� Fast O 2 Fast O 3 Mass balance for [O]: small 10 Slow (R 4) The Stratosphere – Ozone Production CHEM 196 Petrucci

Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Stratospheric O 3 Production and Loss NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone 11 12/19/2021 The Stratosphere – Ozone Production CHEM 196 Petrucci

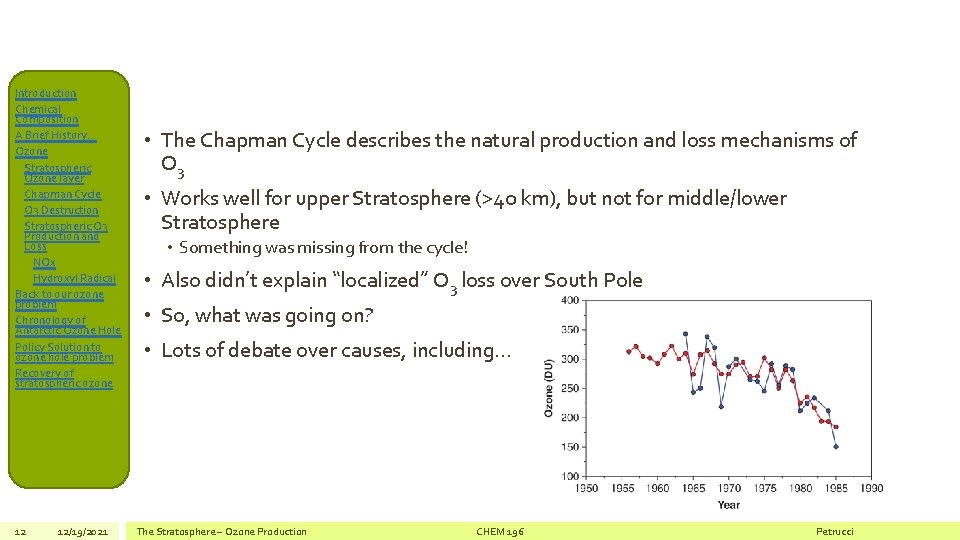
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Stratospheric O 3 Production and Loss NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone 12 12/19/2021 • The Chapman Cycle describes the natural production and loss mechanisms of O 3 • Works well for upper Stratosphere (>40 km), but not for middle/lower Stratosphere • Something was missing from the cycle! • Also didn’t explain “localized” O 3 loss over South Pole • So, what was going on? • Lots of debate over causes, including… The Stratosphere – Ozone Production CHEM 196 Petrucci

Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Stratospheric O 3 Loss NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone Stratospheric Ozone Loss 1986! • There was also a real scientific debate over the relative roles of chemistry and meteorology …Turns out to be both chemistry and meteorology (of course!), but mainly because the meteorology facilitates the chemistry 13 12/19/2021 The Stratosphere – Ozone Destruction CHEM 196 Petrucci

Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone Halides Mario Molina and F. Sherwood Rowland at UC, Irvine 14 12/19/2021 The Stratosphere – Ozone Destruction CHEM 196 Petrucci

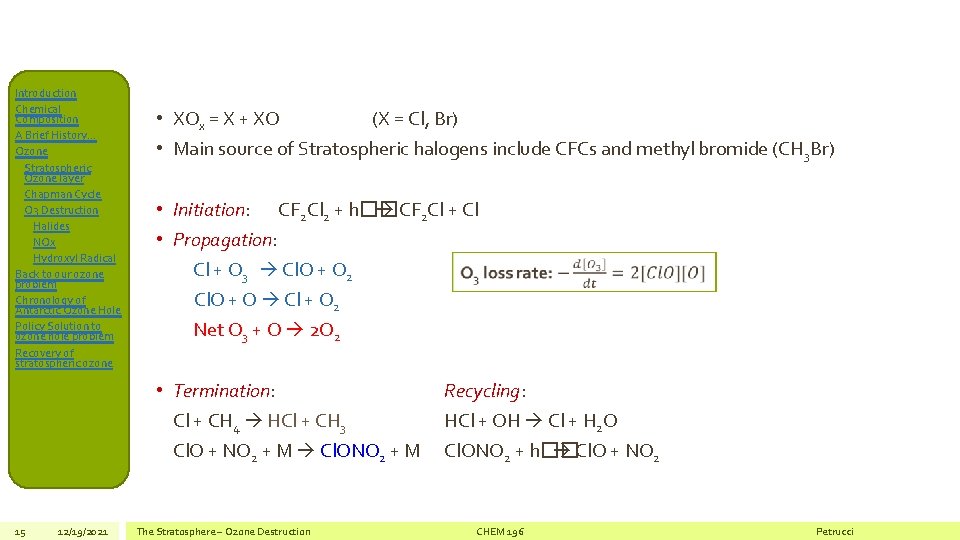
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone • XOx = X + XO (X = Cl, Br) • Main source of Stratospheric halogens include CFCs and methyl bromide (CH 3 Br) • Initiation: CF 2 Cl 2 + h�� CF 2 Cl + Cl • Propagation: Cl + O 3 Cl. O + O 2 Cl. O + O Cl + O 2 Net O 3 + O 2 O 2 • Termination: Cl + CH 4 HCl + CH 3 Cl. O + NO 2 + M Cl. ONO 2 + M 15 12/19/2021 The Stratosphere – Ozone Destruction Recycling: HCl + OH Cl + H 2 O Cl. ONO 2 + h�� Cl. O + NO 2 CHEM 196 Petrucci

