Accuracy Precision Percent Error Practice 1 A student




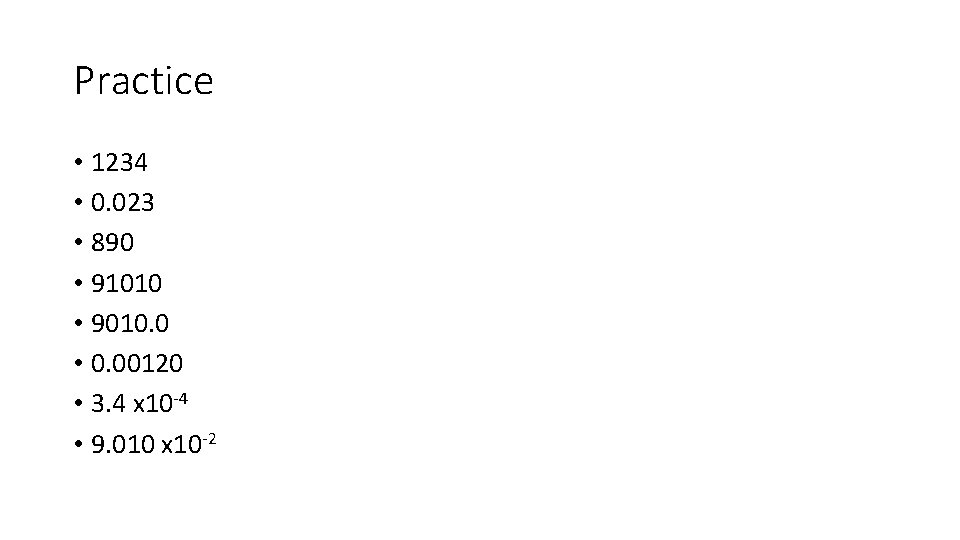







- Slides: 18

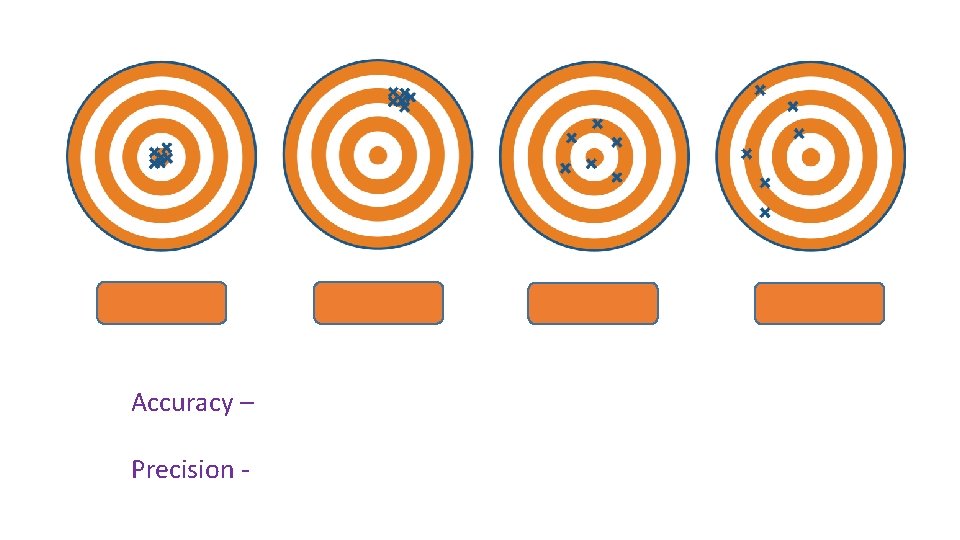
Accuracy – Precision -

Percent Error

Practice 1. A student estimated a mass to be 250 grams, but upon carefully measuring it, found the actual mass to be 240 grams. What is the percent error? 2. The experimental calculation of the specific heat of aluminum was found to be. 156 cal/g◦C, but the actual value was given as. 185 cal/g◦C. What is the percent error?

