Chemical Composition Chemical Composition The identities and relative



- Slides: 10

Chemical Composition

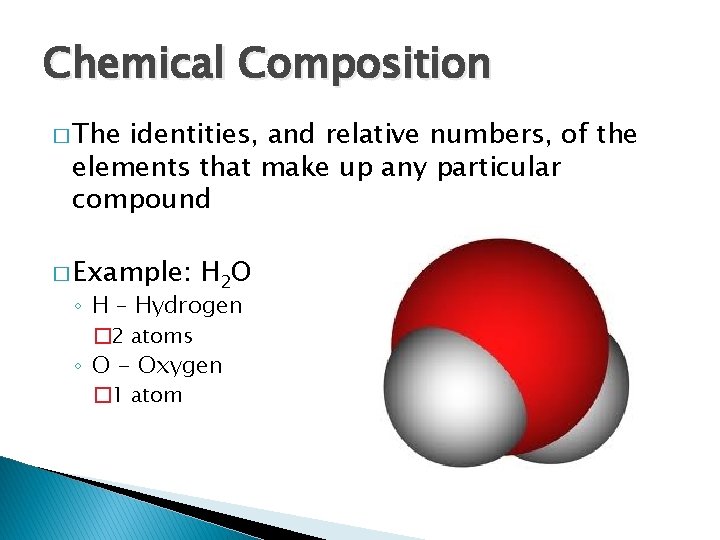
Chemical Composition � The identities, and relative numbers, of the elements that make up any particular compound � Example: H 2 O ◦ H – Hydrogen � 2 atoms ◦ O - Oxygen � 1 atom

Counting Atoms by Grams � Atoms are extremely small so we use a larger number ◦ Mole (mol) = 6. 022 x 1023 �Avogadro’s number ◦ Can specify a mole of anything �Example: Atoms, Ions, Molecules

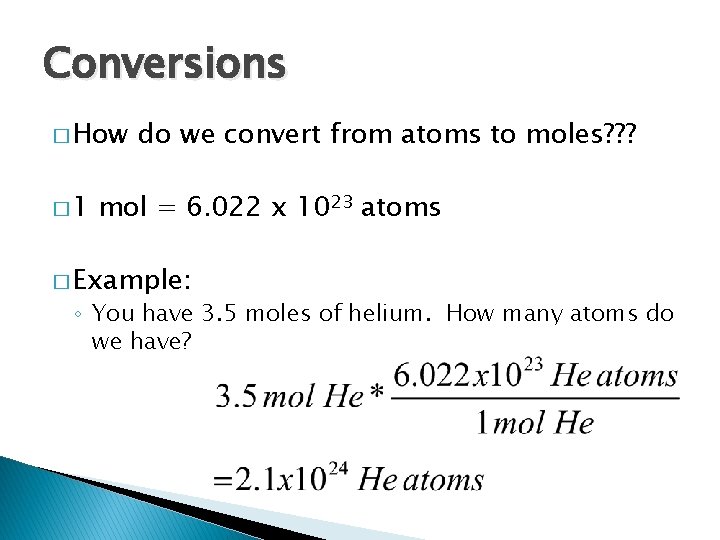
Conversions � How � 1 do we convert from atoms to moles? ? ? mol = 6. 022 x 1023 atoms � Example: ◦ You have 3. 5 moles of helium. How many atoms do we have?

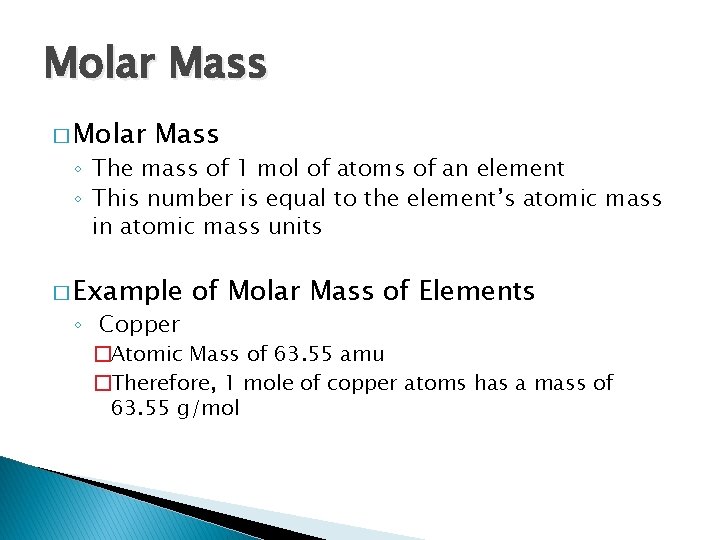
Molar Mass � Molar Mass ◦ The mass of 1 mol of atoms of an element ◦ This number is equal to the element’s atomic mass in atomic mass units � Example ◦ Copper of Molar Mass of Elements �Atomic Mass of 63. 55 amu �Therefore, 1 mole of copper atoms has a mass of 63. 55 g/mol