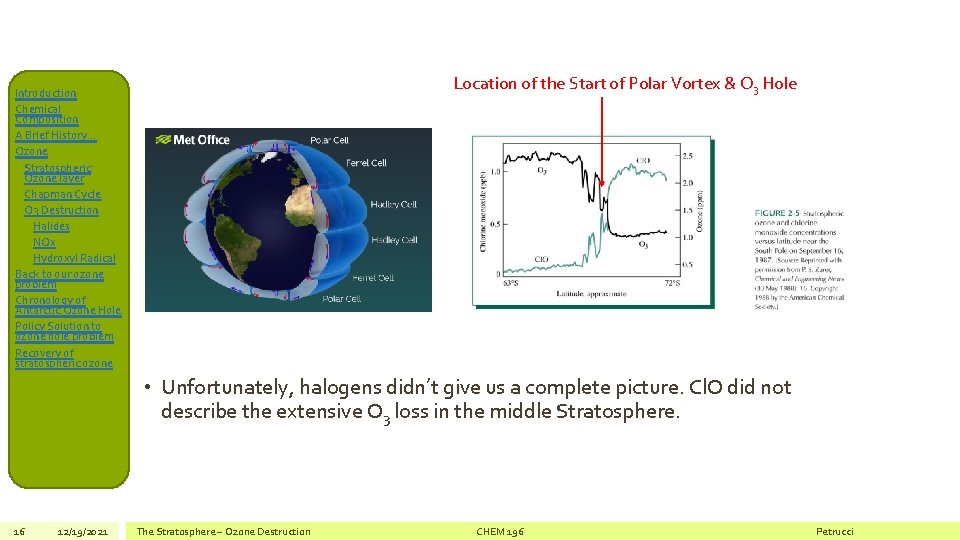
Location of the Start of Polar Vortex & O 3 Hole Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone • Unfortunately, halogens didn’t give us a complete picture. Cl. O did not describe the extensive O 3 loss in the middle Stratosphere. 16 12/19/2021 The Stratosphere – Ozone Destruction CHEM 196 Petrucci

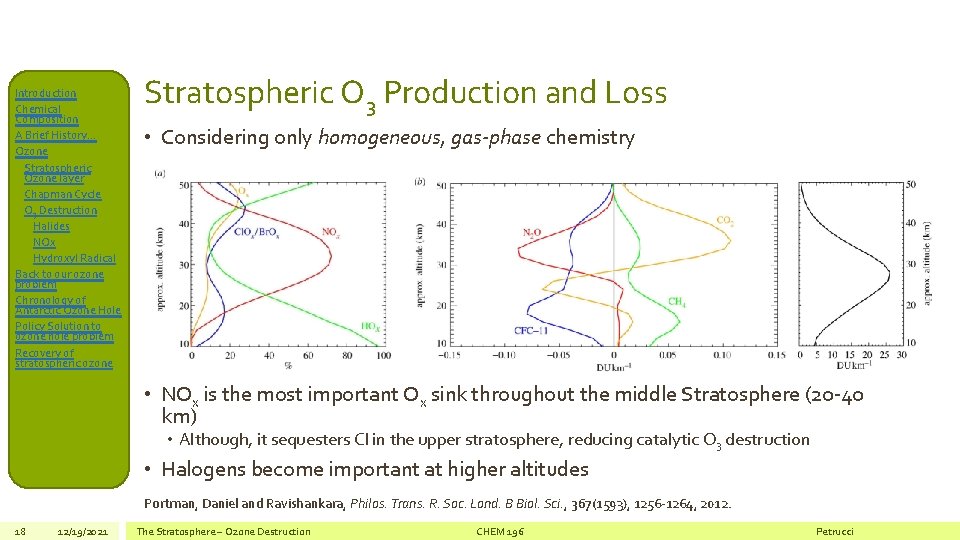
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone Stratospheric O 3 Production and Loss • Considering only homogeneous, gas-phase chemistry • NOx is the most important Ox sink throughout the middle Stratosphere (20 -40 km) • Although, it sequesters Cl in the upper stratosphere, reducing catalytic O 3 destruction • Halogens become important at higher altitudes Portman, Daniel and Ravishankara, Philos. Trans. R. Soc. Lond. B Biol. Sci. , 367(1593), 1256 -1264, 2012. 18 12/19/2021 The Stratosphere – Ozone Destruction CHEM 196 Petrucci

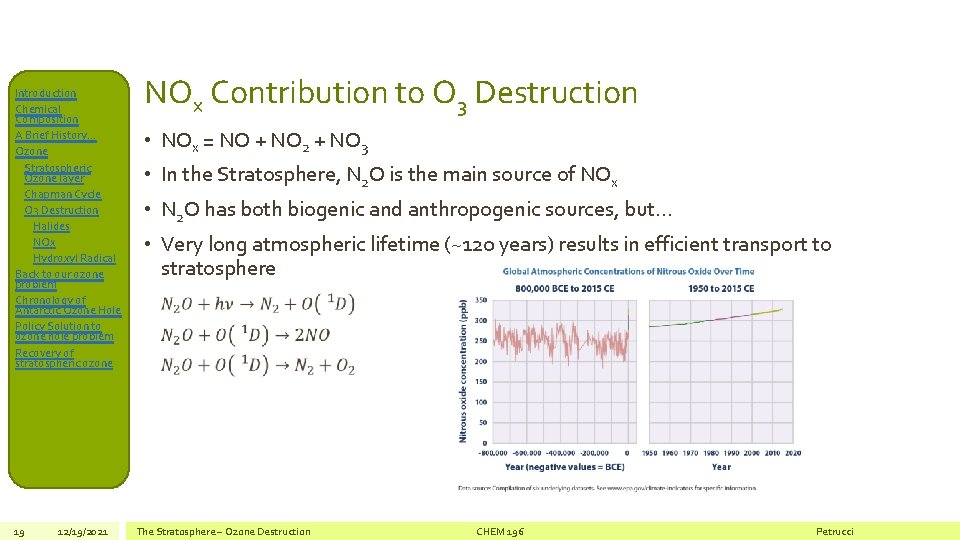
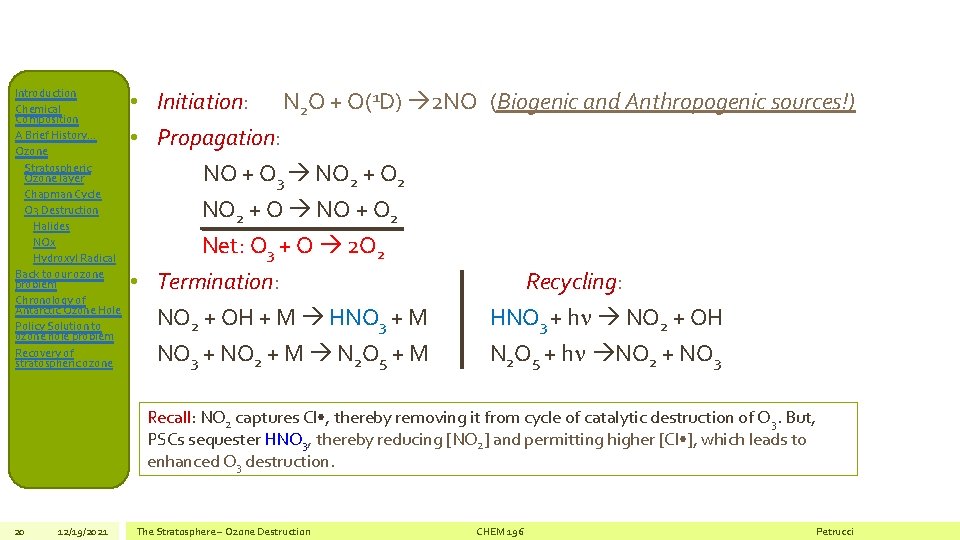
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone 19 12/19/2021 NOx Contribution to O 3 Destruction • NOx = NO + NO 2 + NO 3 • In the Stratosphere, N 2 O is the main source of NOx • N 2 O has both biogenic and anthropogenic sources, but… • Very long atmospheric lifetime (~120 years) results in efficient transport to stratosphere The Stratosphere – Ozone Destruction CHEM 196 Petrucci

Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone • Initiation: N 2 O + O(1 D) 2 NO (Biogenic and Anthropogenic sources!) • Propagation: NO + O 3 NO 2 + O 2 NO 2 + O NO + O 2 Net: O 3 + O 2 O 2 • Termination: Recycling: NO 2 + OH + M HNO 3 + M HNO 3 + h NO 2 + OH NO 3 + NO 2 + M N 2 O 5 + M N 2 O 5 + h NO 2 + NO 3 Recall: NO 2 captures Cl • , thereby removing it from cycle of catalytic destruction of O 3. But, PSCs sequester HNO 3, thereby reducing [NO 2] and permitting higher [Cl • ], which leads to enhanced O 3 destruction. 20 12/19/2021 The Stratosphere – Ozone Destruction CHEM 196 Petrucci

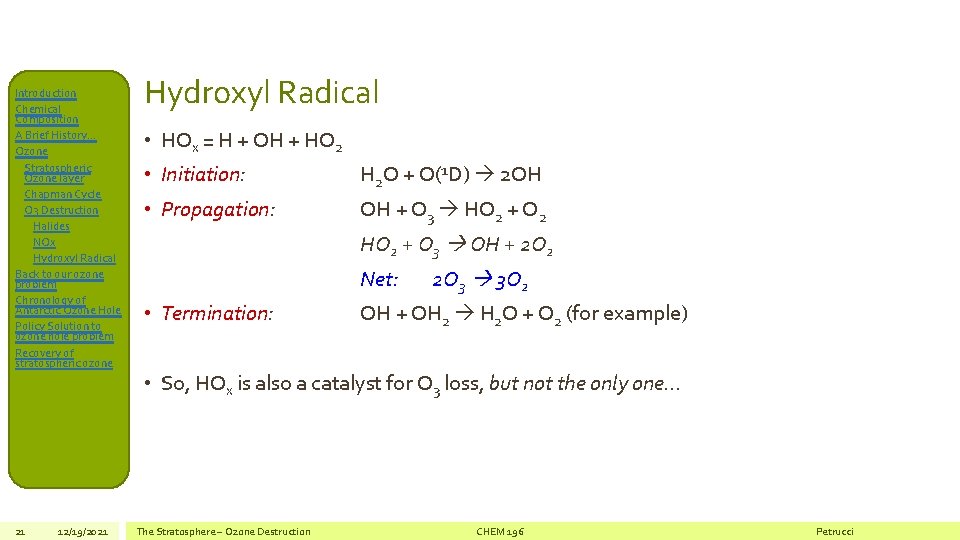
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone 21 12/19/2021 Hydroxyl Radical • HOx = H + OH + HO 2 • Initiation: H 2 O + O(1 D) 2 OH • Propagation: OH + O 3 HO 2 + O 2 HO 2 + O 3 OH + 2 O 2 Net: • Termination: 2 O 3 3 O 2 OH + OH 2 H 2 O + O 2 (for example) • So, HOx is also a catalyst for O 3 loss, but not the only one… The Stratosphere – Ozone Destruction CHEM 196 Petrucci

Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone 22 12/19/2021 Back to our ozone problem • Nothing we’ve discussed so far explains the cyclic behavior of the ozone hole • Why is O 3 depletion enhanced in austral Spring • What about the role of PSCs in stratospheric chemistry? The Stratosphere – Ozone Destruction CHEM 196 Petrucci

