CHEMISTRY Matter and Change Chapter 16 Reaction Rates












![Convert min to sec = -[0. 00933 -0. 01756]/[(20 x 60 )-0] Units= mol Convert min to sec = -[0. 00933 -0. 01756]/[(20 x 60 )-0] Units= mol](https://slidetodoc.com/presentation_image_h2/bd7e4ef0183e658c92de8af35eec95ac/image-17.jpg)














- Slides: 42

CHEMISTRY Matter and Change Chapter 16: Reaction Rates

CHAPTER 16 Table Of Contents Section 16. 1 A Model for Reaction Rates Section 16. 2 Factors Affecting Reaction Rates Exit

Section 16. 1 A Model for Reaction Rates

1 6. 1 SECTION A Model for Reaction Rates Reaction rate Collision theory Activated complex Activation energy

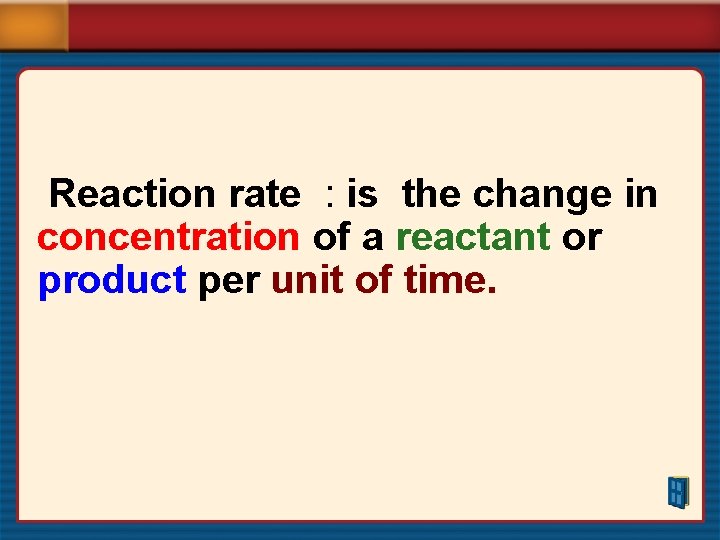
Reaction rate : is the change in concentration of a reactant or product per unit of time.


1 6. 1 SECTION

1 6. 1 SECTION Reaction rates are determined experimentally.





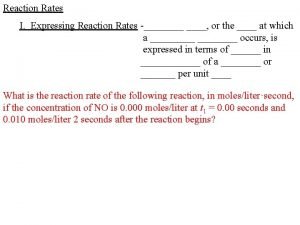
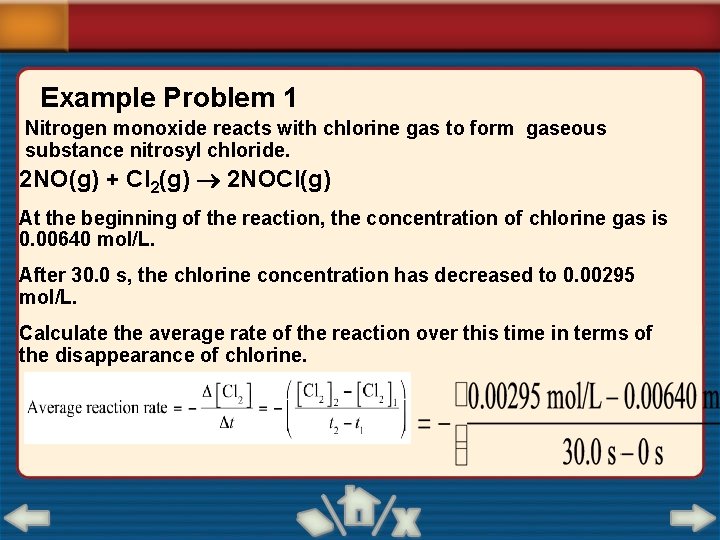
Example Problem 1 Nitrogen monoxide reacts with chlorine gas to form gaseous substance nitrosyl chloride. 2 NO(g) + Cl 2(g) 2 NOCl(g) At the beginning of the reaction, the concentration of chlorine gas is 0. 00640 mol/L. After 30. 0 s, the chlorine concentration has decreased to 0. 00295 mol/L. Calculate the average rate of the reaction over this time in terms of the disappearance of chlorine.


