Chemistry and Calculations Chemistry Honors Accuracy Precision how











- Slides: 53

Chemistry and Calculations Chemistry Honors

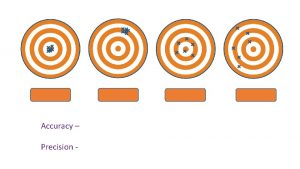
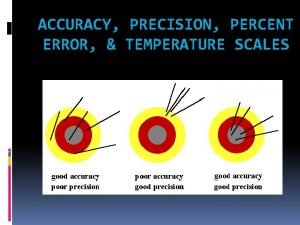
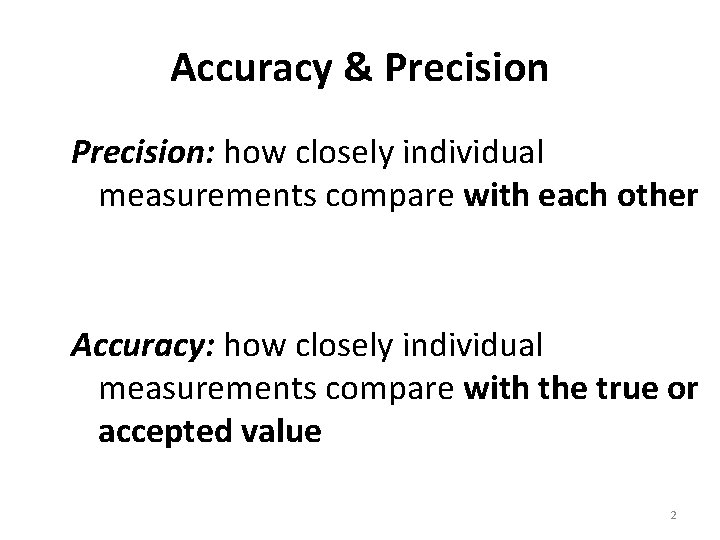
Accuracy & Precision: how closely individual measurements compare with each other Accuracy: how closely individual measurements compare with the true or accepted value 2

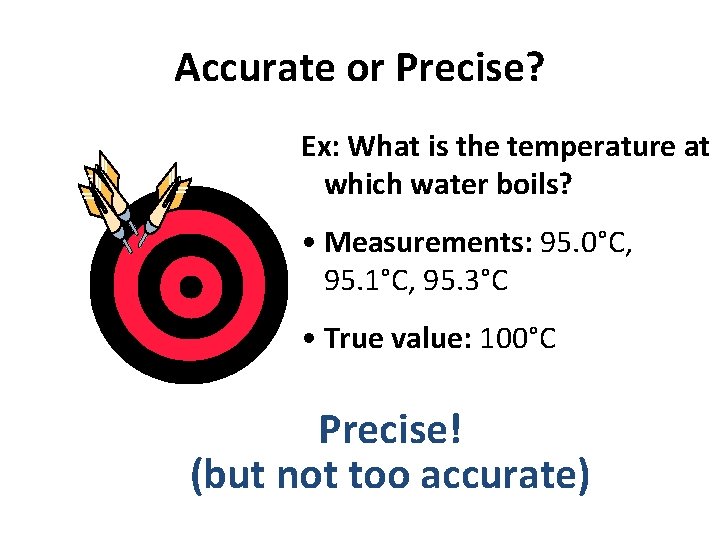
Accurate or Precise? Ex: What is the temperature at which water boils? • Measurements: 95. 0°C, 95. 1°C, 95. 3°C • True value: 100°C Precise! (but not too accurate)

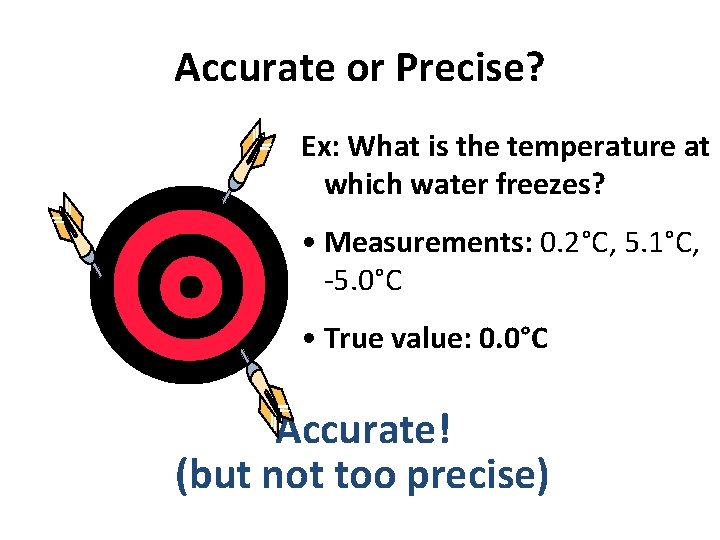
Accurate or Precise? Ex: What is the temperature at which water freezes? • Measurements: 0. 2°C, 5. 1°C, -5. 0°C • True value: 0. 0°C Accurate! (but not too precise)

Accurate or Precise? Ex: What is the mass of one Liter of water? • Measurements: 1. 000 kg, 0. 999 kg, 1. 002 kg • True value: 1. 000 kg Accurate & Precise (it’s time to go pro)

Accurate or Precise? Ex: What is the atmospheric pressure at sea level? • Measurements: 10. 01 atm, 0. 25 atm, 234. 5 atm • True value: 1. 00 atm Not Accurate & Not Precise (don’t quit your day job)

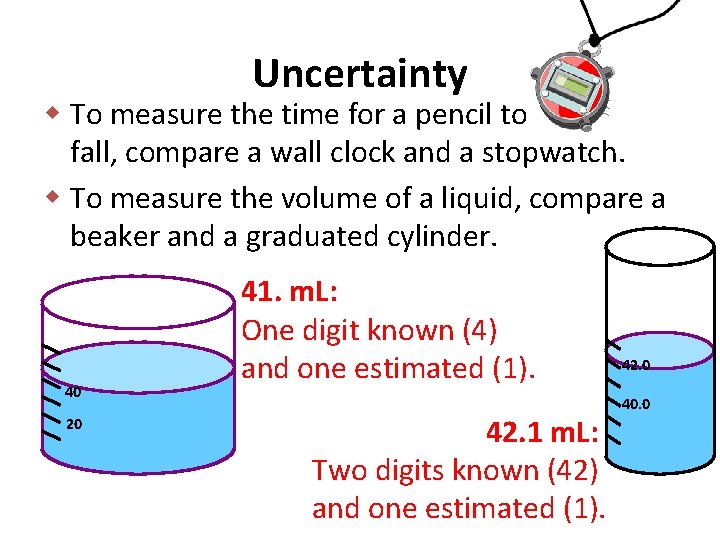
Uncertainty w To measure the time for a pencil to fall, compare a wall clock and a stopwatch. w To measure the volume of a liquid, compare a beaker and a graduated cylinder. 40 20 41. m. L: One digit known (4) and one estimated (1). 42. 1 m. L: Two digits known (42) and one estimated (1). 42. 0 40. 0

The stopwatch and graduated cylinder… • Are more precise instruments (are more certain. ) • Give measurements that are known to more decimal places. .

Significant Figures (“sig figs”): All the digits known with certainty plus one final digit, which is somewhat uncertain. In a correctly reported measured value, the final digit is significant but not certain. If the number 31. 2 is reported. 3 & 1 are known with certainty, the 2 is significant but uncertain. A more precise instrument will give more sig figs in its measurement