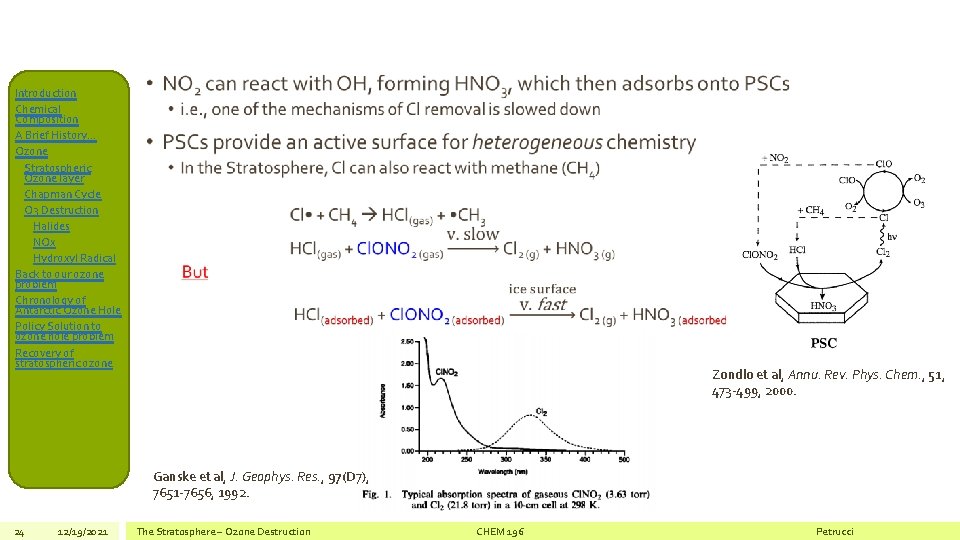
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone • PSCs form in the cold (T < 70 o. C) winter season in the South Pole • They are composed primarily of nitric acid/water crystals • They sequester gas-phase stratospheric NO 2 and, • Provide a surface for heterogeneous chemistry • Recall the catalytic role of Cl atoms in stratospheric O 3 destruction: Cl + O 3 Cl. O + O 2 Cl. O + O Cl + O 2 • Also recall that one possible termination pathway to removing Cl from this chemistry is Cl. O + NO 2 + M Cl. ONO 2 + M • Cl. ONO 2 is a reservoir species for Cl, trapping it • But… 23 12/19/2021 The Stratosphere – Ozone Destruction CHEM 196 Petrucci

Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone • Zondlo et al, Annu. Rev. Phys. Chem. , 51, 473 -499, 2000. Ganske et al, J. Geophys. Res. , 97(D 7), 7651 -7656, 1992. 24 12/19/2021 The Stratosphere – Ozone Destruction CHEM 196 Petrucci

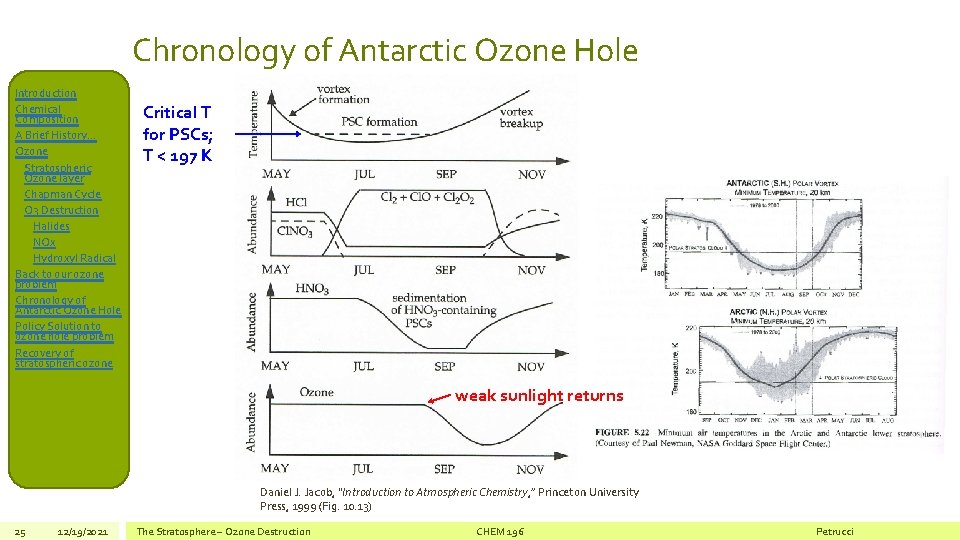
Chronology of Antarctic Ozone Hole Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone Critical T for PSCs; T < 197 K weak sunlight returns Daniel J. Jacob, “Introduction to Atmospheric Chemistry, ” Princeton University Press, 1999 (Fig. 10. 13) 25 12/19/2021 The Stratosphere – Ozone Destruction CHEM 196 Petrucci

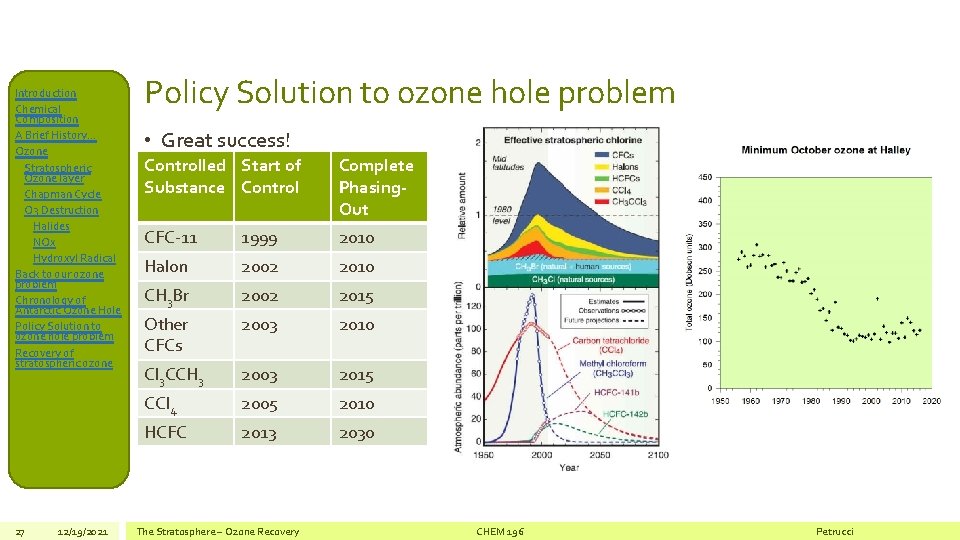
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone 27 12/19/2021 Policy Solution to ozone hole problem • Great success! Controlled Start of Substance Control Complete Phasing. Out CFC-11 1999 2010 Halon 2002 2010 CH 3 Br 2002 2015 Other CFCs 2003 2010 Cl 3 CCH 3 2003 2015 CCl 4 2005 2010 HCFC 2013 2030 The Stratosphere – Ozone Recovery CHEM 196 Petrucci