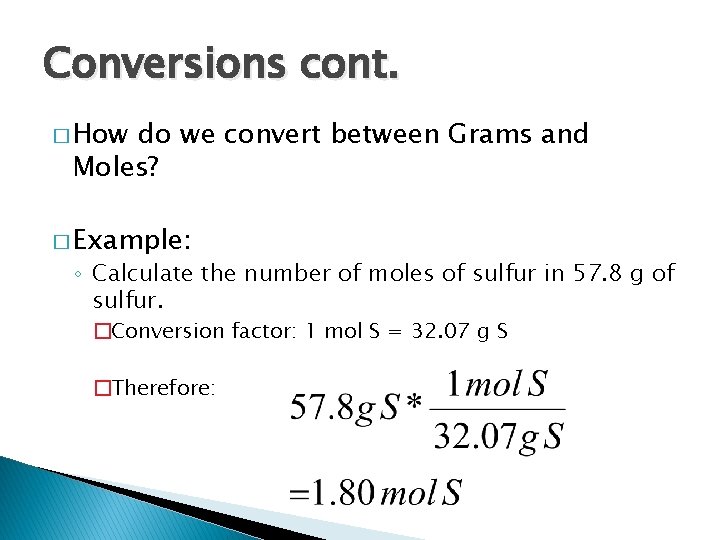
Conversions cont. � How do we convert between Grams and Moles? � Example: ◦ Calculate the number of moles of sulfur in 57. 8 g of sulfur. �Conversion factor: 1 mol S = 32. 07 g S �Therefore:

Finding Atoms � How many aluminum atoms are there in an aluminum can with a mass of 16. 2 grams? ◦ Conversion Factor: � 26. 98 g Al = 1 mol Al � 1 mol Al = 6. 022 x 1023 Al atoms

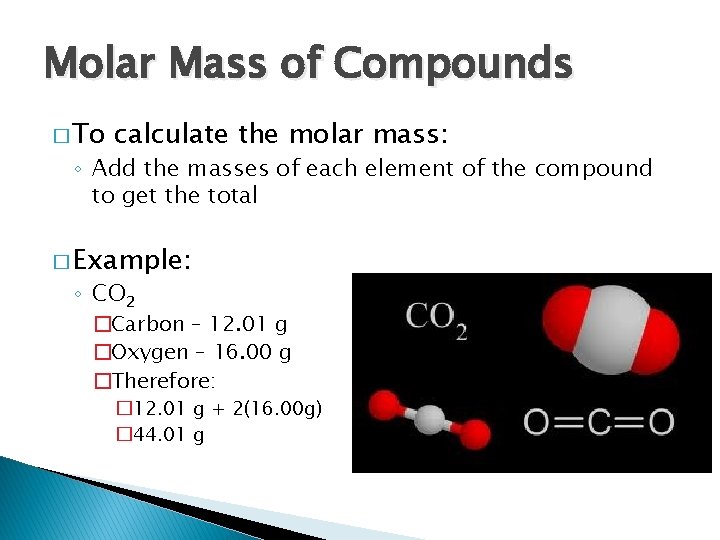
Molar Mass of Compounds � To calculate the molar mass: ◦ Add the masses of each element of the compound to get the total � Example: ◦ CO 2 �Carbon – 12. 01 g �Oxygen – 16. 00 g �Therefore: � 12. 01 g + 2(16. 00 g) � 44. 01 g

Conversions cont. � How do we convert between grams and moles of a compound? 1. 2. 3. Calculate the molar mass of the compound Find conversion factors Solve

Example � Calculate water. the mass (in grams) of 1. 75 mol of Molar Mass of H 2 O = 2(Atomic Mass of H) + 1(Atomic Mass of O) = 2(1. 01 g/mol) + 1(16. 00 g/mol) = 18. 02 g/mol