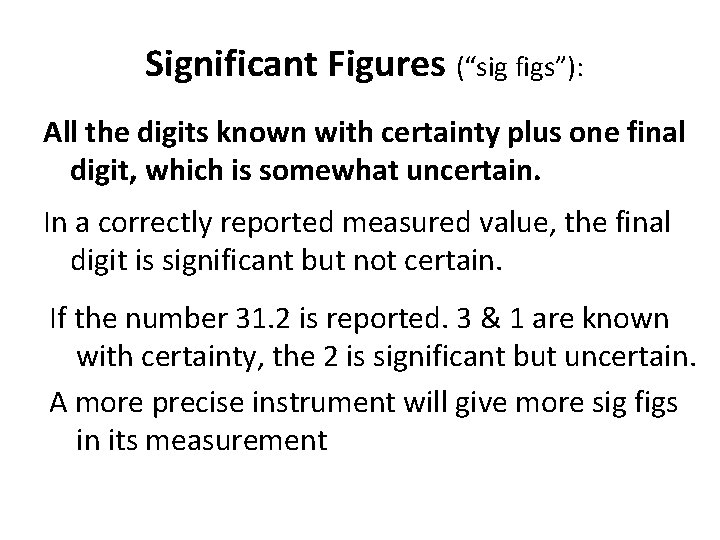
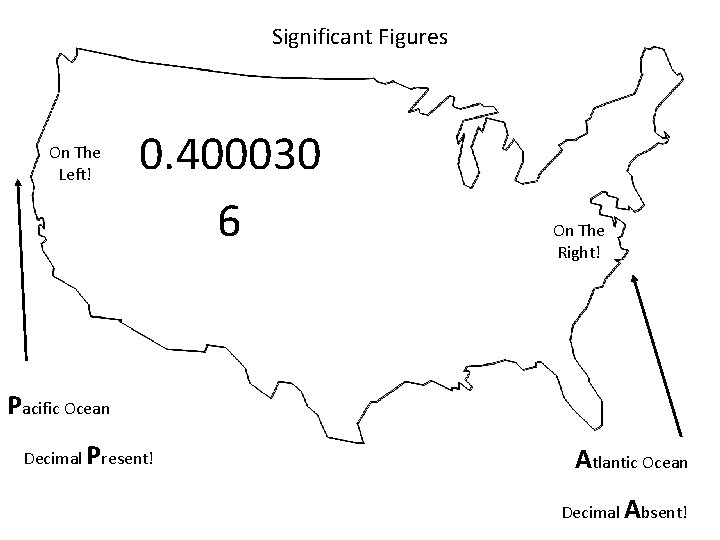
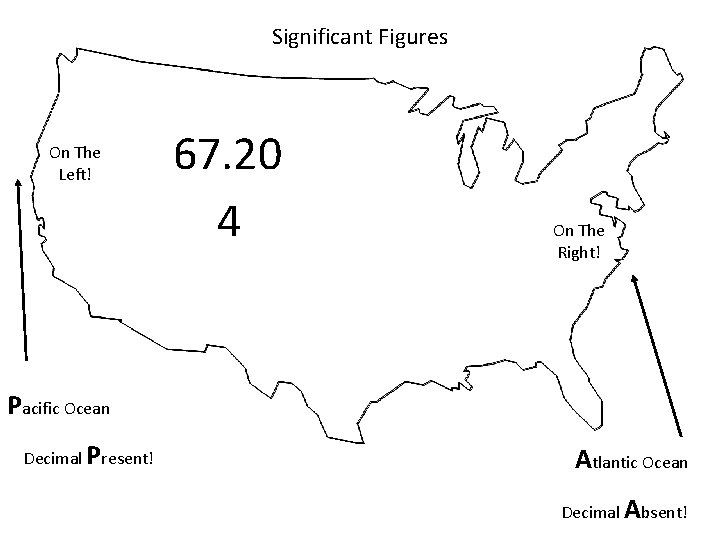
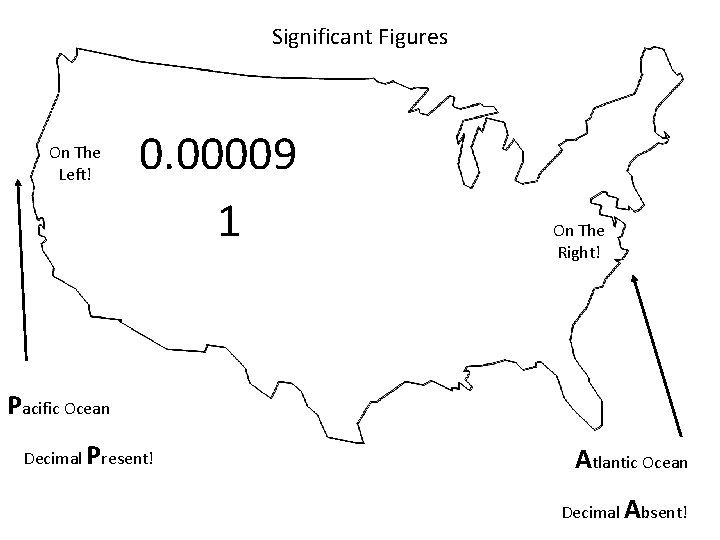
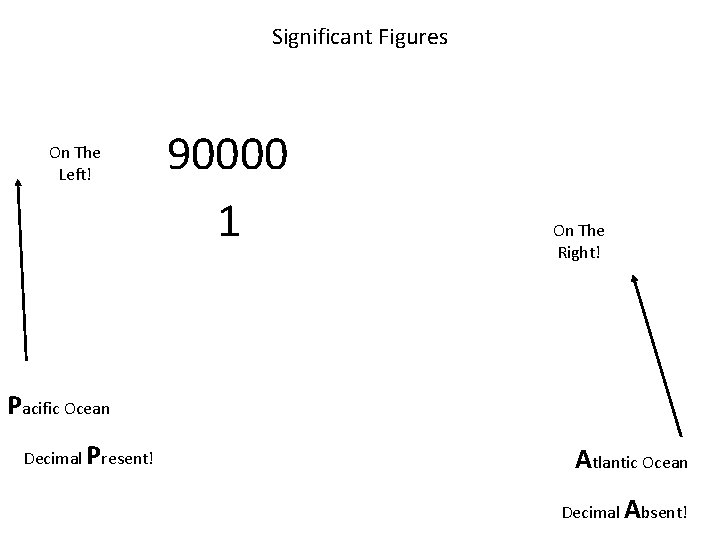
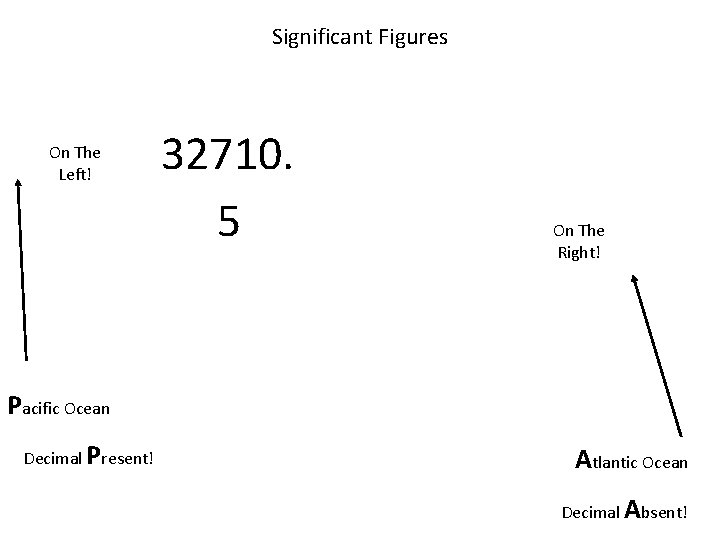
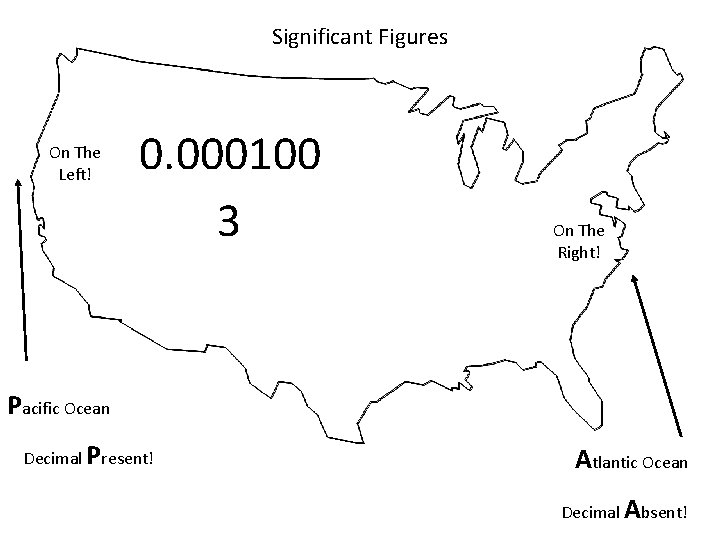
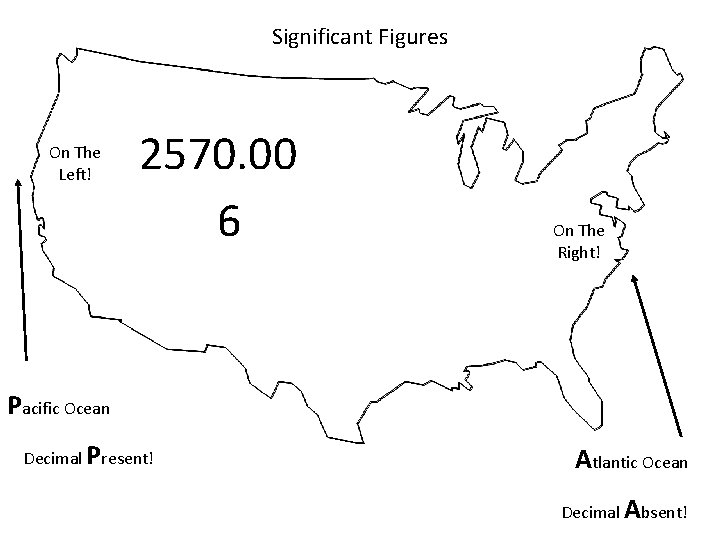
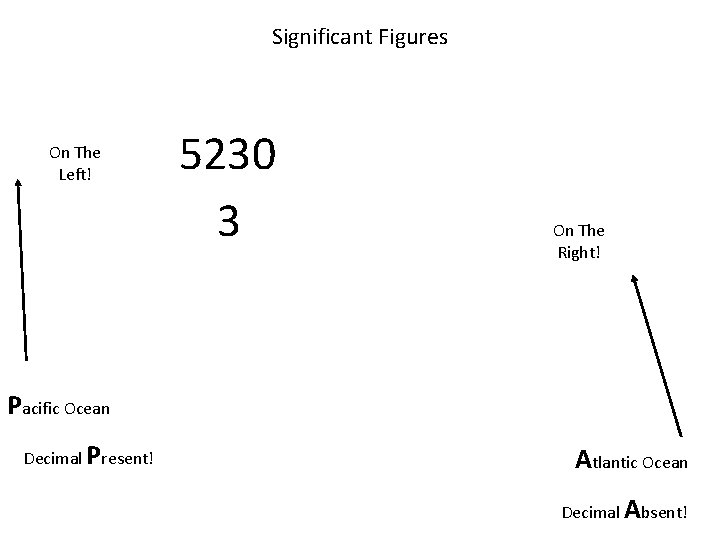
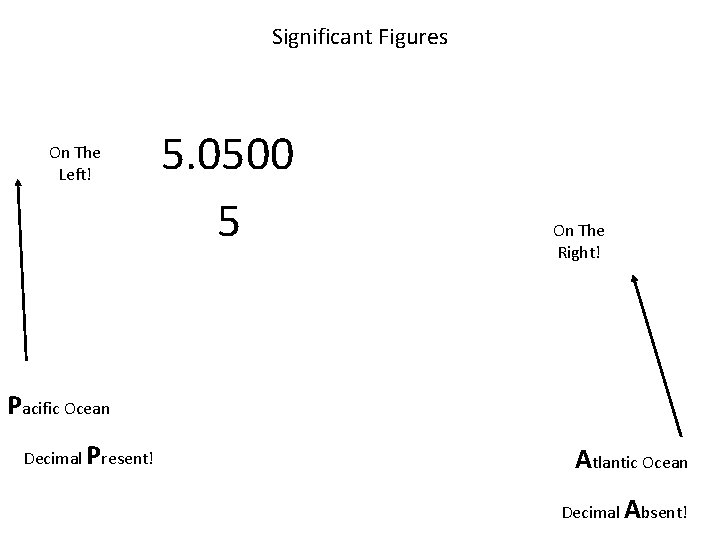
When are digits “significant”? The “Atlantic-Pacific” Rule “PACIFIC” Decimal point is PRESENT. Count digits from left side, starting with the first nonzero digit. PACIFIC 40603. 23 ft 2 = 7 sig figs 0. 01586 m. L = 4 sig figs