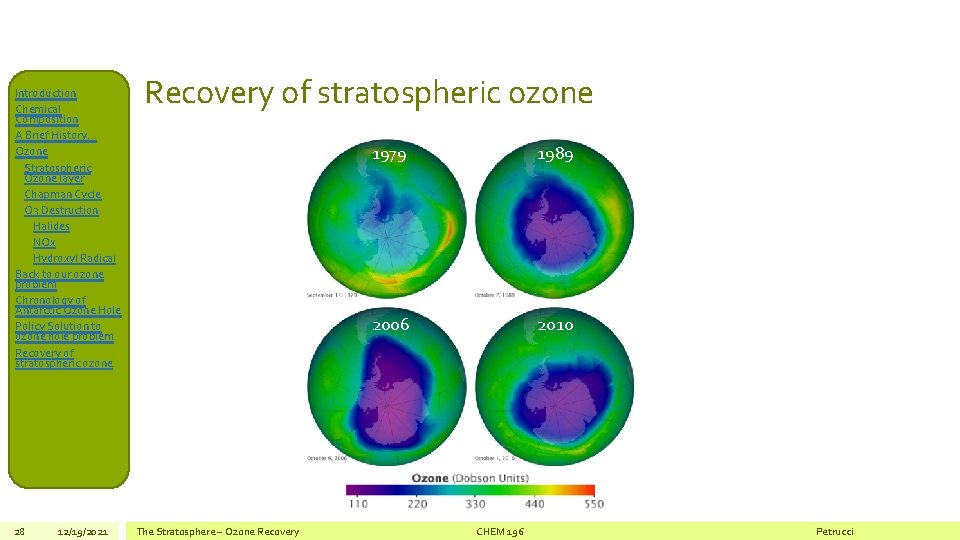
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone 28 12/19/2021 Recovery of stratospheric ozone The Stratosphere – Ozone Recovery 1979 1989 2006 2010 CHEM 196 Petrucci

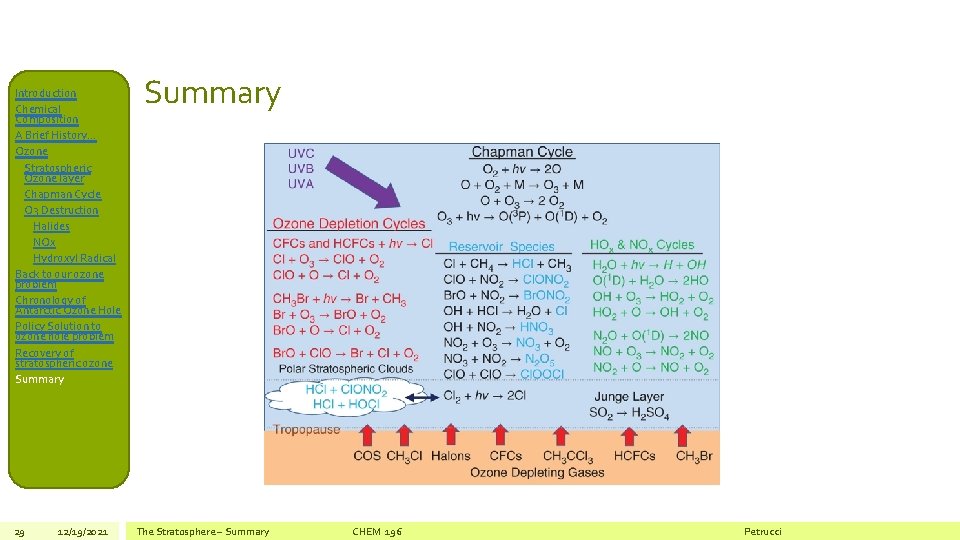
Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone Summary 29 12/19/2021 Summary The Stratosphere – Summary CHEM 196 Petrucci

Introduction Chemical Composition A Brief History… Ozone Stratospheric Ozone layer Chapman Cycle O 3 Destruction Halides NOx Hydroxyl Radical Back to our ozone problem Chronology of Antarctic Ozone Hole Policy Solution to ozone hole problem Recovery of stratospheric ozone Summary 30 12/19/2021 • Chemistry may be the “central science” to describe O 3 depletion in the upper atmosphere, but as is often the case with most questions of environmental importance, it is not the only player! The Stratosphere – Summary CHEM 196 Petrucci

31 Extra Slides

1. The original Chapman mechanism included a fifth reaction: O + M O 2 + M What would be the effect of this reaction on ozone? Is it more important in the lower or in the upper stratosphere? 2. [O]~109 molec/cm 3 in the upper stratosphere and ~ 106 in the lower stratosphere, if the lifetime of O 3 w. r. t. transport away from equator is ~ month, is it fair to assume O 3 is in a chemical steady state throughout the stratosphere? k. O+O 3 (US) ~ 4 x 10 -15 cm 3/molec/s; k. O+O 3(LS) ~ 3 x 10 -16 cm 3/molec/

Questions 1. Of the ozone loss mechanisms we have examined so far, can any operate at night? 2. There are several variations possible for NOx and HOx catalyzed O 3 destruction cycles. Given that NO 3 photolysis can proceed via one of two channels a) NO 3 + hv NO 2 + O b) NO 3 + hv NO + O 2 Come up with at least one additional catalytic cycle for NOx. 3. A minor oxidation pathway for NO is HO 2 + NO -> OH + NO 2 What is the net effect of this reaction on ozone? 33


Nitrogen Oxide (NOx) Family – move to • NOx = NO + NO 2 Tropospheric Chemistry topic • NO is one of a very few atmospheric molecules that absorb and photolyze in the visible range 2 • Photolysis occurs with nearly 100% yield below 398 nm • Photolysis rates can be very large! Roehl et al, J. Phys. Chem. , 98: 1 -5 (1994) • This will become especially important when we talk about Tropospheric chemistry Volz-Thomas et al, J. Geophys. Res. , 101: 18613 (1996)

The Chapman Model • Valid for mid-Stratosphere • Underestimates ξO 3 in other layers of Stratosphere • Overestimates ξO 3 in middle layer by factor of 2 • What’s missing chemically?