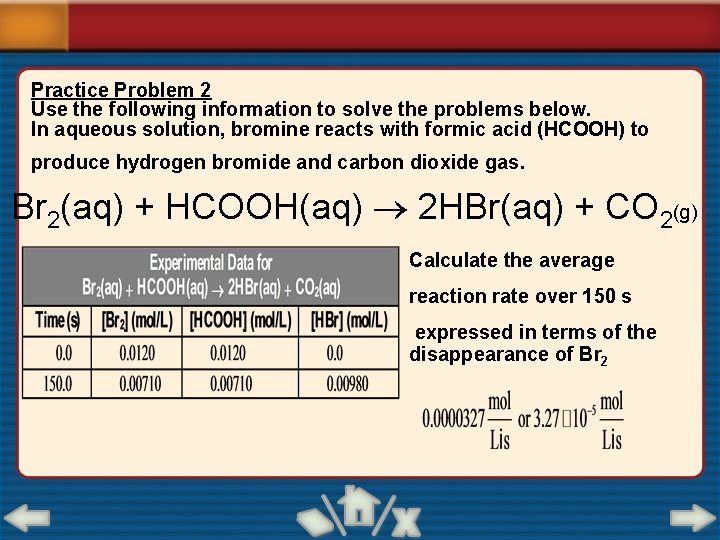
Practice Problem 2 Use the following information to solve the problems below. In aqueous solution, bromine reacts with formic acid (HCOOH) to produce hydrogen bromide and carbon dioxide gas. Br 2(aq) + HCOOH(aq) 2 HBr(aq) + CO 2(g) Calculate the average reaction rate over 150 s expressed in terms of the disappearance of Br 2

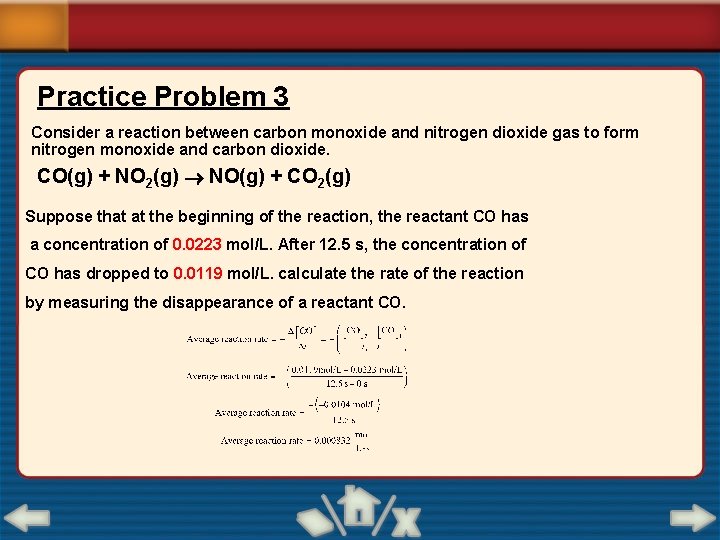
Practice Problem 3 Consider a reaction between carbon monoxide and nitrogen dioxide gas to form nitrogen monoxide and carbon dioxide. CO(g) + NO 2(g) NO(g) + CO 2(g) Suppose that at the beginning of the reaction, the reactant CO has a concentration of 0. 0223 mol/L. After 12. 5 s, the concentration of CO has dropped to 0. 0119 mol/L. calculate the rate of the reaction by measuring the disappearance of a reactant CO.
![Convert min to sec 0 00933 0 0175620 x 60 0 Units mol Convert min to sec = -[0. 00933 -0. 01756]/[(20 x 60 )-0] Units= mol](https://slidetodoc.com/presentation_image_h2/bd7e4ef0183e658c92de8af35eec95ac/image-17.jpg)
Convert min to sec = -[0. 00933 -0. 01756]/[(20 x 60 )-0] Units= mol /(L. s) Find the answer. And in same way calculate the rate at time interval 80 -100