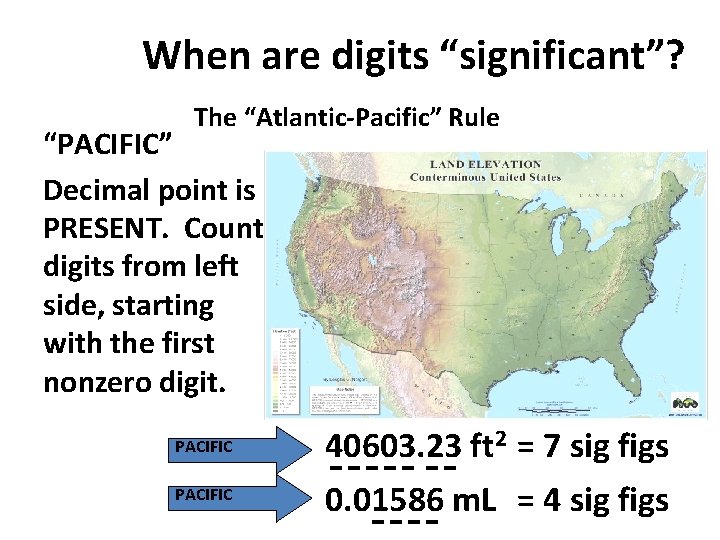
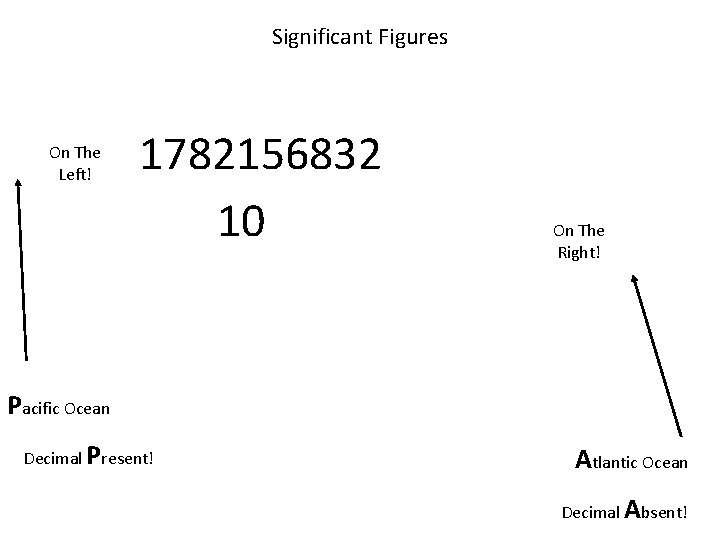
When are digits “significant”? “ATLANTIC” Decimal point is ABSENT. Count digits from right side, starting with the first nonzero digit. 3 sig figs = 40600 ft 2 1 sig fig = 1000 m. L ATLANTIC

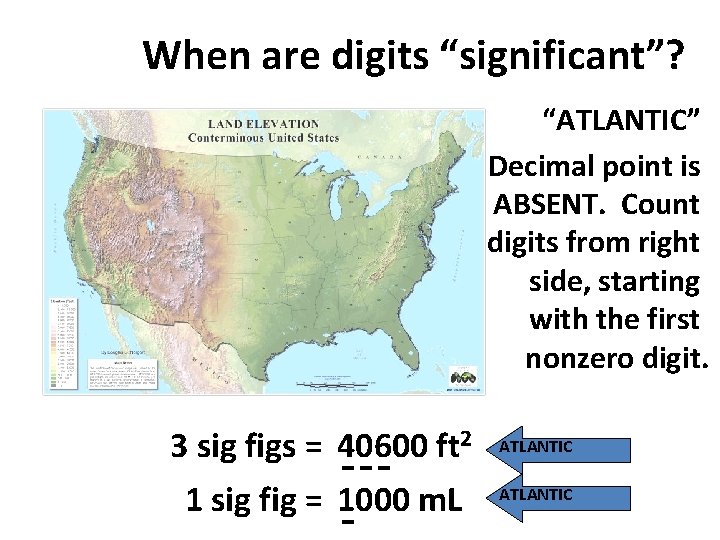
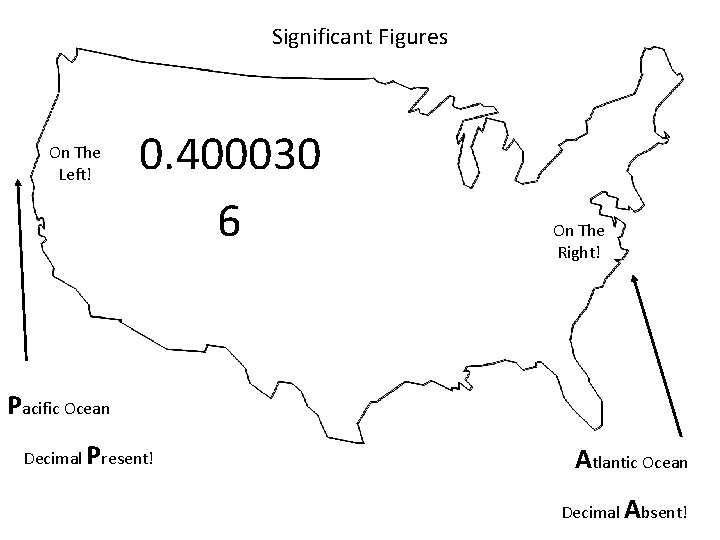
Significant Figures On The Left! 0. 400030 6 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