Role of NOx in O 3 Destruction From j. O 3 below 300 nm Reactive NOx Rapid interconversion of NO and NO 2 (i. e. , NOx) O(1 D) can react with both NO 2 and O 3 However, even though ξO 3 ~ 100 x ξNO 2, the rate of reaction of O with NO 2 is much faster and reaction of O-atom with NO 2 is favored.
![NO 2 NO Interconversion z km T K NONO 2 0 288 • NO 2 -NO Interconversion z (km) T (K) [NO]/[NO 2] 0 288](https://slidetodoc.com/presentation_image_h2/19b257c482cf5c3c9ff1ea07a04e579e/image-36.jpg)
• NO 2 -NO Interconversion z (km) T (K) [NO]/[NO 2] 0 288 0. 72 1. 8 10 256 2. 6 4. 2 50 223 12. 6 18. 6

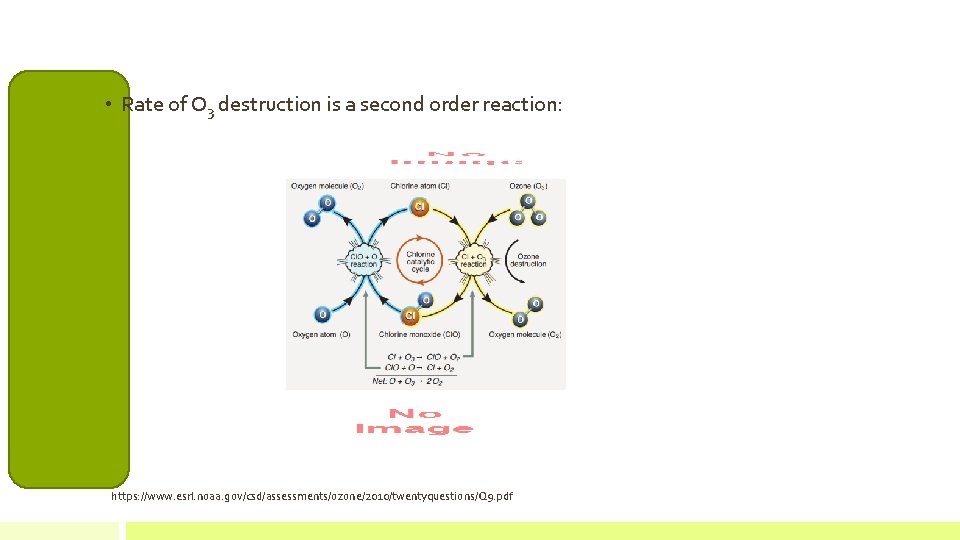
• Rate of O 3 destruction is a second order reaction: https: //www. esrl. noaa. gov/csd/assessments/ozone/2010/twentyquestions/Q 9. pdf

Main Seek To Answer in Part 1 of the Course. What are their • What are. Questions the species We (gases & particles) present in the atmosphere? natural and anthropogenic sources? • What chemical reactions do these species undergo in the atmosphere? • What are the products from the atmospheric transformations of these species? • What effects do the presence of these species and their chemical transformation products have on the atmosphere, climate, and human health?

• Three gases make up ~99% of total atmospheric mass (~5. 14 x 1018 kg) • N 2 (78%) – diatomic nitrogen • O 2 (21%) – diatomic oxygen • Ar (1%) – argon • These 3 gases are relatively un-reactive and their mean residence times are much longer that the rate of atmospheric mixing • As a result, their concentrations are relatively uniform globally • Water vapor is the next most abundant constituent • Found in the lowest part of the atmosphere • Concentrations are variable • Can be as high as 3% in some areas • Remaining gaseous constituents, trace gases, represent <1% of atmosphere, but play critical role in Earth’s radiative balance

Structure of Earth’s • Defined by temperature Atmosphere Chapman Cycle (responsible for steady-state conc. O 3): Rxn 1: O 2 + h (< 240 nm) 2 O Rxn 2: O 2 + O + M O 3 + M + heat Rxn 3: O 3 + O 2 O 2 Rxn 4: O 3 + h O + O 2 Stable Air – Vertical Mixing Slow Ozone Layer Stratospheric Aerosol Lifetimes ~ 1 – 2 years Contains ~ 80% of total atmospheric mass Unstable Air – Rapid Vertical Mixing (all weather here) Most of the H 2 O (gas) here Contains Most Atmospheric Aerosol Mass (< ~ 10 km) Tropospheric Aerosol Lifetimes ~ hours – 2 weeks

