How Science Works Accuracy Precision Sensitivity SSER Ltd










- Slides: 22

How Science Works Accuracy, Precision & Sensitivity © SSER Ltd.

Accuracy is how close the measurement is to the true value of the variable. You will normally be taking three or more readings of the same measurement, and using the mean of these values as your result. So, accuracy can also be described as… How close the mean of a set of measurements is to the true value of the variable.

How to Achieve Accuracy

How to Achieve Accuracy No-one knows what the ‘true value’ of a measurement is. However, the following steps will help you improve your accuracy: § Use a well calibrated, precise and sensitive instrument. § You may need to set an instrument to zero before use. § Make sure that the measuring instrument is appropriate for the task, with small enough divisions along the axis.

How to Achieve Accuracy No-one knows what the ‘true value’ of a measurement is. However, the following steps will help you improve your accuracy: § Take the readings as carefully as possible. § Repeat the measurements at least three times. Let different members of the team each take a measurement if possible. § Look out for anomalous results – those that are inconsistent with the rest of the data. Repeat a measurement if necessary.

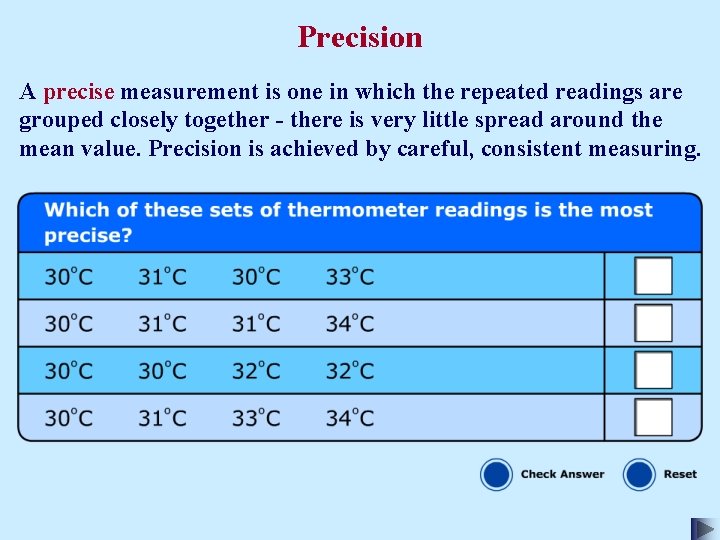
Precision

Precision A precise measurement is one in which the repeated readings are grouped closely together - there is very little spread around the mean value. Precision is achieved by careful, consistent measuring.

Precision A precise measurement is one in which the repeated readings are grouped closely together - there is very little spread around the mean value. Precision is achieved by careful, consistent measuring.

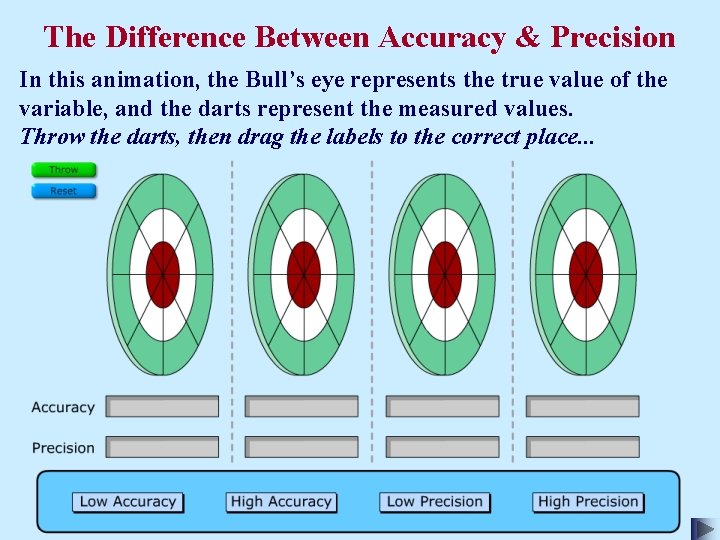
The Difference Between Accuracy & Precision

The Difference Between Accuracy & Precision Measurement can be precise but not necessarily accurate. . . § If three measurements of the same length are taken very carefully from the end of a ruler rather than from the zero mark, the measurements will be precise (there will be very little spread from the mean). However, as all the measurements were taken using an incorrect method, they will be inaccurate. Measurement can be accurate but not necessarily precise. . . § If three measurements of the same length are taken carelessly (but correctly using the zero mark of the ruler), the measurements will vary a lot, so they will not be precise. The mean of these measurements may be close to the true length (by chance) – they are therefore accurate. You should always try to achieve accuracy and precision measurements which vary little and are close to the true value.