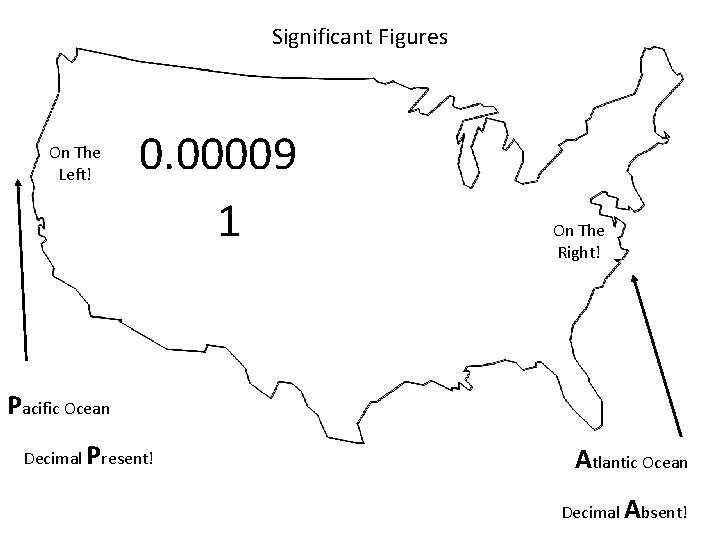
Significant Figures On The Left! 0. 00009 1 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

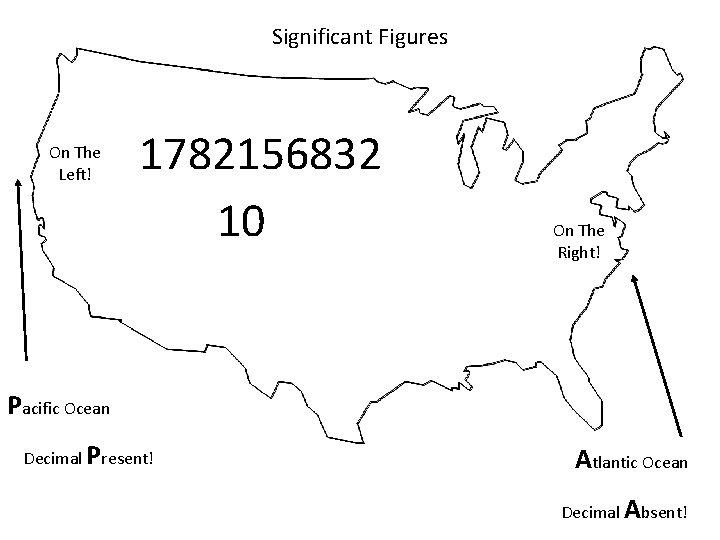
Significant Figures On The Left! 1782156832 10 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

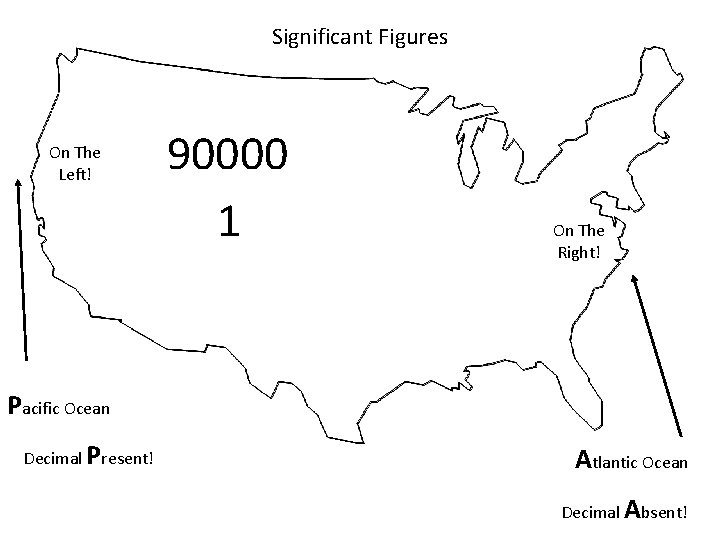
Significant Figures On The Left! 90000 1 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

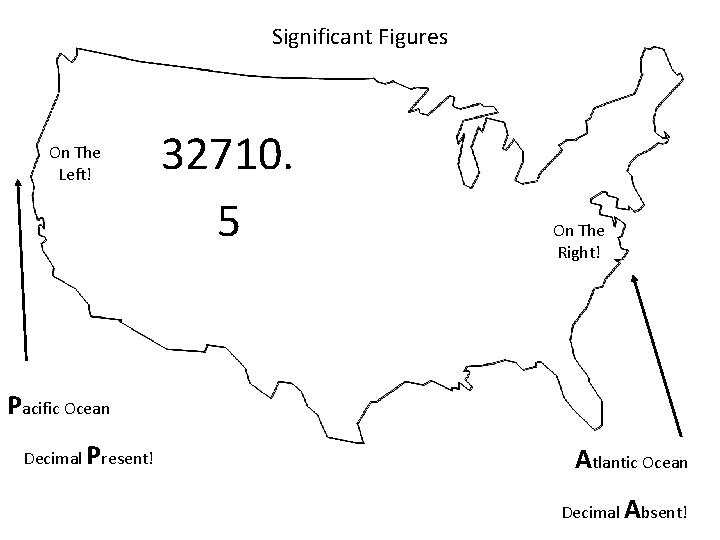
Significant Figures On The Left! 32710. 5 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

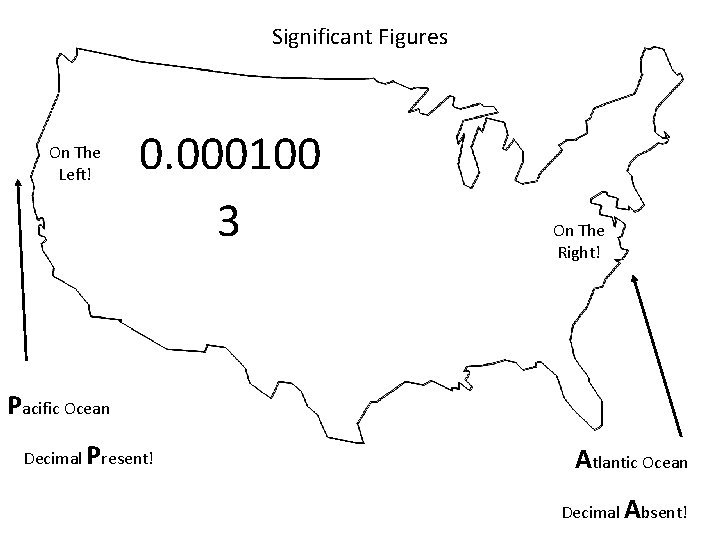
Significant Figures On The Left! 0. 000100 3 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

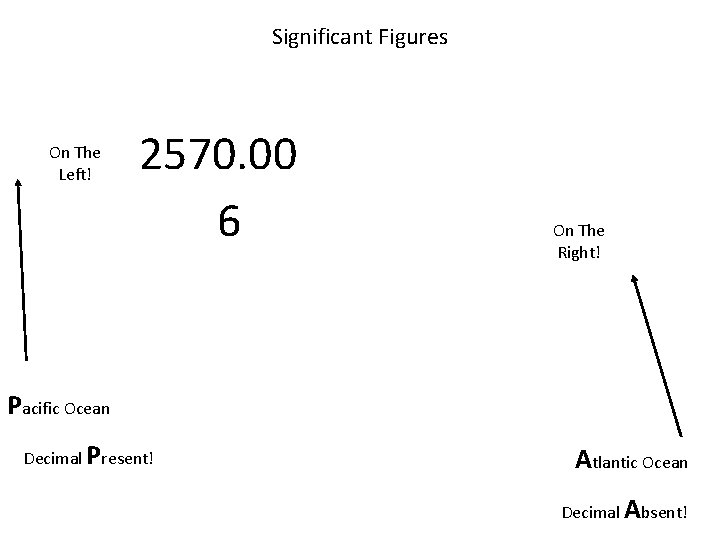
Significant Figures On The Left! 2570. 00 6 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