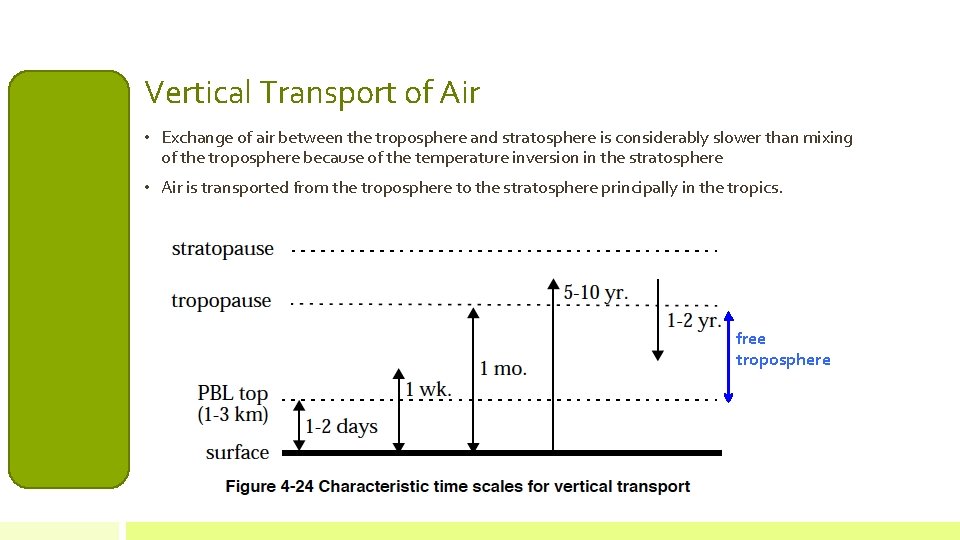
Vertical Transport of Air • Exchange of air between the troposphere and stratosphere is considerably slower than mixing of the troposphere because of the temperature inversion in the stratosphere • Air is transported from the troposphere to the stratosphere principally in the tropics. free troposphere

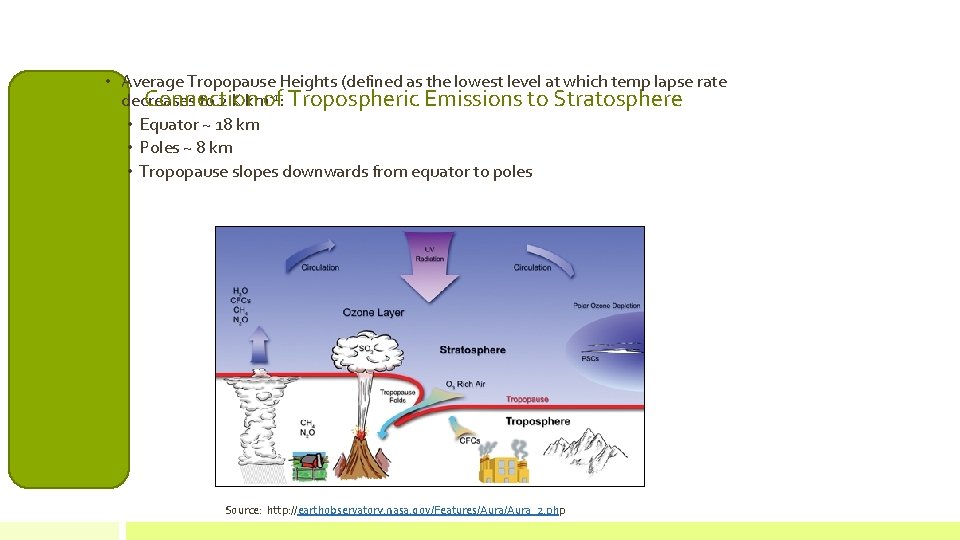
• Average Tropopause Heights (defined as the lowest level at which temp lapse rate -1: Tropospheric Emissions to Stratosphere Connection decreases to 2 K kmof • Equator ~ 18 km • Poles ~ 8 km • Tropopause slopes downwards from equator to poles Source: http: //earthobservatory. nasa. gov/Features/Aura_2. php

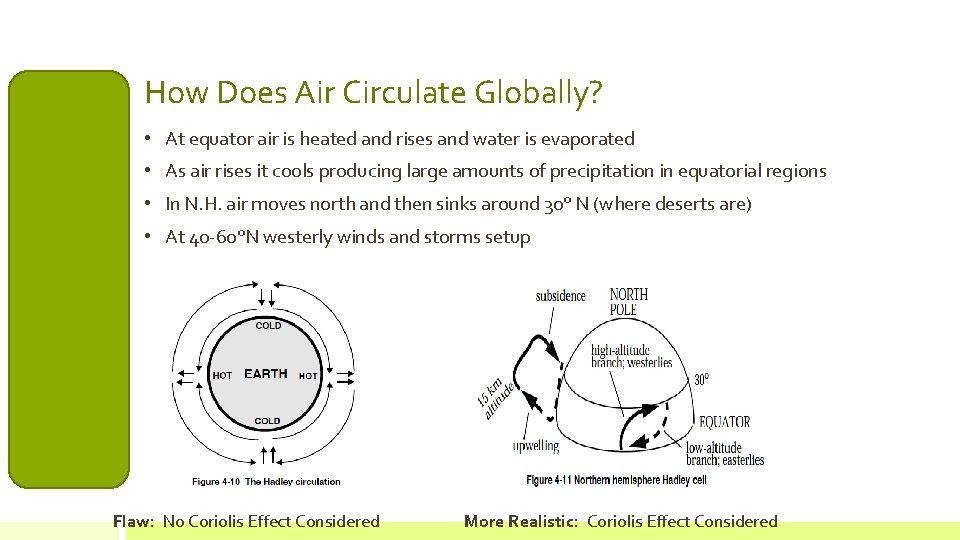
How Does Air Circulate Globally? • At equator air is heated and rises and water is evaporated • As air rises it cools producing large amounts of precipitation in equatorial regions • In N. H. air moves north and then sinks around 30° N (where deserts are) • At 40 -60°N westerly winds and storms setup Flaw: No Coriolis Effect Considered More Realistic: Coriolis Effect Considered