The Difference Between Accuracy & Precision In this animation, the Bull’s eye represents the true value of the variable, and the darts represent the measured values. Throw the darts, then drag the labels to the correct place. . .

Precision of Instruments A precise instrument will give very close readings for the same value of a variable, e. g. if a 1 kg mass is repeatedly weighed on a precise balance, it will give very close (if not exactly the same) readings each time. Precision in instruments is about the quality and reliability of the instrument. Using a more precise instrument will lead to more precise measurements, because precision is about reducing the range of results when measuring the same value of a variable.

Sensitivity of Instruments Sensitivity is the smallest change that an instrument can measure. A set of scales calibrated in milligrams is more sensitive than a set of scales calibrated in grams. A more sensitive instrument that is calibrated with smaller divisions along its scale, allows the user to be more precise. . .

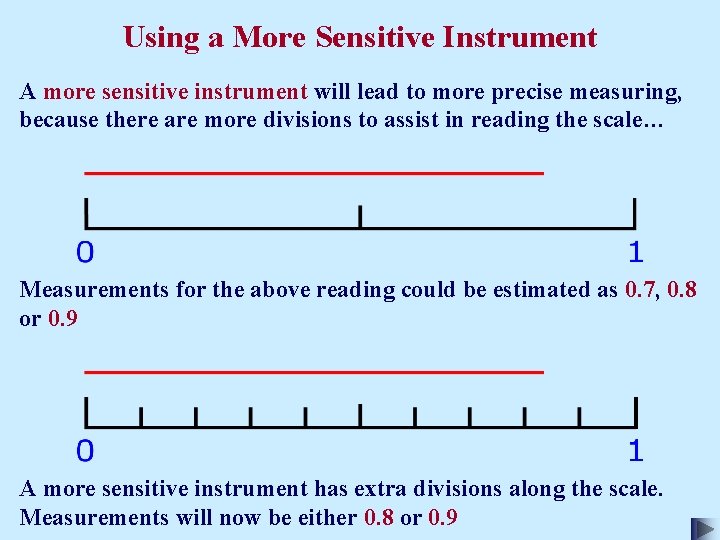
Using a More Sensitive Instrument A more sensitive instrument will lead to more precise measuring, because there are more divisions to assist in reading the scale… Measurements for the above reading could be estimated as 0. 7, 0. 8 or 0. 9 A more sensitive instrument has extra divisions along the scale. Measurements will now be either 0. 8 or 0. 9

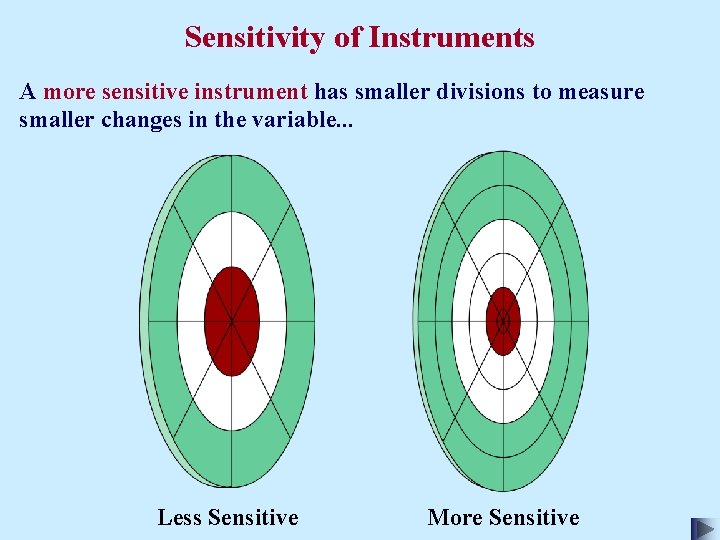
Sensitivity of Instruments A more sensitive instrument has smaller divisions to measure smaller changes in the variable. . . Less Sensitive More Sensitive