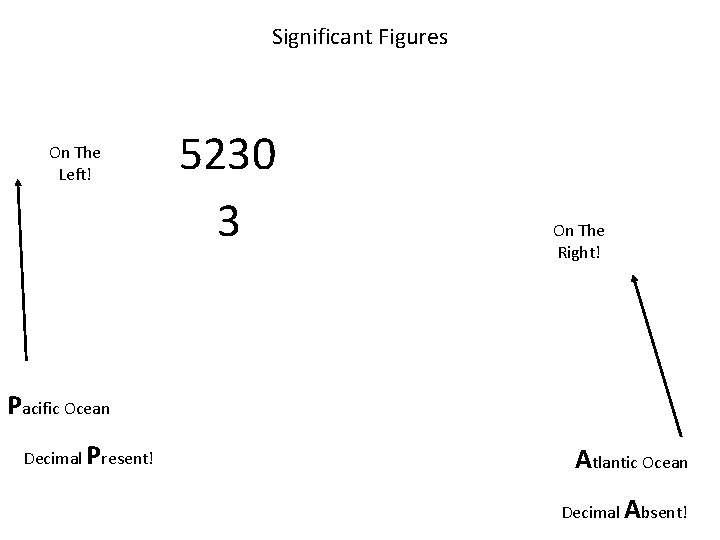
Significant Figures On The Left! 5230 3 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

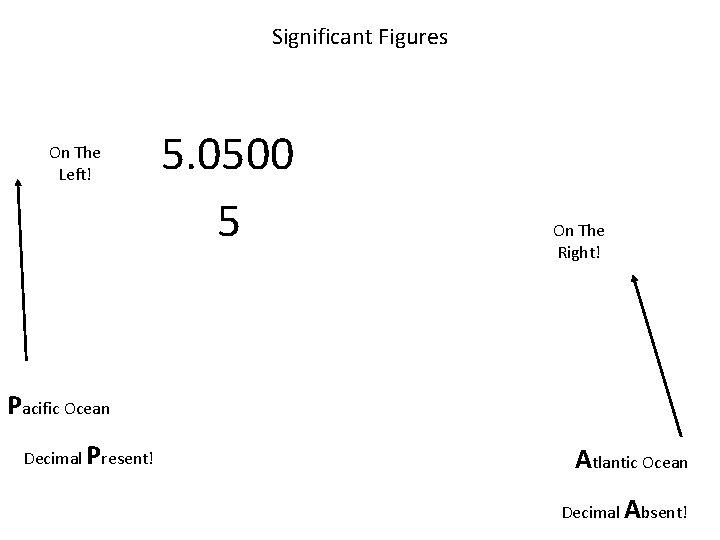
Significant Figures On The Left! 5. 0500 5 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

Examples • 0. 00932 Decimal point present → “Pacific” → count digits from left, starting with first nonzero digit = 3 sig figs • 27510 Decimal point absent → “Atlantic” → count digits from right, starting with first nonzero digit = 4 sig figs • If number is obtained by counting, ex: 8 beakers, or is used in a conversion factor, ex: 1000 mm= 1 meter it is an exact number. = unlimited number of significant figures.

Significant Figures On The Left! 0. 400030 6 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

Significant Figures On The Left! 67. 20 4 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

Significant Figures On The Left! 0. 00009 1 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

Significant Figures On The Left! 1782156832 10 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

Significant Figures On The Left! 90000 1 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

Significant Figures On The Left! 32710. 5 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

Significant Figures On The Left! 0. 000100 3 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

Significant Figures On The Left! 2570. 00 6 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

Significant Figures On The Left! 5230 3 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

Significant Figures On The Left! 5. 0500 5 On The Right! Pacific Ocean Decimal Present! Atlantic Ocean Decimal Absent!

Addition and Subtraction • The answer has the same number of digits to the right of the decimal point as there are in the measurement having the fewest digits to the right of the decimal point. • Ex: 56. 31 g – 14. 1 g = • Answer must be rounded so has only one number to the right of the decimal point.

Multiplication and Division • The answer has no more sig. figs. than are in the measurement with the fewest number of sig. figs. • 7. 2 cm X 8. 141 cm =58 cm 2 The answer can only have 2 sig. Figs.

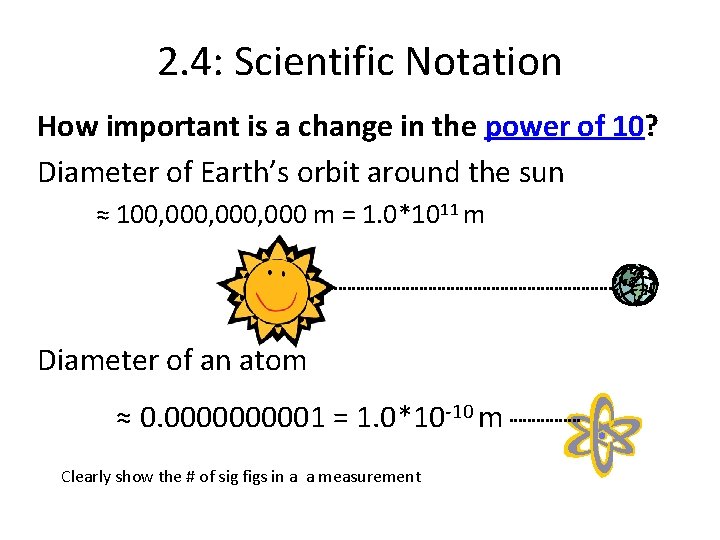
2. 4: Scientific Notation How important is a change in the power of 10? Diameter of Earth’s orbit around the sun ≈ 100, 000, 000 m = 1. 0*1011 m Diameter of an atom ≈ 0. 000001 = 1. 0*10 -10 m Clearly show the # of sig figs in a a measurement

Writing in scientific notation 1. Move the decimal point in the original number so that it is located to the right of the first nonzero digit. 2. Multiply the new number by 10 raised to the proper power that is equal to the number of places the decimal moved. The form is M x 10 n 3. If the decimal point moves: w To the left, the power of 10 is positive. w To the right, the power of 10 is negative.

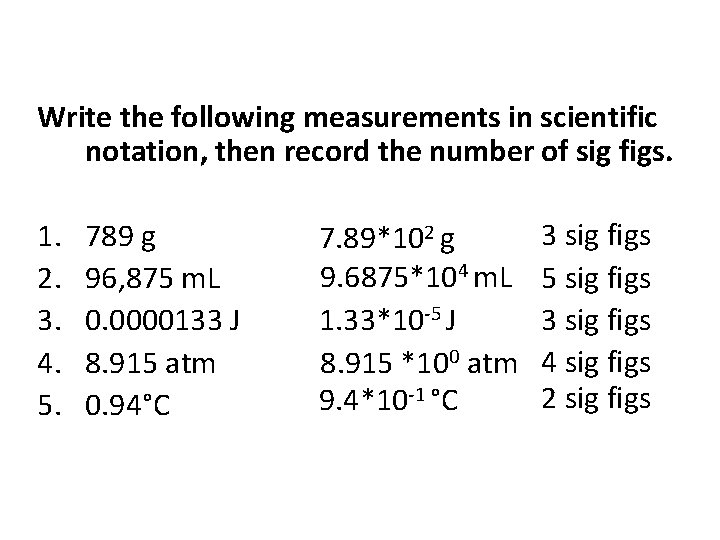
Write the following measurements in scientific notation, then record the number of sig figs. 1. 2. 3. 4. 5. 789 g 96, 875 m. L 0. 0000133 J 8. 915 atm 0. 94°C 7. 89*102 g 9. 6875*104 m. L 1. 33*10 -5 J 8. 915 *100 atm 9. 4*10 -1 °C 3 sig figs 5 sig figs 3 sig figs 4 sig figs 2 sig figs

When Adding & Subtracting • All values must have same exponent • Ex: 4. 71 X 103 L+ 3. 3 X 104 L = 4. 71 X 103 L + 33. X 103 L = 37. 71 X 103 L OR. 471 X 104 L + 03. 3 X 104 L =3. 771 X 104 L Answer = 3. 8 X 104 L Convert answer to appropriate scientific notation. Least number of places past decimal