https: //www. metoffice. gov. uk/learning/atmosphere/global-circulation-patterns

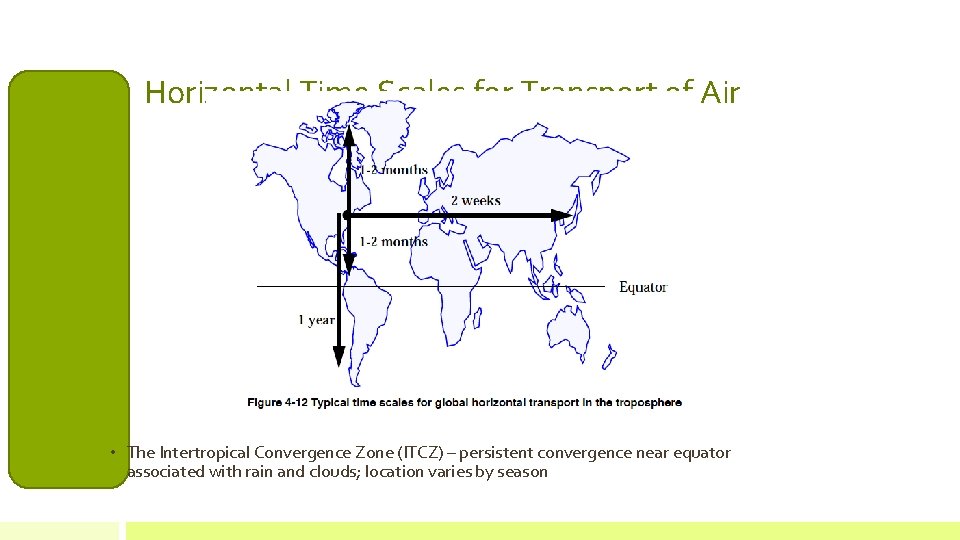
Horizontal Time Scales for Transport of Air • The Intertropical Convergence Zone (ITCZ) – persistent convergence near equator associated with rain and clouds; location varies by season

• Units of Atmospheric Concentration

Chemical Composition of the Atmosphere

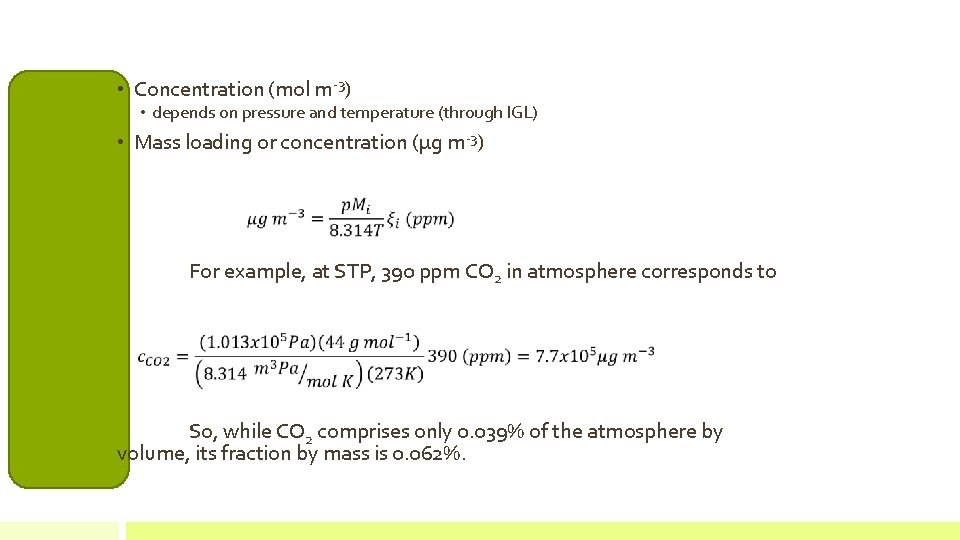
• Concentration (mol m-3) • depends on pressure and temperature (through IGL) • Mass loading or concentration (µg m-3) For example, at STP, 390 ppm CO 2 in atmosphere corresponds to So, while CO 2 comprises only 0. 039% of the atmosphere by volume, its fraction by mass is 0. 062%.

 Layer closest to earth
Layer closest to earth How cold is the stratosphere
How cold is the stratosphere Stratosphere
Stratosphere Stratosphere height
Stratosphere height Ionosphere
Ionosphere Percent composition examples
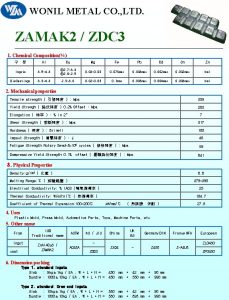
Percent composition examples Zamak 5 chemical composition
Zamak 5 chemical composition Mining company profile
Mining company profile Chemical composition
Chemical composition Chemical composition of human body
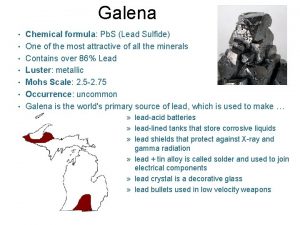
Chemical composition of human body What is the chemical formula for galena
What is the chemical formula for galena Composition of fungi
Composition of fungi What is the chemical composition of saliva
What is the chemical composition of saliva Zamak 5 chemical composition
Zamak 5 chemical composition Mesosphere chemical composition
Mesosphere chemical composition Chemical composition of solid waste
Chemical composition of solid waste Urea reactor design
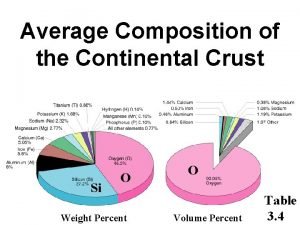
Urea reactor design Chemical composition of continental crust
Chemical composition of continental crust Zdc v2
Zdc v2 Gram structure
Gram structure Size of chromosome in micrometers
Size of chromosome in micrometers Chemical composition of gene
Chemical composition of gene Filament bending
Filament bending Definite chemical composition
Definite chemical composition Spider goat silk
Spider goat silk Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry Definition of chemical kinetics in chemistry
Definition of chemical kinetics in chemistry Pixl knowit gcse chemistry chemical changes
Pixl knowit gcse chemistry chemical changes Chemistry grade 11 unit 4
Chemistry grade 11 unit 4 Chapter 8 review chemical equations and reactions section 2
Chapter 8 review chemical equations and reactions section 2 A shorthand way to represent a molecule
A shorthand way to represent a molecule Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Ap chemistry equilibrium
Ap chemistry equilibrium Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Empirical formula pogil
Empirical formula pogil Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes What is the chemical formula for tetranitrogen heptoxide?
What is the chemical formula for tetranitrogen heptoxide? Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia