Chemistry I Chapter 25 Chemistry I Honors Chapter































- Slides: 59

Chemistry I – Chapter 25 Chemistry I Honors – Chapter 19 ICP – Chapter 18 1 Nuclear Chemistry SAVE PAPER AND INK!!! When you print out the notes on Power. Point, print "Handouts" instead of "Slides" in the print setup. Also, turn off the backgrounds (Tools>Options>Print>UNcheck "Background Printing")!

Radioactivity • One of the pieces of evidence for the fact that atoms are made of smaller particles came from the work of ____ (1876 -1934). • She discovered ____, the spontaneous disintegration of some elements into smaller pieces. 2

Nuclear Reactions vs. Normal Chemical Changes • Nuclear reactions involve the nucleus • The nucleus opens, and protons and neutrons are rearranged • The opening of the nucleus releases a tremendous amount of energy that holds the nucleus together – called binding energy • “Normal” Chemical Reactions involve electrons, not protons and neutrons 3

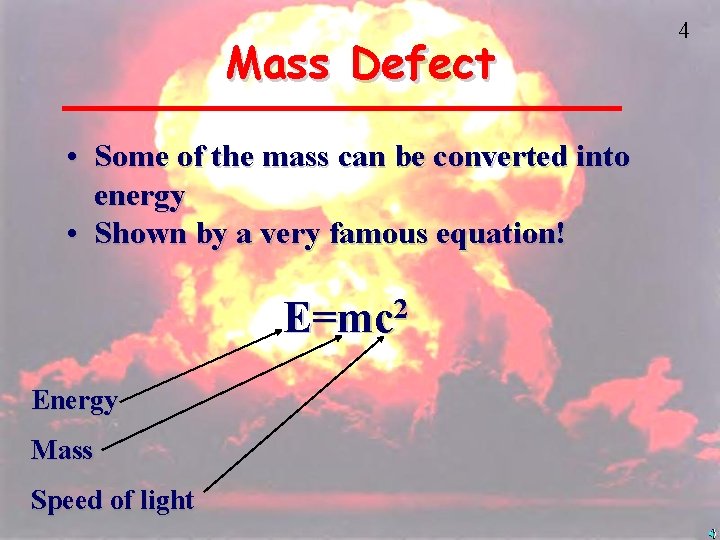
Mass Defect • Some of the mass can be converted into energy • Shown by a very famous equation! E=mc 2 Energy Mass Speed of light 4

5 Types of Radiation • Alpha (ά) – a positively charged helium isotope - we usually ignore the charge because it involves electrons, not protons and neutrons • Beta (β) – an electron • Gamma (γ) – pure energy; called a ray rather than a particle

6 Other Nuclear Particles • Neutron • Positron – a positive electron • Proton – usually referred to as hydrogen-1 • Any other elemental isotope

Balancing Nuclear Reactions • In the reactants (starting materials – on the left side of an equation) and products (final products – on the right side of an equation) Atomic numbers must balance and Mass numbers must balance • Use a particle or isotope to fill in the missing protons and neutrons 7

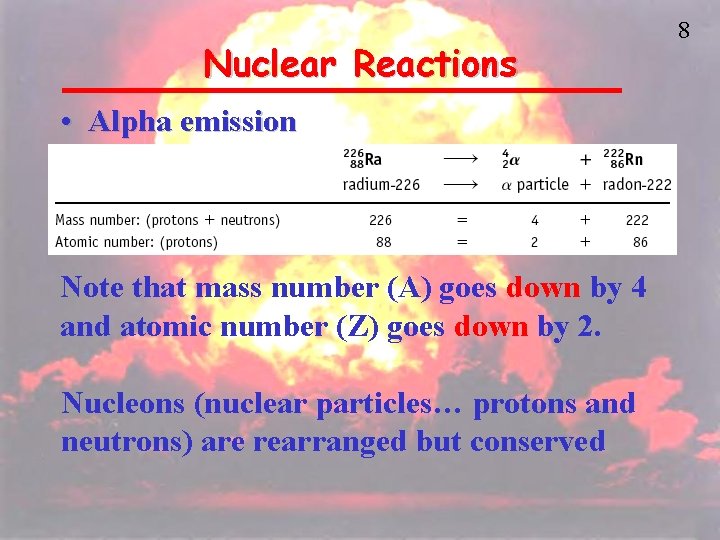
Nuclear Reactions • Alpha emission Note that mass number (A) goes down by 4 and atomic number (Z) goes down by 2. Nucleons (nuclear particles… protons and neutrons) are rearranged but conserved 8

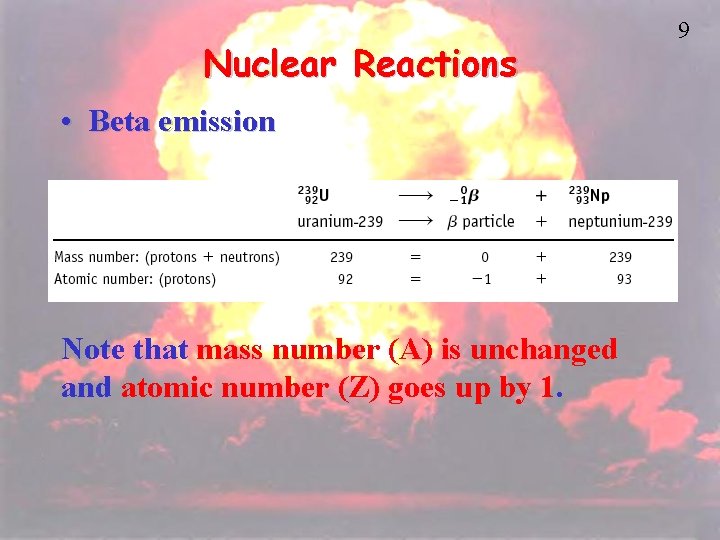
Nuclear Reactions • Beta emission Note that mass number (A) is unchanged and atomic number (Z) goes up by 1. 9

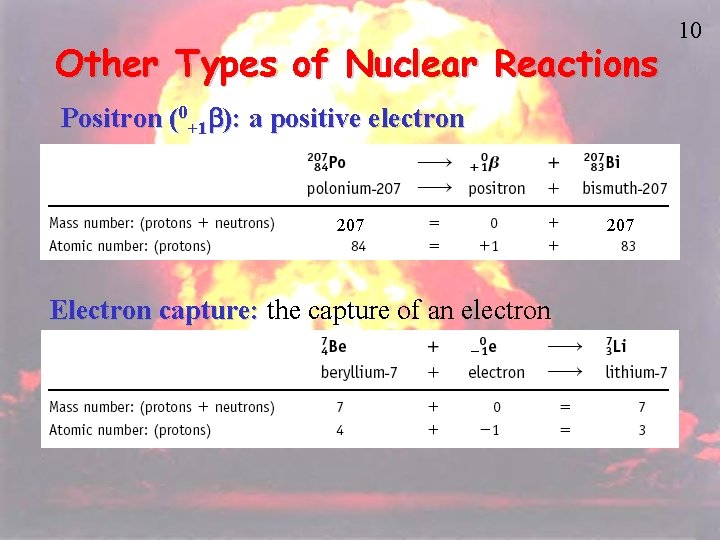
Other Types of Nuclear Reactions Positron (0+1 b): a positive electron 207 Electron capture: the capture of an electron 207 10

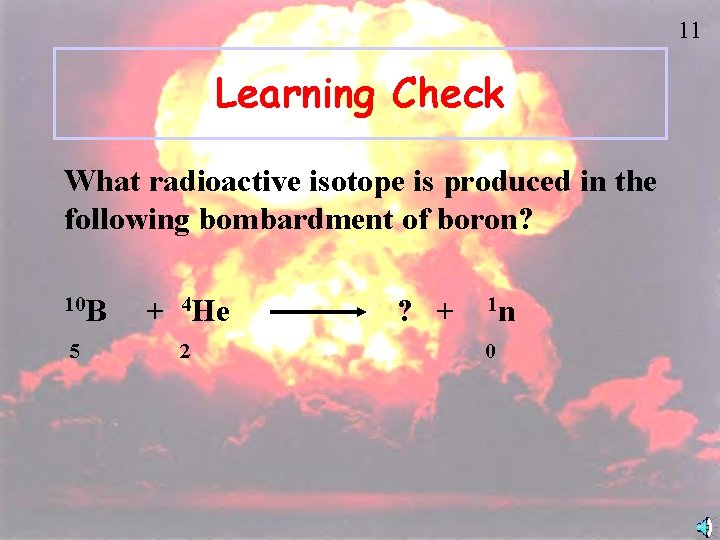
11 Learning Check What radioactive isotope is produced in the following bombardment of boron? 10 B 5 + 4 He 2 ? + 1 n 0

12 Write Nuclear Equations! Write the nuclear equation for the beta emitter Co-60.

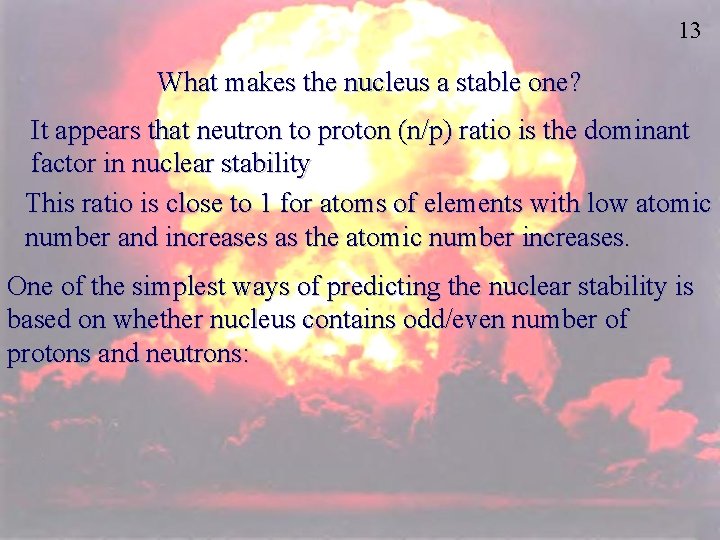
13 What makes the nucleus a stable one? It appears that neutron to proton (n/p) ratio is the dominant factor in nuclear stability This ratio is close to 1 for atoms of elements with low atomic number and increases as the atomic number increases. One of the simplest ways of predicting the nuclear stability is based on whether nucleus contains odd/even number of protons and neutrons:

14

15 Nuclides containing odd numbers of both protons and neutrons are the least stable means more radioactive. Nuclides containing even numbers of both protons and neutrons are most stable means less radioactive. Nuclides contain odd numbers of protons and even numbers of neutrons are less stable than nuclides containing even numbers of protons and odd numbers of neutrons.

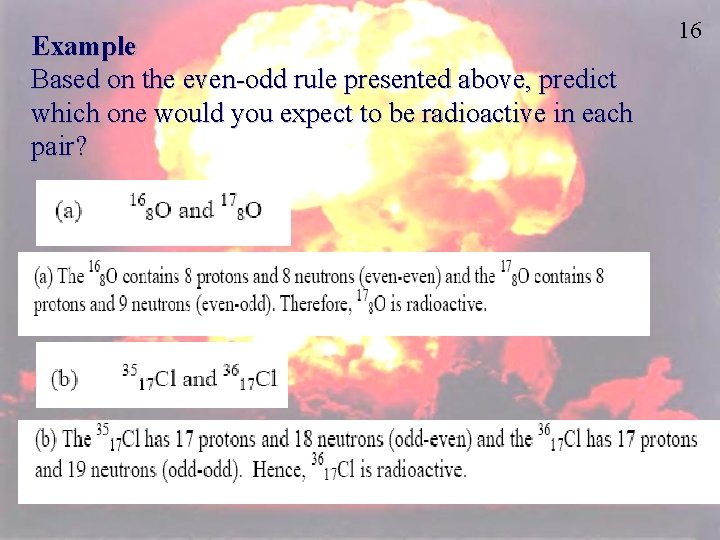
Example Based on the even-odd rule presented above, predict which one would you expect to be radioactive in each pair? 16

17

18 http: //www. brightstorm. com/science/physics/nuclear-stability/

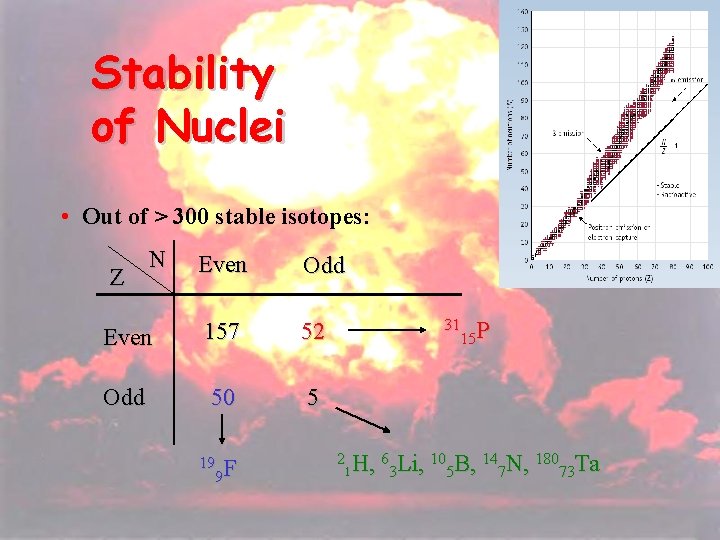
19 Stability of Nuclei • Out of > 300 stable isotopes: N Even Odd Even 157 52 Odd 50 5 Z 19 F 9 31 P 15 2 H, 63 Li, 105 B, 147 N, 18073 Ta 1

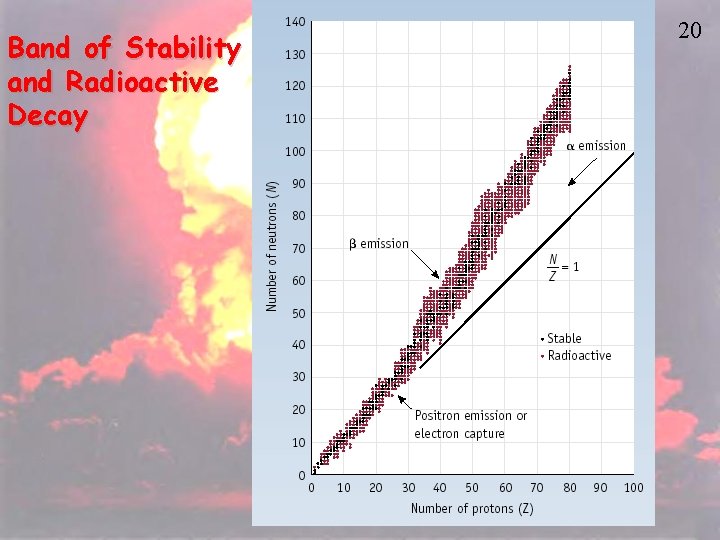
Band of Stability and Radioactive Decay 20

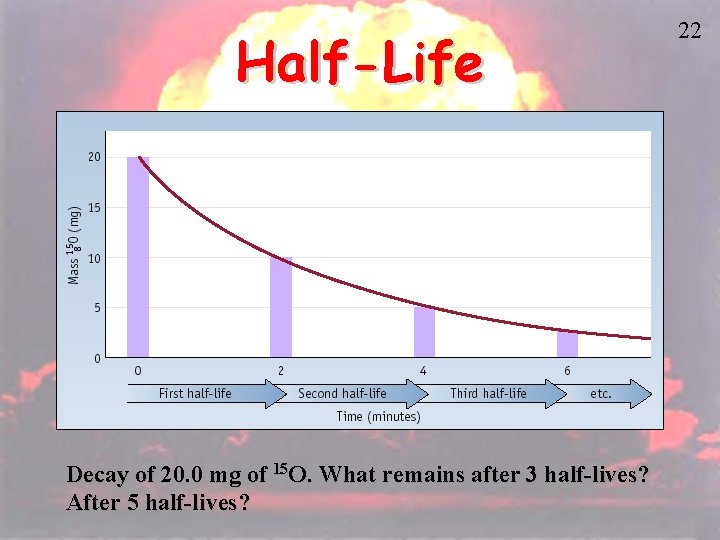
Half-Life • HALF-LIFE is the time that it takes for 1/2 a sample to decompose. • The rate of a nuclear transformation depends only on the “reactant” concentration. 21

Half-Life Decay of 20. 0 mg of 15 O. What remains after 3 half-lives? After 5 half-lives? 22

Kinetics of Radioactive Decay For each duration (half-life), one half of the substance decomposes. For example: Ra-234 has a half-life of 3. 6 days If you start with 50 grams of Ra-234 After 3. 6 days > 25 grams After 7. 2 days > 12. 5 grams After 10. 8 days > 6. 25 grams 23

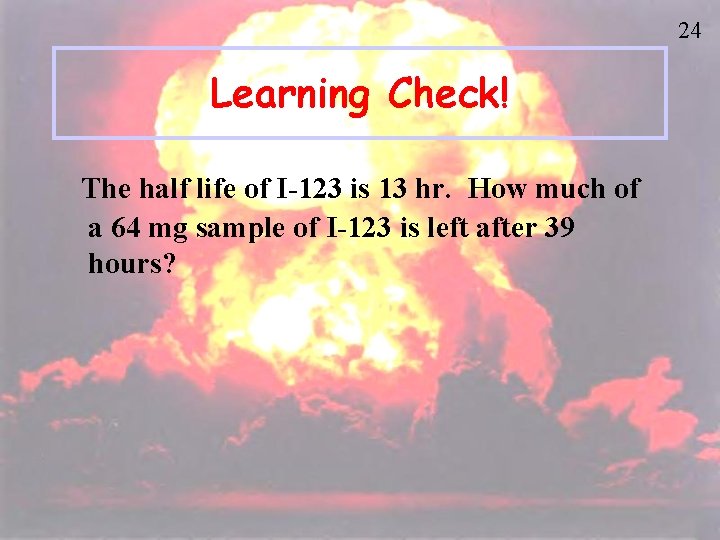
24 Learning Check! The half life of I-123 is 13 hr. How much of a 64 mg sample of I-123 is left after 39 hours?

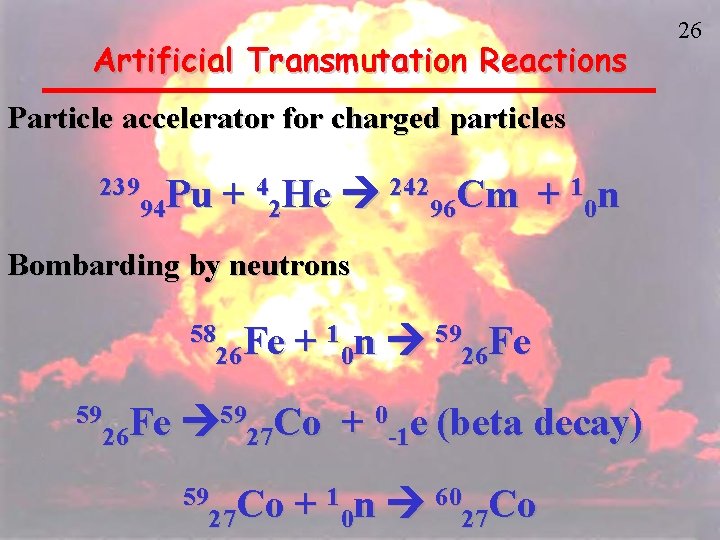
Nuclear Transmutation Reactions New elements or new isotopes of known elements are produced by bombarding an atom with a particle such as a proton, neutron, or even a much heavier nucleus. These reactions are nonspontaneous, they must be induced in some way Three categories are: Artificial Transmutation Nuclear Fusion Nuclear Fission 25

Artificial Transmutation Reactions Particle accelerator for charged particles 239 Pu 94 + 42 He 24296 Cm + 10 n Bombarding by neutrons 58 Fe 26 59 Fe 26 + 10 n 5926 Fe 5927 Co + 0 -1 e (beta decay) 59 Co 27 + 10 n 6027 Co 26

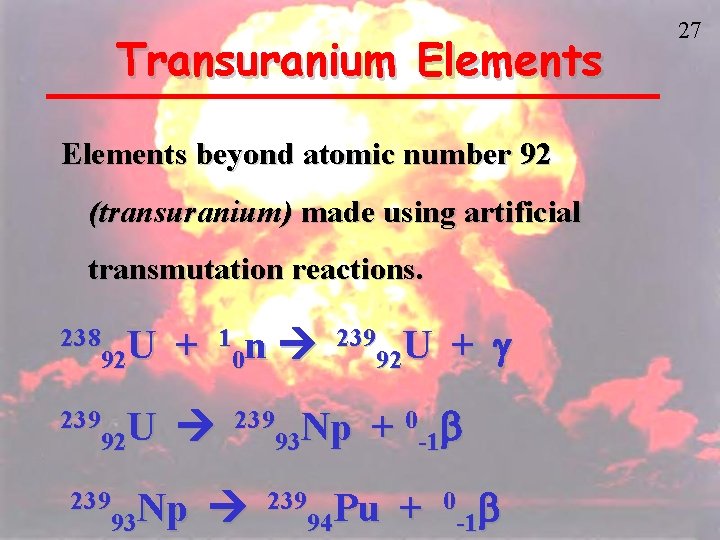
Transuranium Elements beyond atomic number 92 (transuranium) made using artificial transmutation reactions. 238 U 92 + 239 U 92 239 Np 93 1 n 0 239 U 92 239 Np 93 + g + 0 -1 b 239 Pu 94 + 0 b -1 27

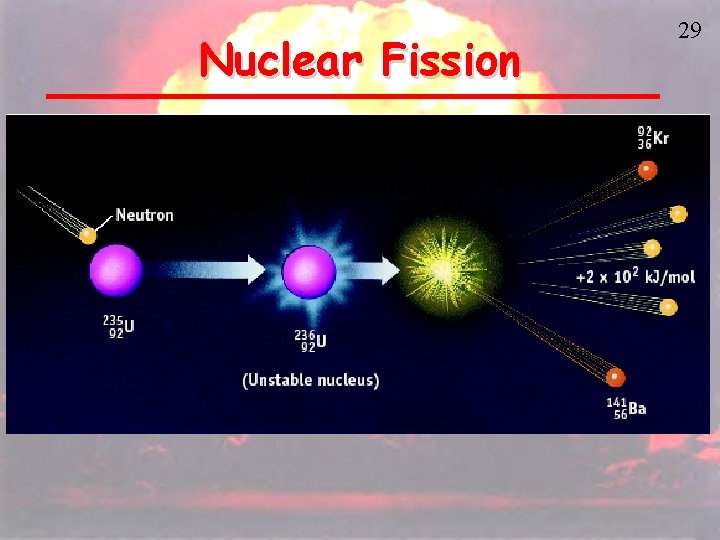
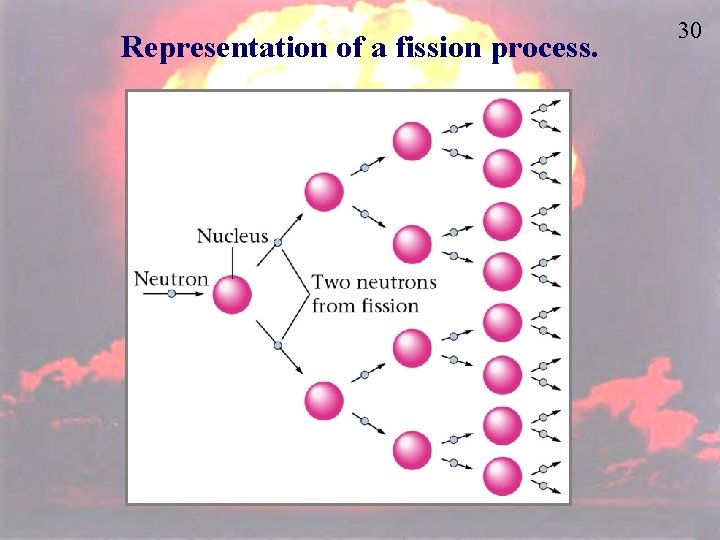
Nuclear Fission is the splitting of atoms These are usually very large, so that they are not as stable Fission chain has two general steps: 1. Initiation. Reaction of a single atom starts the chain (e. g. , 235 U + neutron) 2. Propagation. 236 U fission releases neutrons that initiate other fissions 28

Nuclear Fission 29

Representation of a fission process. 30

31 Nuclear Fission Critical mass – the minimum mass of fissionable material required to generate a self-sustaining nuclear reaction. U-235 and Pu-239 are two isotopes that can undergo fission reactions that has any practical importance. Atomic Bombs: Uses high explosive to force two masses of radioactive materials together to create a critical mass. This neutron source then triggers an uncontrolled chain reaction.

Nuclear Fission & POWER • Currently about 103 nuclear power plants in the U. S. and about 439 worldwide. • 14% of the world’s electricity comes from nuclear fission. 32

Nuclear Fission compared to Coal Power 33 • Pros – Less fuel is needed; Produces more energy per gram of fuel. – Cleaner for the environment • Cons – Waste products are radioactive – Nuclear Meltdown is much more disastrous – Storage, Transportation, Terrorist threats (all of these become much more frustrating) • Similarities – Produce electricity by the same process

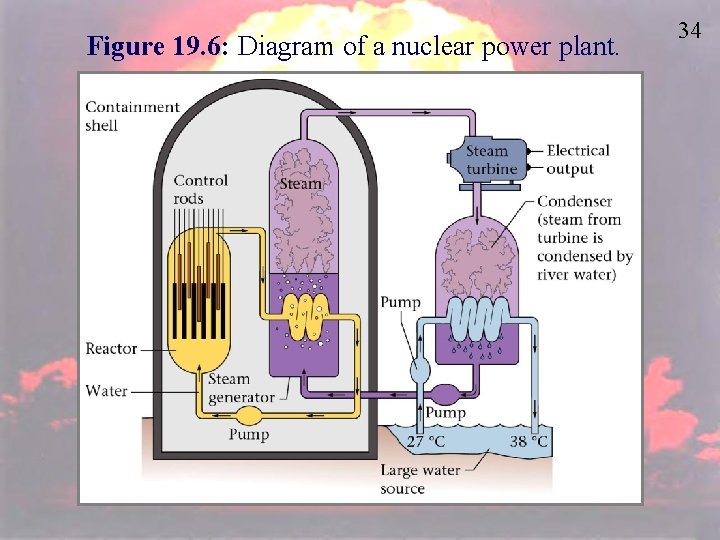
Figure 19. 6: Diagram of a nuclear power plant. 34

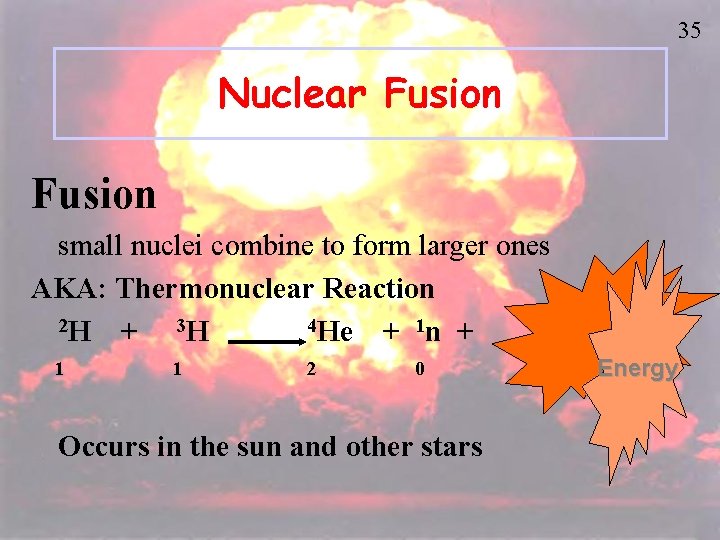
35 Nuclear Fusion small nuclei combine to form larger ones AKA: Thermonuclear Reaction 2 H 4 He + 3 H + 1 n + 1 1 2 0 Occurs in the sun and other stars Energy

36 Nuclear Fusion • The lowest amount of energy for any self-sustaining fusion reaction is that between the fusion of H-2 and H-3, 40, 000 K! • Tokamak only can reach 3, 000 K • Hydrogen Bomb

37 Fusion compared to fission • Pros – Unlimited fuel – Cheaper fuel – Produces more energy per gram of fuel – Little or no radioactive waste – No danger of meltdown • Cons – Containment of reaction – Temperature to sustain the reaction

Radiation • Ionizing (x-rays, gamma rays, beta, alpha, neutrons, protons, heavy nuclei) – Ionizes matter causing free radicals. Free radicals are highly reactive and cause bonds to break. The breaking of bonds in DNA can cause mutations, chromosome aberrations, or cell death • Nonionizing (UV, Visible, IR, microwaves) – Cuases molecules to vibrate and electrons to move between energy levels. 38

39 Radiation • Ionizing v. Nonionizing – Both lead to somatic damage (damage to cells that are non associated with reproduction): sunburn, skin rash, cancer, and cataracts. – Ionizing radiation causes genetic mutation which could show up in offspring.

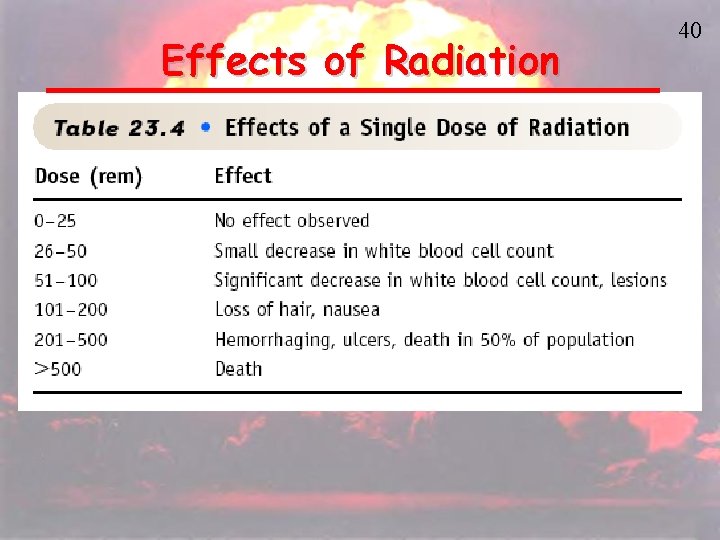
Effects of Radiation 40

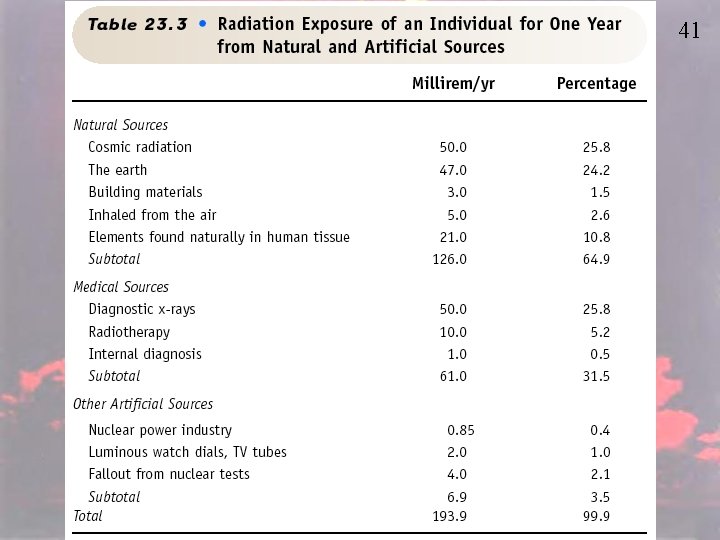
41

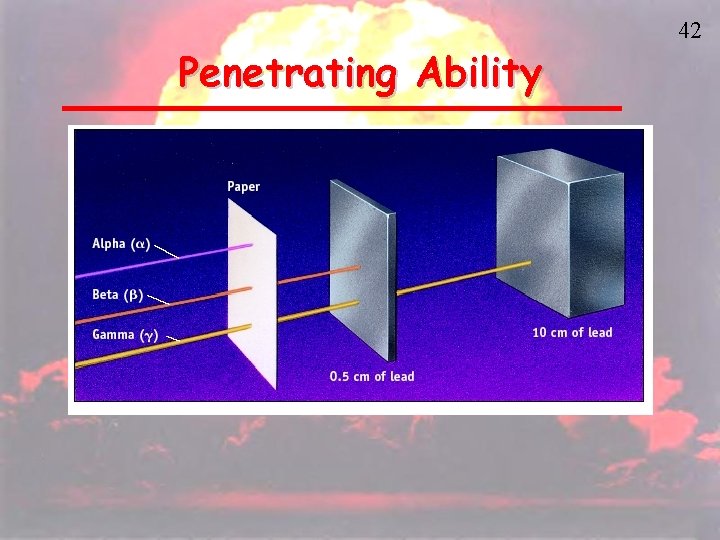
Penetrating Ability 42

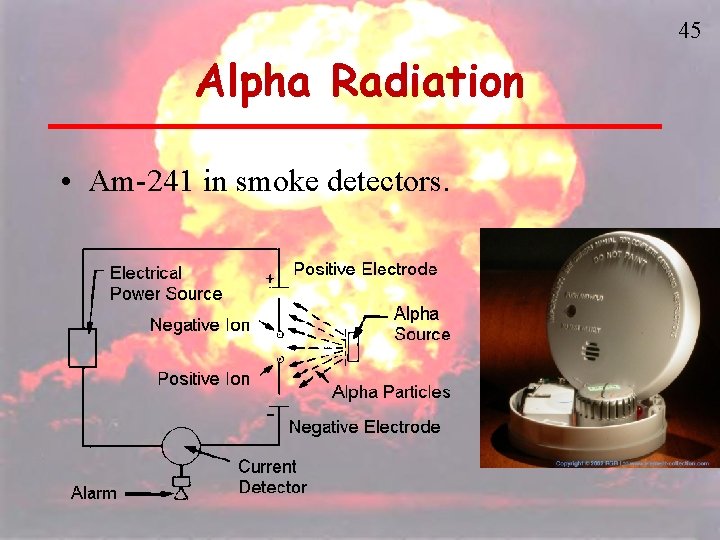
43 Alpha Radiation • Very little penetrating ability • Skin or a piece of paper can shield it. • Dangerous when ingested, can cause ionization. • Rn-222 decays to Po-218. Radon gas is inhaled and exhaled with no effect but the atoms of Po-218 get trapped in lungs emitting alpha radiation. • Am-241 in smoke detectors.

44 Alpha Radiation • Rn-222 decays to Po-218. Radon gas is inhaled and exhaled with no effect but the atoms of Po-218 get trapped in lungs emitting alpha radiation.

45 Alpha Radiation • Am-241 in smoke detectors.

46 Beta Radiation • Penetrates up to a cm in water and a few meters in air. • Can be shielded by 3 inches of paper, Al foil, glass plate, and 2 in of wood. • More dangerous when ingested.

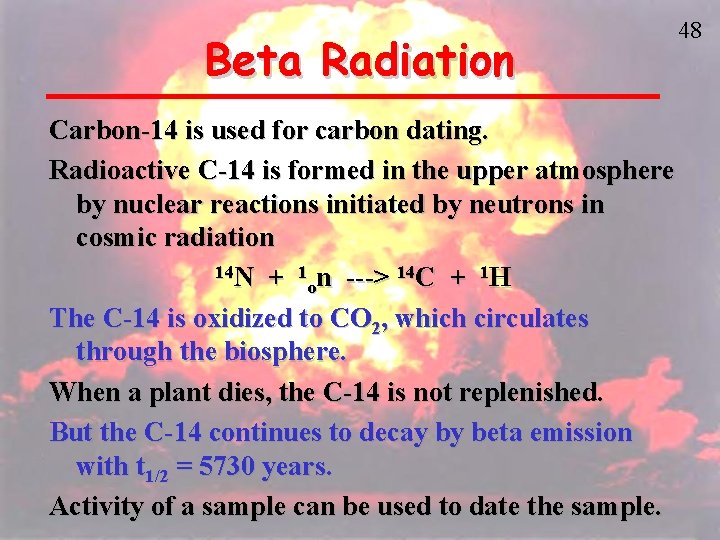
47 Beta Radiation • Carbon-14

Beta Radiation Carbon-14 is used for carbon dating. Radioactive C-14 is formed in the upper atmosphere by nuclear reactions initiated by neutrons in cosmic radiation 14 N + 1 n ---> 14 C + 1 H o The C-14 is oxidized to CO 2, which circulates through the biosphere. When a plant dies, the C-14 is not replenished. But the C-14 continues to decay by beta emission with t 1/2 = 5730 years. Activity of a sample can be used to date the sample. 48

49 Beta Radiation • Strontium-90 present in bone and bone marrow can cause cancer • It is a product of nuclear fission. It will undergo beta decay with a halflife of 64 hours.

50 Gamma Radiation • Penetrates many different kinds of objects. • Shielding: several feet of dense material such as concrete or lead • Kills cells, including cancerous cells.

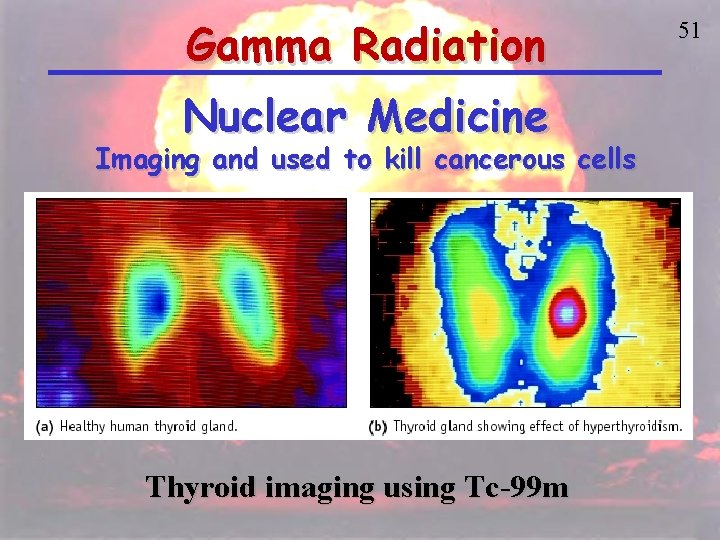
Gamma Radiation Nuclear Medicine Imaging and used to kill cancerous cells Thyroid imaging using Tc-99 m 51

Gamma Radiation Food Irradiation • Food can be irradiated with g rays from 60 Co or 137 Cs. • Irradiated milk has a shelf life of 3 mo. without refrigeration. • USDA has approved irradiation of meats and eggs. 52

Gamma Radiation Therapy • Malignant tumors can be destroyed by exposing them to high energy radiation such as x-rays or gamma rays. 53

54 Dating Rocks • U-238 to Pb-206 used to dated rocks • K-40 to Ar-40 used to date rocks millions and billions of years old. • Compare the ratio of daughter to parent while also knowing the half-life.

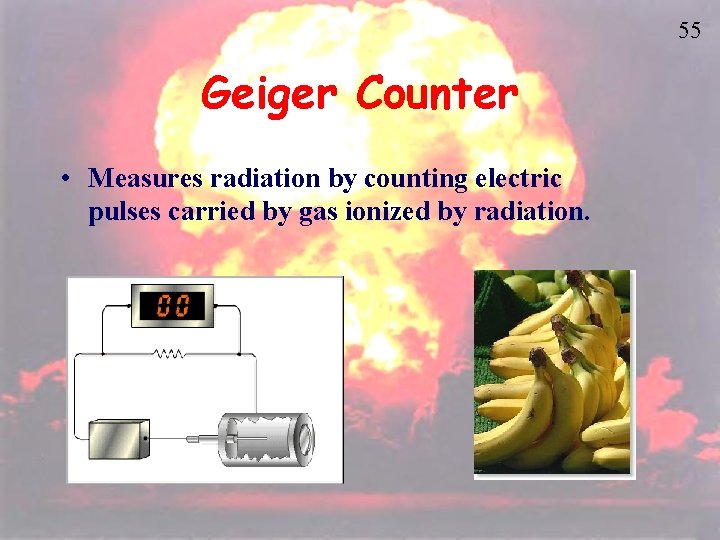
55 Geiger Counter • Measures radiation by counting electric pulses carried by gas ionized by radiation.