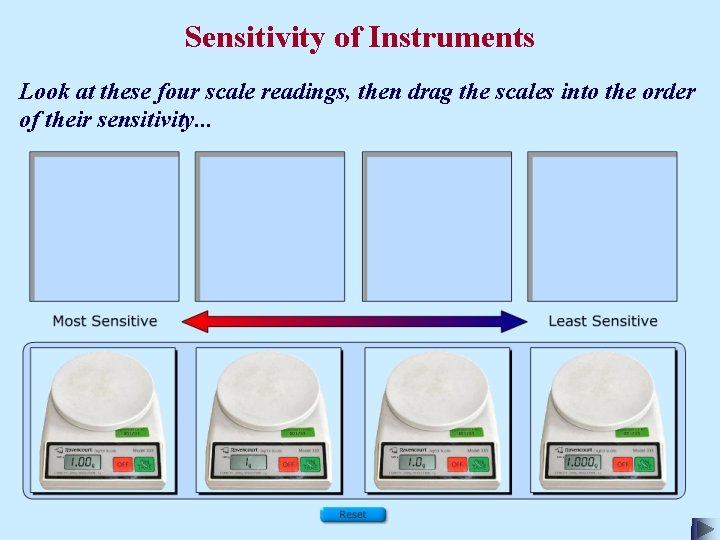
Sensitivity of Instruments Look at these four scale readings, then drag the scales into the order of their sensitivity. . .

Reliable Measuring The smallest unit to which you can reliably measure, will be the smallest division on the apparatus you select. Example: If you want to measure to the nearest tenth of a degree, your thermometer must be calibrated in tenths of degrees, like this one.

Litmus Paper If in your experiment you only need to identify a substance as acid or alkali, litmus paper is sensitive enough. Drag the paper into the beaker to see the colour change. . .

Litmus Paper If in your experiment you only need to identify a substance as acid or alkali, litmus paper is sensitive enough. Drag the paper into the beaker to see the colour change. . .

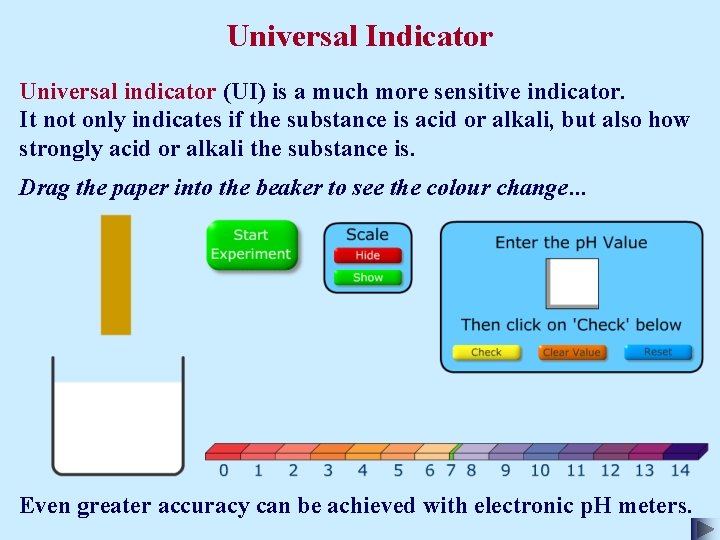
Universal Indicator Universal indicator (UI) is a much more sensitive indicator. It not only indicates if the substance is acid or alkali, but also how strongly acid or alkali the substance is. Drag the paper into the beaker to see the colour change. . .

Universal Indicator Universal indicator (UI) is a much more sensitive indicator. It not only indicates if the substance is acid or alkali, but also how strongly acid or alkali the substance is. Drag the paper into the beaker to see the colour change. . . Even greater accuracy can be achieved with electronic p. H meters.

End of Show Copyright © 2006 SSER Ltd. and its licensors. Images are for viewing purposes only. All rights reserved.