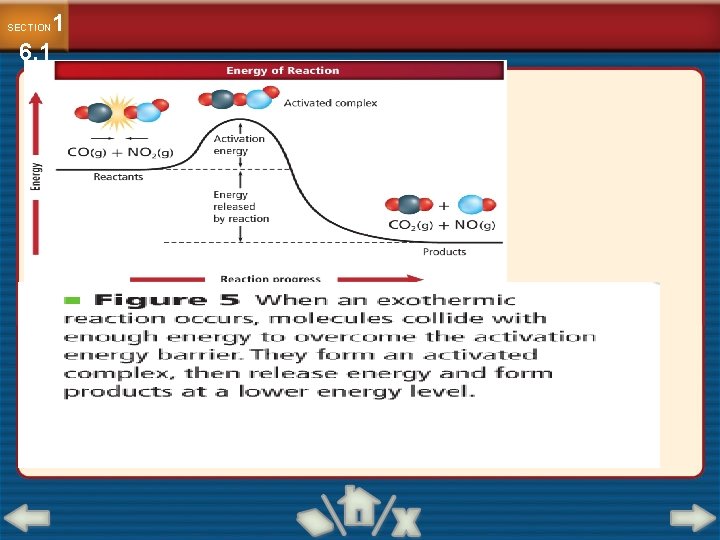
1 6. 1 SECTION A Model for Reaction Rates Collision Theory Collision theory states that atoms, ions, and molecules must collide in order to react.

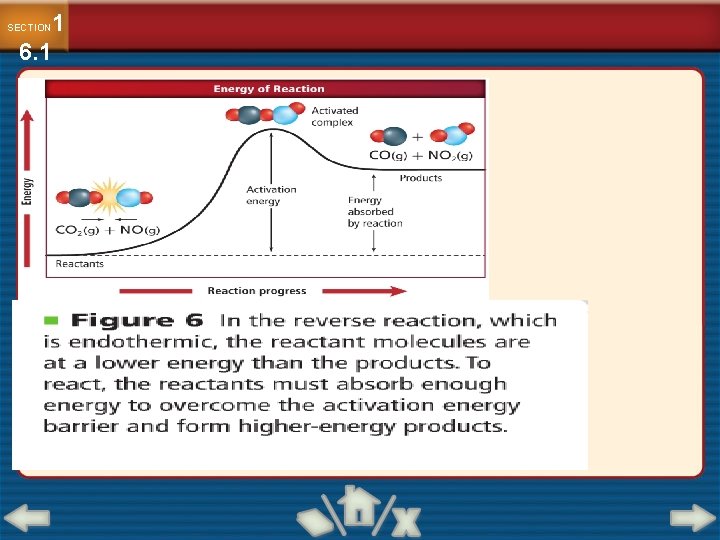
The reacting particles need a minimum amount of energy to form the activated complex and lead to a reaction. Activated complex : it is a temporary unstable arrangement of atoms that may form Products

Not always a reactants can yelled a product

1 6. 1 SECTION

1 6. 1 SECTION

1 6. 1 SECTION

DH is the enthalpy change DH is Energy released by the reaction. For an exothermic reaction, DH is negative. For an endothermic reaction, DH is positive.

change in energy can be plotted against progress of a reaction Reactants turn into products

1 6. 1 SECTION Section Check Which of the following is NOT a requirement for a reaction to occur, according to the collision theory? A. Reacting substances must collide. B. Reacting substances must be in an exothermic reaction. C. Reacting substances must collide in the correct orientation. D. Reacting substances must collide with sufficient energy to form an activated complex.