Practice 1. Joe measured the volume of an object 5 times, and got the results of: 34. 5 m. L, 34. 9 m. L, 34. 2 m. L, 33. 4 m. L, and 35. 9 m. L. What is his average experimental value? 2. The actual volume of the object was 34. 1 m. L. What is the percent error of his average result? 3. A student’s calculation was found to have a 15. 6% error, and the actual value was determined to be 25. 7 m. L. What are two possible values for the student’s experimental measurement?

Scientific Notation Why use it?

Practice • Write the following in scientific notation: • • • 61, 500 0. 0000568 321 64, 960, 000 0. 07085 • Express in standard form: • 4. 22715 x 108 • 9. 004 x 10 -2

Significant Figures Rules: 1. Every non-zero digit is significant. 2. Zero’s in between non-zero digits are significant. 3. Leading zeros are not significant. 4. Zeros that are at the end of a number and to the right of a decimal are significant. (ex: 43. 00 or 1. 010 have four sig figs) 5. Ending zeros without a decimal are not significant (ex: 7000 has one sig fig)
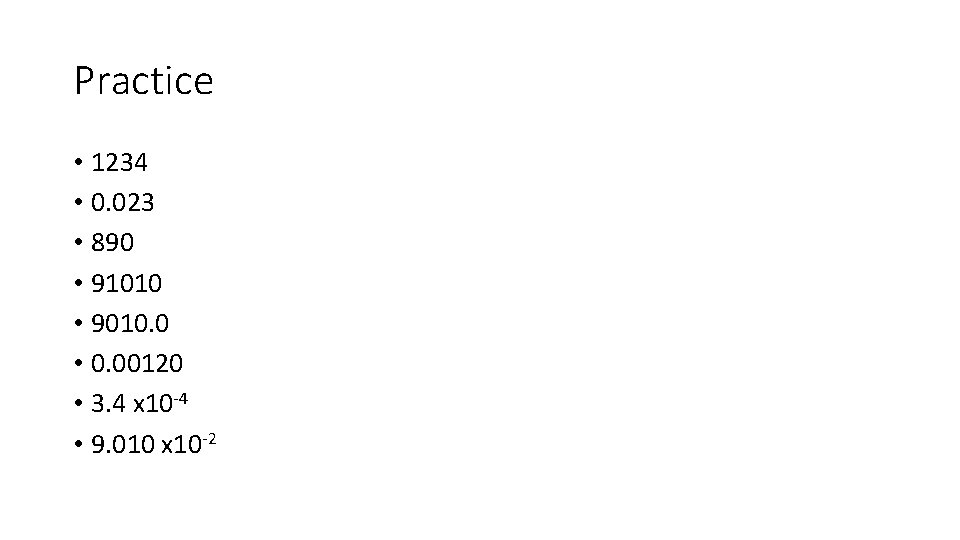
Practice • 1234 • 0. 023 • 890 • 91010 • 9010. 0 • 0. 00120 • 3. 4 x 10 -4 • 9. 010 x 10 -2

Addition and Subtraction • Round to the same number of decimal places as that of the numberin the calculation with the least decimal places. • 2. 5 cm + 0. 50 cm + 0. 055 cm • 416 g - 210 g

Multiplication and Division • Round to the same number of significant figures as the number with the least number of significant figures. • 0. 020 cm x 50 cm x 11. 1 cm • 0. 530 g / 0. 1010 m. L

SI Units SI Measurement System • Le Systeme International d’Unites : SI • System of measurement agreed on by scientists from all over the world in 1960 (related to the metric system) • A decimal system based on 10 • Contains 7 base units and its units are defined in terms of standards of measurement that are objects or natural occurrence that are of constant value or are easily reproducible • We still use some non-SI units

Exact or Measured Numbers • Exact examples: number of eggs in a carton, 1000 milliliters in a liter, 2. 54 cm in an inch, etc. • Measured examples: volume of a substance measured in a graduated cylinder, temperature of a liquid recorded using a thermometer. • Known digit plus one estimated digit