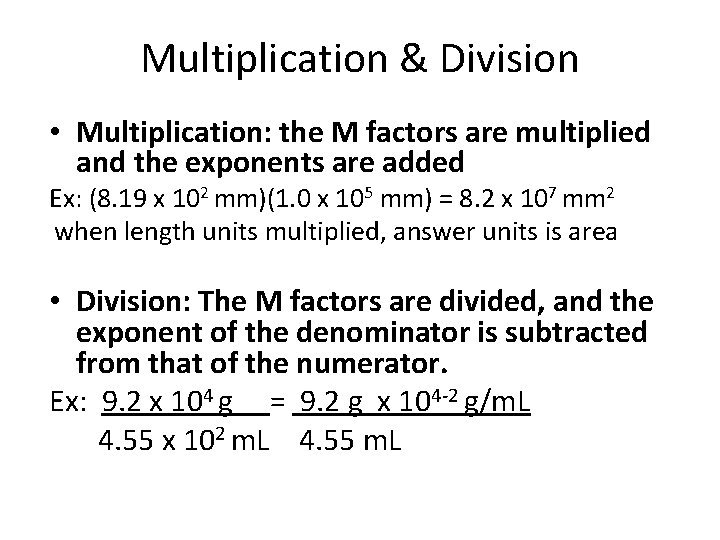
Multiplication & Division • Multiplication: the M factors are multiplied and the exponents are added Ex: (8. 19 x 102 mm)(1. 0 x 105 mm) = 8. 2 x 107 mm 2 when length units multiplied, answer units is area • Division: The M factors are divided, and the exponent of the denominator is subtracted from that of the numerator. Ex: 9. 2 x 104 g = 9. 2 g x 104 -2 g/m. L 4. 55 x 102 m. L 4. 55 m. L

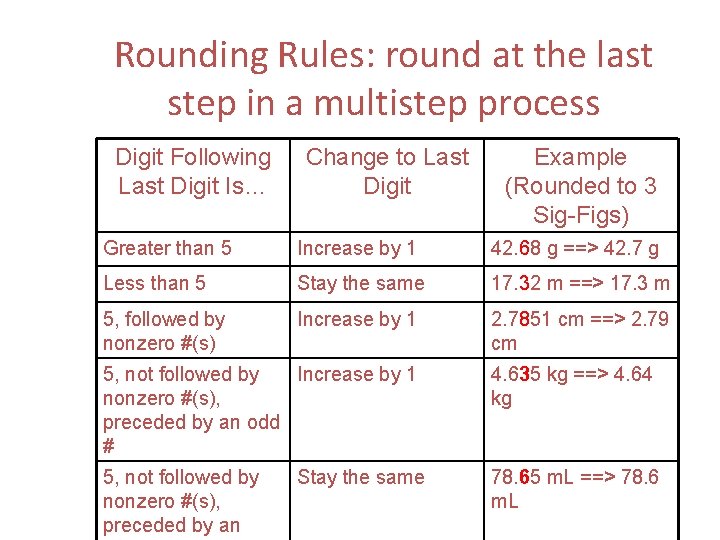
Rounding Rules: round at the last step in a multistep process Digit Following Last Digit Is… Change to Last Digit Example (Rounded to 3 Sig-Figs) Greater than 5 Increase by 1 42. 68 g ==> 42. 7 g Less than 5 Stay the same 17. 32 m ==> 17. 3 m 5, followed by nonzero #(s) Increase by 1 2. 7851 cm ==> 2. 79 cm 5, not followed by Increase by 1 nonzero #(s), preceded by an odd # 4. 635 kg ==> 4. 64 kg 5, not followed by nonzero #(s), preceded by an 78. 65 m. L ==> 78. 6 m. L Stay the same

Système International d'Unités • The metric system or Système International d'Unités (S. I. ), was first organized in Paris as part of the French Revolution & adopted by France in 1795. At that time, the meter & kilogram were standardized. • Every country in the world uses SI units except the USA, Myanmar, & Liberia. • By 2009, all products sold in Europe must use the metric system. No dual-labeling will be permitted.

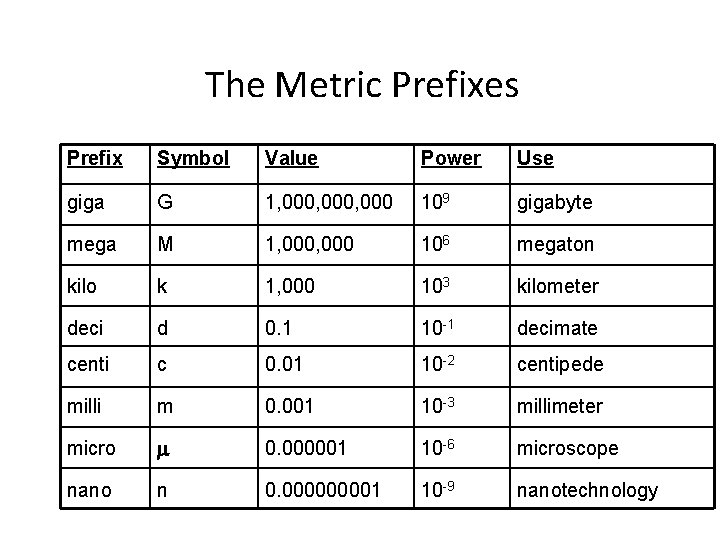
The Metric Prefixes Prefix Symbol Value Power Use giga G 1, 000, 000 109 gigabyte mega M 1, 000 106 megaton kilo k 1, 000 103 kilometer deci d 0. 1 10 -1 decimate centi c 0. 01 10 -2 centipede milli m 0. 001 10 -3 millimeter micro m 0. 000001 10 -6 microscope nano n 0. 00001 10 -9 nanotechnology

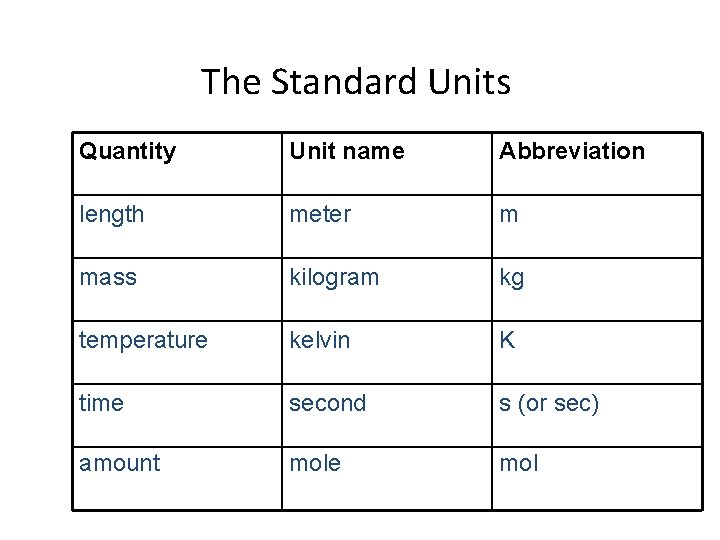
The Standard Units Quantity Unit name Abbreviation length meter m mass kilogram kg temperature kelvin K time second s (or sec) amount mole mol

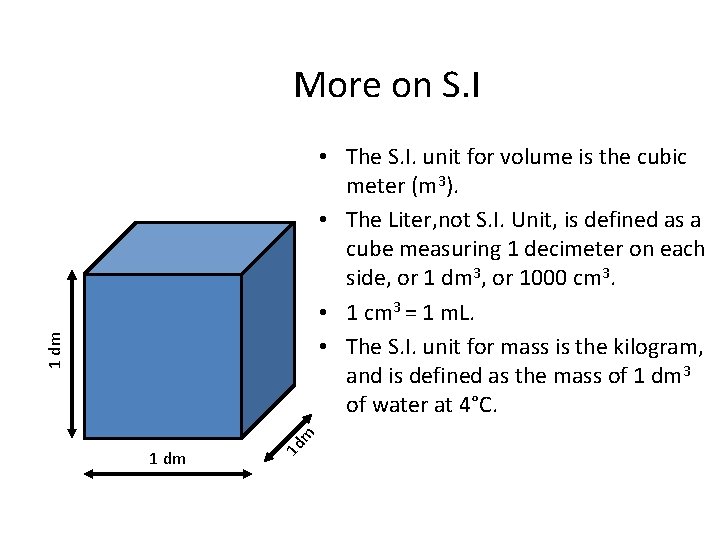
More on S. I 1 dm 1 d m 1 dm • The S. I. unit for volume is the cubic meter (m 3). • The Liter, not S. I. Unit, is defined as a cube measuring 1 decimeter on each side, or 1 dm 3, or 1000 cm 3. • 1 cm 3 = 1 m. L. • The S. I. unit for mass is the kilogram, and is defined as the mass of 1 dm 3 of water at 4°C.

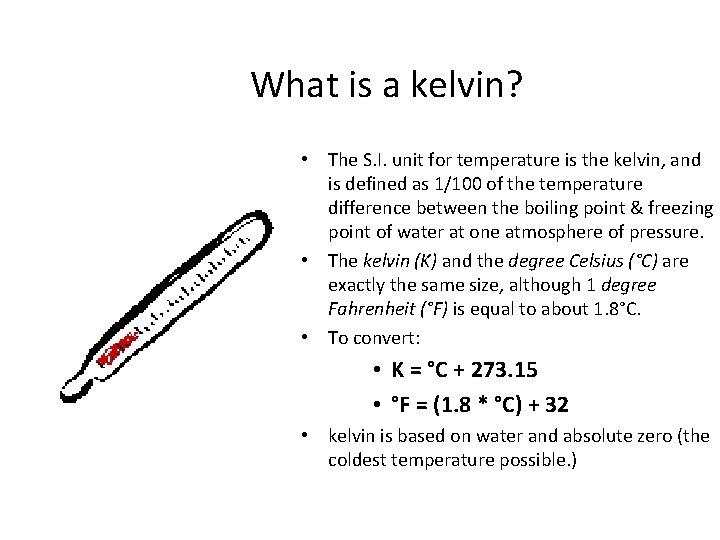
What is a kelvin? • The S. I. unit for temperature is the kelvin, and is defined as 1/100 of the temperature difference between the boiling point & freezing point of water at one atmosphere of pressure. • The kelvin (K) and the degree Celsius (°C) are exactly the same size, although 1 degree Fahrenheit (°F) is equal to about 1. 8°C. • To convert: • K = °C + 273. 15 • °F = (1. 8 * °C) + 32 • kelvin is based on water and absolute zero (the coldest temperature possible. )

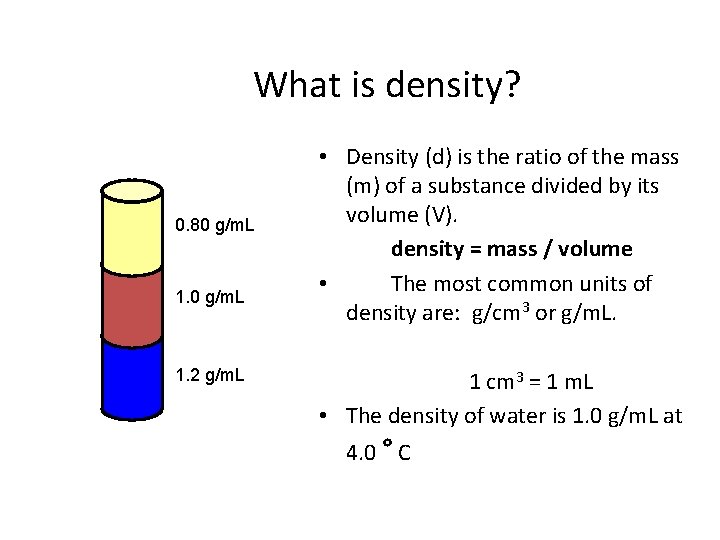
What is density? 0. 80 g/m. L 1. 2 g/m. L • Density (d) is the ratio of the mass (m) of a substance divided by its volume (V). density = mass / volume • The most common units of density are: g/cm 3 or g/m. L. 1 cm 3 = 1 m. L • The density of water is 1. 0 g/m. L at 4. 0 ° C

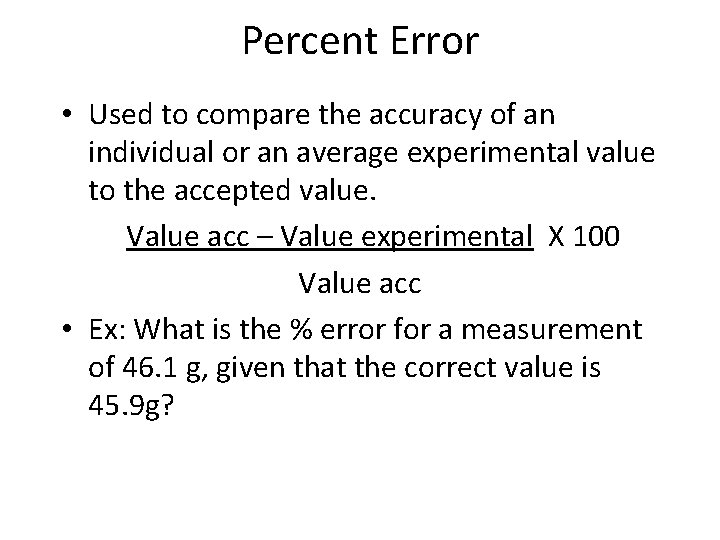
Percent Error • Used to compare the accuracy of an individual or an average experimental value to the accepted value. Value acc – Value experimental X 100 Value acc • Ex: What is the % error for a measurement of 46. 1 g, given that the correct value is 45. 9 g?

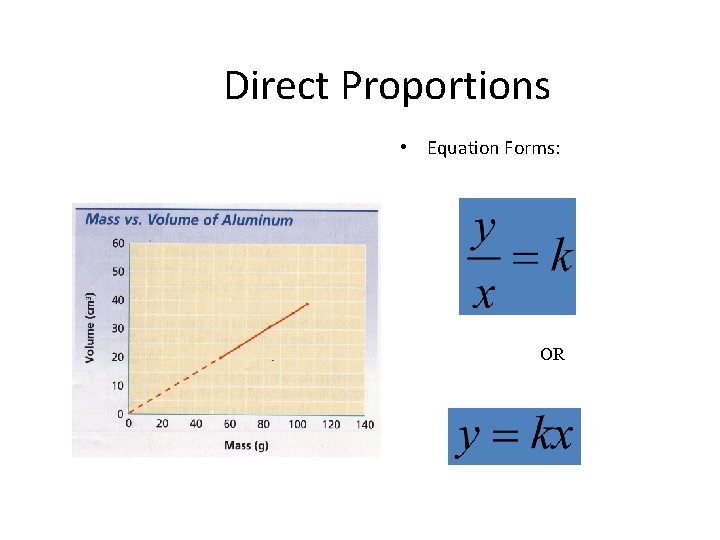
Direct Proportions • Equation Forms: OR

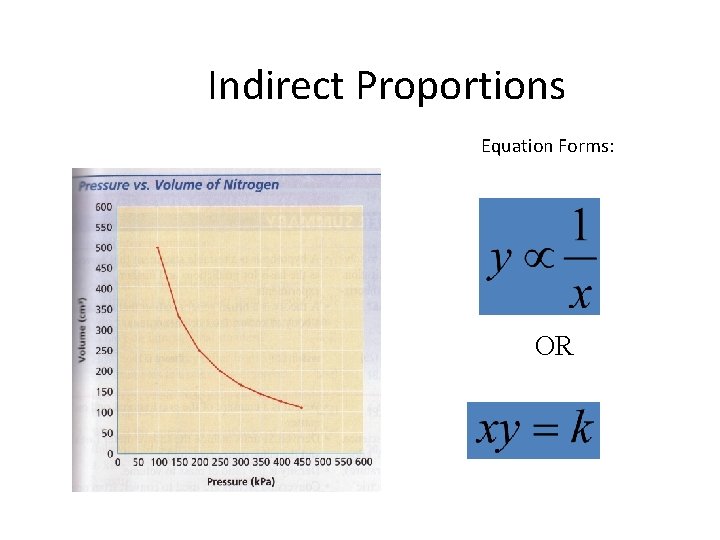
Indirect Proportions Equation Forms: OR

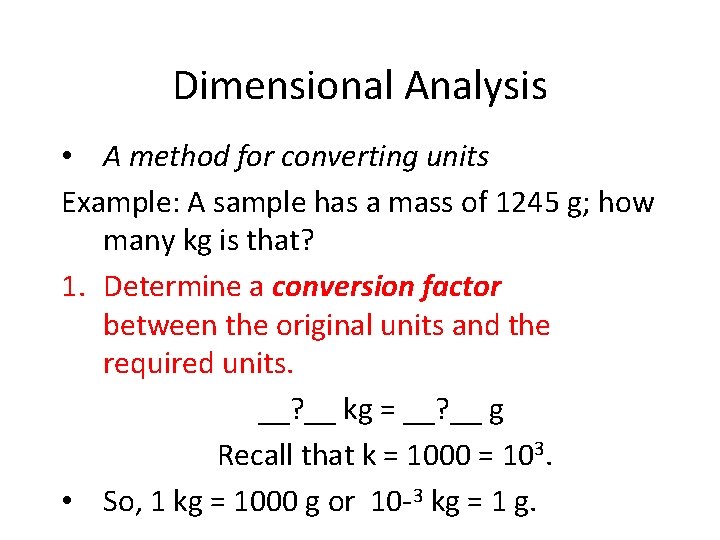
Dimensional Analysis • A method for converting units Example: A sample has a mass of 1245 g; how many kg is that? 1. Determine a conversion factor between the original units and the required units. __? __ kg = __? __ g Recall that k = 1000 = 103. • So, 1 kg = 1000 g or 10 -3 kg = 1 g.