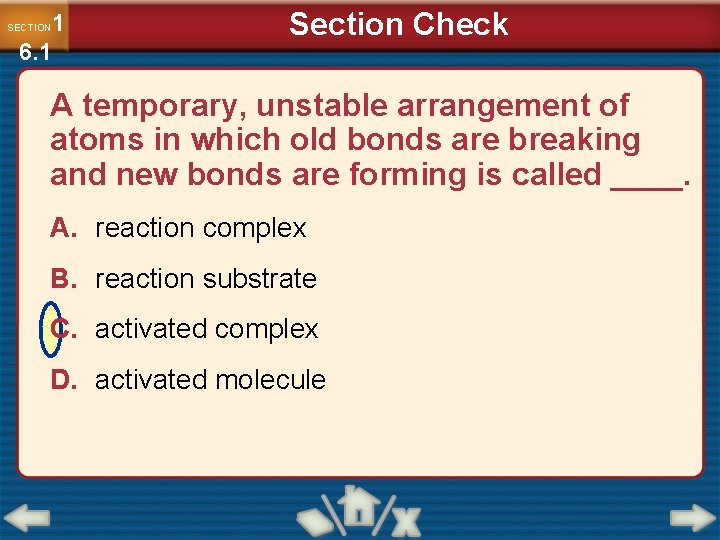
1 6. 1 SECTION Section Check A temporary, unstable arrangement of atoms in which old bonds are breaking and new bonds are forming is called ____. A. reaction complex B. reaction substrate C. activated complex D. activated molecule

What is an activated complex ? An activated complex is a temporary, unstable arrangement of atoms in which old bonds are breaking and new bonds are forming.

Section 6. 2 Factors Affecting Reaction Rates

Some substances react more readily (faster)than others.

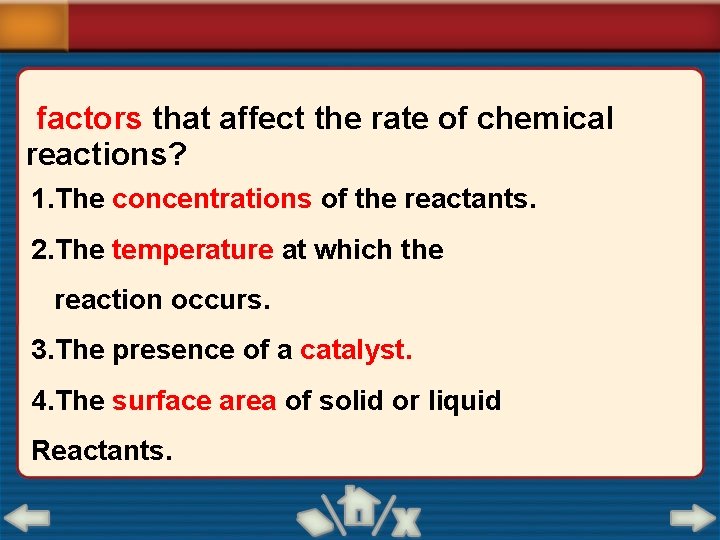
factors that affect the rate of chemical reactions? 1. The concentrations of the reactants. 2. The temperature at which the reaction occurs. 3. The presence of a catalyst. 4. The surface area of solid or liquid Reactants.

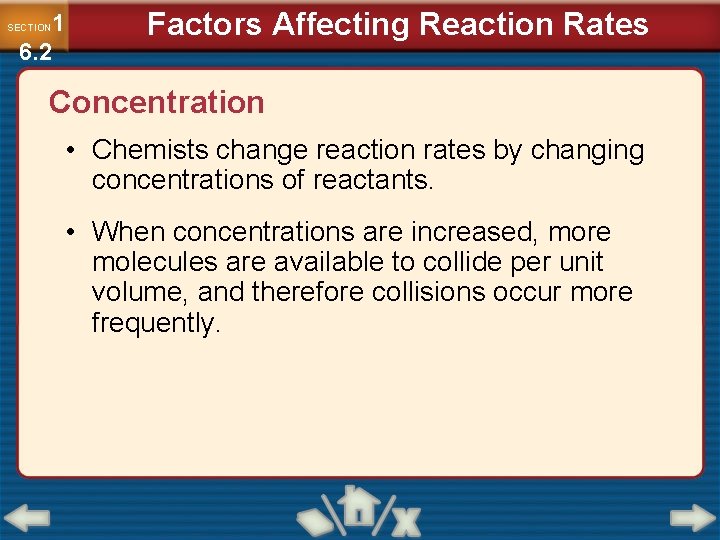
1 6. 2 SECTION Factors Affecting Reaction Rates Concentration • Chemists change reaction rates by changing concentrations of reactants. • When concentrations are increased, more molecules are available to collide per unit volume, and therefore collisions occur more frequently.