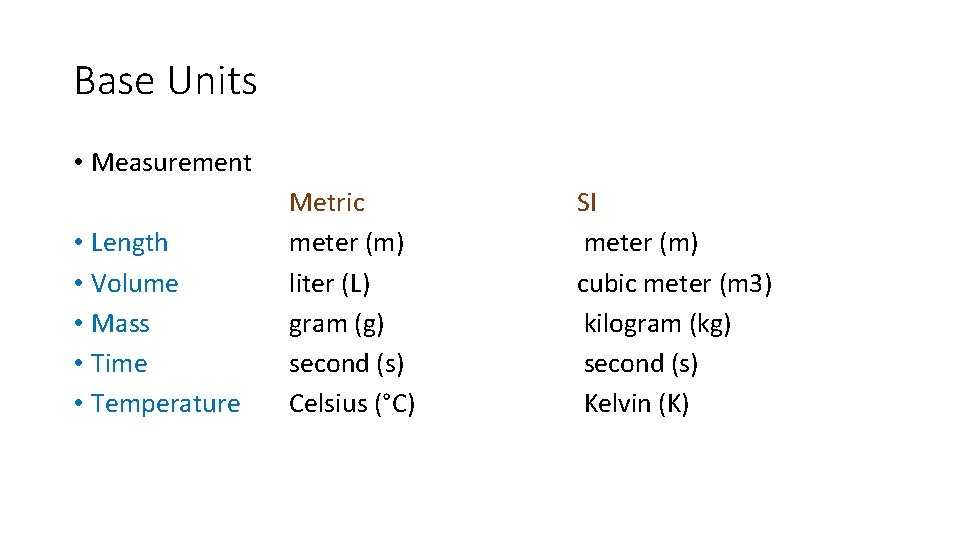
Base Units • Measurement • Length • Volume • Mass • Time • Temperature Metric meter (m) liter (L) gram (g) second (s) Celsius (°C) SI meter (m) cubic meter (m 3) kilogram (kg) second (s) Kelvin (K)

Temperature • Measure of how hot or cold something is. • Temperature determines the direction of heat transfer. • Celsius based upon freezing and boiling point of water • Kelvin developed from Lord Kevin (physicist and mathematician) –based on absolute zero. • K= C◦ + 273. 15 • C◦ = K – 273. 15

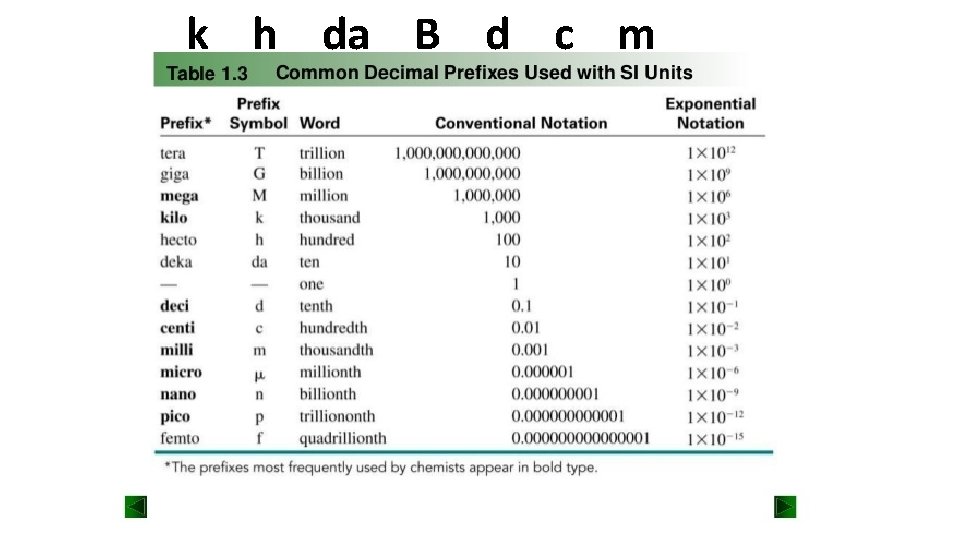
k h da B d c m

Format for Conversions

Practice • How many seconds are in 8 hours? • Students need 1. 84 grams of copper wire to perform an experiment. The lab has a 50 gram spool of copper wire. How many students can perform the experiment? • Express 750 dg in grams.

Practice • What is 0. 073 cm in micrometers? • What is 49◦C in Kelvin?