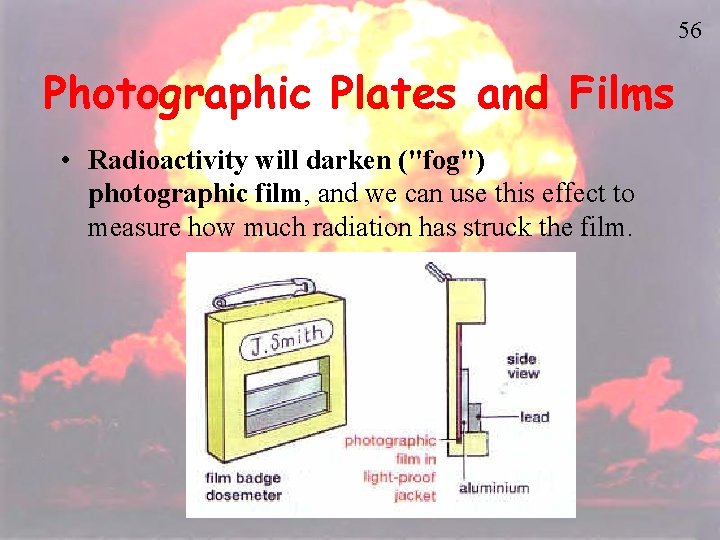
56 Photographic Plates and Films • Radioactivity will darken ("fog") photographic film, and we can use this effect to measure how much radiation has struck the film.

Scintillation Detectors Work by the radiation striking a suitable material (such as Sodium Iodide), and producing a tiny flash of light. This is amplified by a "photomultiplier tube" which results in a burst of electrons large enough to be detected. Scintillation detectors form the basis of the hand-held instruments used to monitor contamination in nuclear power stations. They can recognize the difference between a, b and g radiation, and make different noises (such as bleeps or clicks) accordingly. 57

Solid-State Detectors The most up-to-date instruments. They are used in particleaccelerator laboratories to show the results of high-energy collisions, with banks of them clustered around the collision site, feeding data into huge computers. 58

59 Detecting Radiation • http: //www. darvill. clara. net/nucrad/detect. ht m
 Kuei honors chemistry
Kuei honors chemistry Chemistry unit 4 review answers
Chemistry unit 4 review answers Honors chemistry summer assignment
Honors chemistry summer assignment Bond order
Bond order Chapter 3 geometry test
Chapter 3 geometry test Precalculus honors chapter 1 test
Precalculus honors chapter 1 test Creighton university honors program
Creighton university honors program James scholar uiuc aces
James scholar uiuc aces Do no. 36 s. 2016
Do no. 36 s. 2016 Ucsb frap
Ucsb frap Physics semester 1 review
Physics semester 1 review Honors biology ecology test
Honors biology ecology test Economics 4.05 uncle sam's toolbox
Economics 4.05 uncle sam's toolbox Honors geometry quadrilaterals test
Honors geometry quadrilaterals test Honors earth science
Honors earth science Mrs blueprint
Mrs blueprint Hilton honors military program
Hilton honors military program Honors project
Honors project Honors biology properties of water lab
Honors biology properties of water lab Honors geometry parallel lines and transversals worksheet
Honors geometry parallel lines and transversals worksheet Tulane honors program
Tulane honors program Honors physics projectile motion test
Honors physics projectile motion test Honors biology unit 4 test
Honors biology unit 4 test Smc scholars classes
Smc scholars classes Golden opulence sundae
Golden opulence sundae Math 3 honors
Math 3 honors Chem
Chem Algebra 1 honors
Algebra 1 honors Mahurin honors college
Mahurin honors college Honors algebra 2 final exam
Honors algebra 2 final exam Umes honors program
Umes honors program Latin honors smith college
Latin honors smith college Pathfinder shooting star
Pathfinder shooting star Physics honors notes
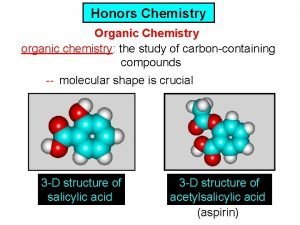
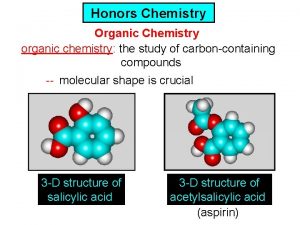
Physics honors notes Ib chemistry organic chemistry
Ib chemistry organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Approach chemistry chalk chapter
Approach chemistry chalk chapter Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Thermodynamic vs kinetic control
Thermodynamic vs kinetic control Organic chemistry introduction
Organic chemistry introduction Modern chemistry textbook answers chapter 9
Modern chemistry textbook answers chapter 9 Love formula in chemistry
Love formula in chemistry Modern chemistry chapter 15 review answers
Modern chemistry chapter 15 review answers Chapter 14 review acids and bases
Chapter 14 review acids and bases Chapter 13 review ions in aqueous solutions
Chapter 13 review ions in aqueous solutions Chapter 12 review solutions section 1
Chapter 12 review solutions section 1 Law of combining volumes
Law of combining volumes Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Chapter 7 chemistry review
Chapter 7 chemistry review 12x12x12x12x12x12x12
12x12x12x12x12x12x12 Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Chapter 12 basics of chemistry cosmetology
Chapter 12 basics of chemistry cosmetology Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Chapter 25 nuclear chemistry answer key
Chapter 25 nuclear chemistry answer key Carboxylic acid h3o+ reaction
Carboxylic acid h3o+ reaction Modern chemistry chapter 4
Modern chemistry chapter 4