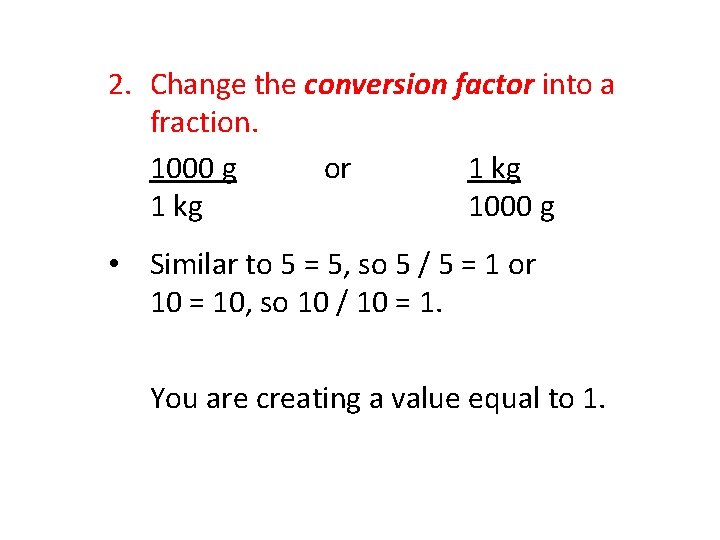
2. Change the conversion factor into a fraction. 1000 g or 1 kg 1000 g • Similar to 5 = 5, so 5 / 5 = 1 or 10 = 10, so 10 / 10 = 1. You are creating a value equal to 1.

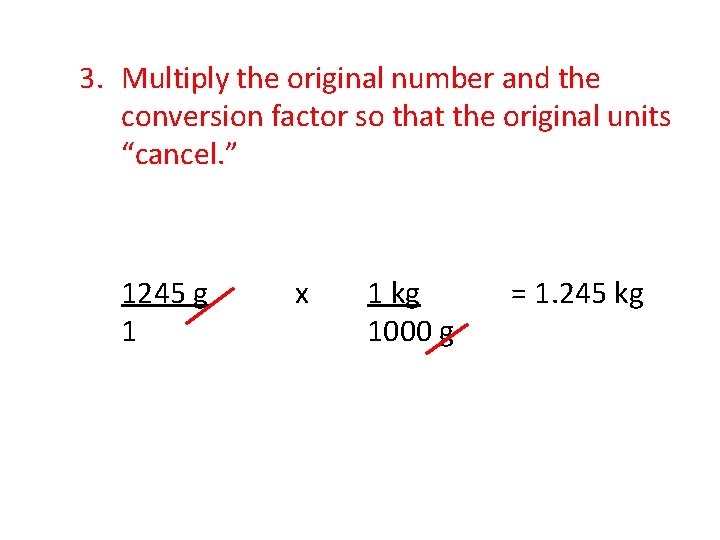
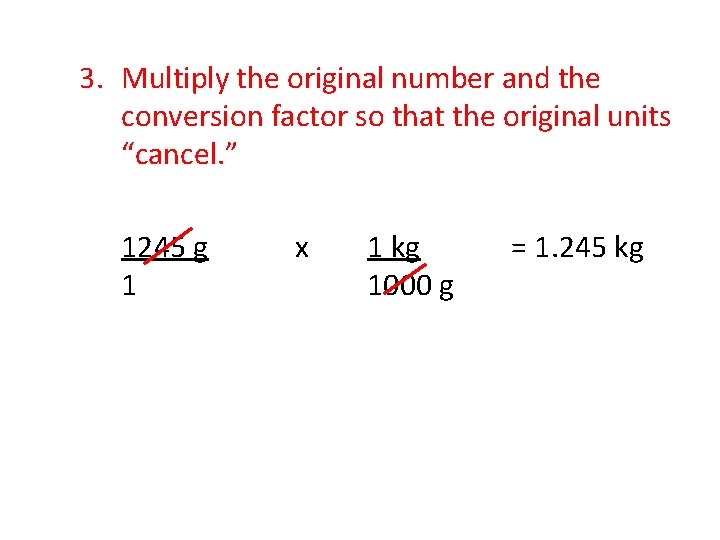
3. Multiply the original number and the conversion factor so that the original units “cancel. ” 1245 g 1 x 1 kg 1000 g = 1. 245 kg

3. Multiply the original number and the conversion factor so that the original units “cancel. ” 1245 g 1 x 1 kg 1000 g = 1. 245 kg

Practice • Convert 1. 65 L to m. L. • Convert 3. 5 mm to m. • Convert 2. 00 L to quarts. (1 qt = 946 m. L)
 Precision definition chemistry
Precision definition chemistry Accuracy v precision in chemistry
Accuracy v precision in chemistry Precision vs accuracy chemistry
Precision vs accuracy chemistry Difference accuracy and precision
Difference accuracy and precision Precision and significant figures
Precision and significant figures Relative uncertainty
Relative uncertainty Lab measurement accuracy
Lab measurement accuracy Quick lab accuracy and precision answers
Quick lab accuracy and precision answers Accuracy vs precision graph
Accuracy vs precision graph Brainpop precision and accuracy quiz answers
Brainpop precision and accuracy quiz answers Precision definition
Precision definition Classification of instrumentation
Classification of instrumentation Precision and accuracy
Precision and accuracy K h da b d c m
K h da b d c m Accuracy and precision
Accuracy and precision Precision vs accuracy
Precision vs accuracy Brainpop mindfulness quiz answers
Brainpop mindfulness quiz answers Sensitivity accuracy precision
Sensitivity accuracy precision Is accuracy qualitative
Is accuracy qualitative Pmi quality management
Pmi quality management High precision vs high accuracy
High precision vs high accuracy C to k formula
C to k formula Difference between precision and non precision instruments
Difference between precision and non precision instruments Semi precision attachment
Semi precision attachment Types of connections in steel structures
Types of connections in steel structures Negatif sayıların binary gösterimi
Negatif sayıların binary gösterimi Kuei honors chemistry
Kuei honors chemistry What is the answer
What is the answer Honors chemistry summer assignment
Honors chemistry summer assignment Bond order formula
Bond order formula Osmolar gap
Osmolar gap With high honors
With high honors Ucsb honors college
Ucsb honors college Honors geometry parallel lines and transversals worksheet
Honors geometry parallel lines and transversals worksheet Creighton honors program
Creighton honors program Aces james scholar
Aces james scholar Physics semester 1 review
Physics semester 1 review Honors biology ecology test
Honors biology ecology test 04.05 uncle sams toolbox-honors
04.05 uncle sams toolbox-honors Quadrilateral test review
Quadrilateral test review Honors earth science
Honors earth science Mrs blueprint
Mrs blueprint Hilton honors military program
Hilton honors military program Honors project
Honors project Honors biology properties of water lab
Honors biology properties of water lab Tulane honors program
Tulane honors program Chapter 3 geometry test
Chapter 3 geometry test Honors precalculus chapter 1 test
Honors precalculus chapter 1 test Vyi physics
Vyi physics Honors biology unit 4 test
Honors biology unit 4 test Santa monica college honors program
Santa monica college honors program Golden opulence sundae
Golden opulence sundae Math 3 honors
Math 3 honors Honors its atomic
Honors its atomic