1 6. 2 SECTION Factors Affecting Reaction Rates Surface Area • Greater surface area allows particles to collide with many more particles per unit of time. • For the same mass, many small particles have more surface area than one large particle. • Reaction rate increases with increasing surface area.

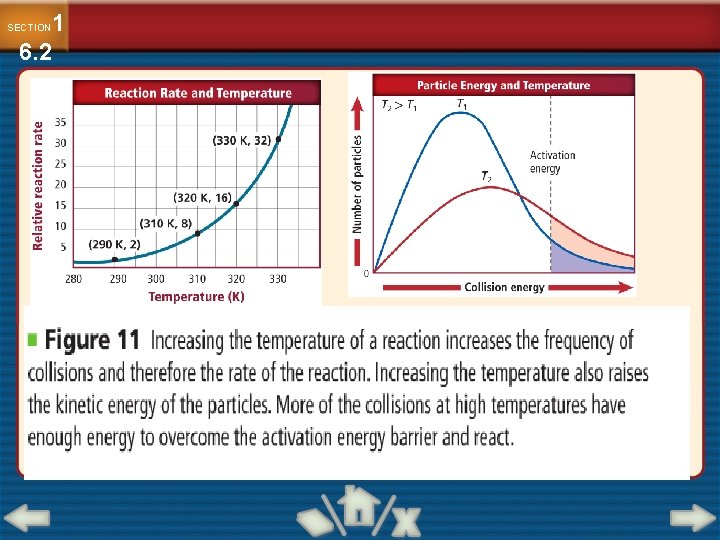
1 6. 2 SECTION Factors Affecting Reaction Rates Temperature • Increasing temperature generally increases reaction rate. • Increasing temperature increases the kinetic energy of the particles. • Reacting particles collide more frequently at higher temperatures.

1 6. 2 SECTION Factors Affecting Reaction Rates Temperature (cont. ) • High-energy collisions are more frequent at a higher temperature. • As temperature increases, reaction rate increases.

1 6. 2 SECTION

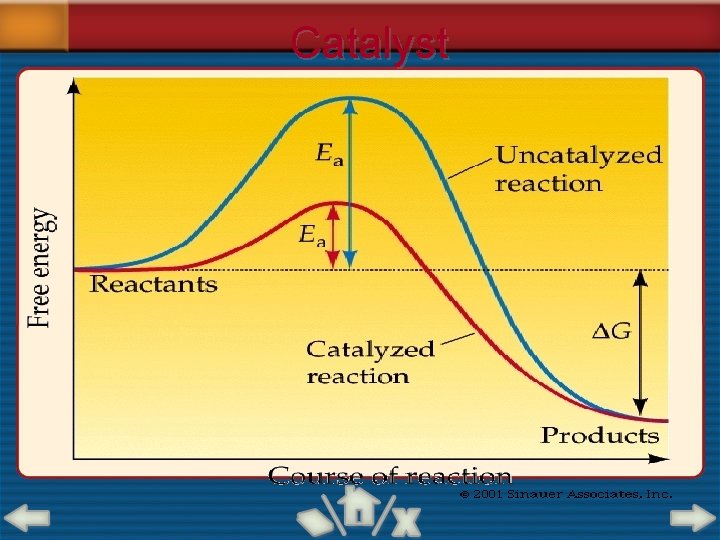
1 6. 2 SECTION Factors Affecting Reaction Rates Catalysts and Inhibitors • A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the reaction. • An inhibitor is a substance that slows or prevents a reaction.

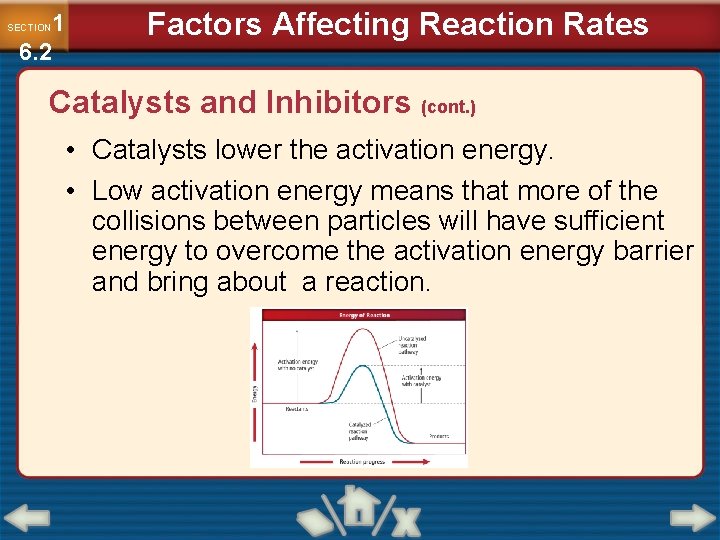
1 6. 2 SECTION Factors Affecting Reaction Rates Catalysts and Inhibitors (cont. ) • Catalysts lower the activation energy. • Low activation energy means that more of the collisions between particles will have sufficient energy to overcome the activation energy barrier and bring about a reaction.

Catalyst

1 6. 2 SECTION Section Check Which of the following generally does not increase the rate of a chemical reaction? A. increasing concentration B. adding a catalyst C. adding an inhibitor D. increasing temperature

1 6. 2 SECTION Section Check High-energy particle collisions are more frequent: A. when an inhibitor is present B. when temperature is decreased C. when activation energy is higher D. when temperature is increased
 Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 10 the mole answer key
Chemistry matter and change chapter 10 the mole answer key 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chemistry matter and change chapter 10 the mole answer key
Chemistry matter and change chapter 10 the mole answer key Chemistry matter and change chapter 2 answer key
Chemistry matter and change chapter 2 answer key What is a unit ratio
What is a unit ratio Ratios guided notes
Ratios guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Chapter 18 review chemical equilibrium section 3 answer key
Chapter 18 review chemical equilibrium section 3 answer key Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Chapter 2 matter section 1 classifying matter answer key
Chapter 2 matter section 1 classifying matter answer key Chapter 1 review matter and change
Chapter 1 review matter and change Circle the solute and underline the solvent
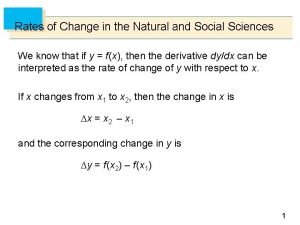
Circle the solute and underline the solvent Rates of change in the natural and social sciences
Rates of change in the natural and social sciences 1-4 extrema and average rates of change answer key
1-4 extrema and average rates of change answer key Linear models and rates of change
Linear models and rates of change Rate of change vocabulary
Rate of change vocabulary Expressing reaction rates
Expressing reaction rates Rates of reaction can be positive or negative. true false
Rates of reaction can be positive or negative. true false Expressing reaction rates
Expressing reaction rates Reaction rates
Reaction rates Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter Basic food chemistry the nature of matter
Basic food chemistry the nature of matter Meysam golmohammadi
Meysam golmohammadi Optic tract
Optic tract Gray matter and white matter
Gray matter and white matter What is gray matter in the brain
What is gray matter in the brain Chemistry matter and its changes
Chemistry matter and its changes Addition reaction and substitution reaction
Addition reaction and substitution reaction Difference between nuclear reaction and chemical reaction
Difference between nuclear reaction and chemical reaction Cartoon chemistry and reaction types
Cartoon chemistry and reaction types Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Composition of matter section 1
Composition of matter section 1 Composition of matter section 1
Composition of matter section 1 Flow energy review
Flow energy review Changes in matter grade 5
Changes in matter grade 5