Accuracy and Precision Accuracy and Precision You will


- Slides: 11

Accuracy and Precision

Accuracy and Precision You will be conducting an experiment to determine the area of your desk as precisely and accurately as possible. You will be given an ‘instrument’ to determine the length and the width of your desk. You will then calculate the area. Set up a table of values so that you can do several trials We will compile class data, and determine which measurement is best… what is the difference between being accurate and being precise?

Accuracy and Precision The terms “accuracy” and “precision” mean the same thing to most people, but to a scientist, their meanings are quite distinct. Accuracy refers to how close a measurement is to an accepted value. Precision refers to how close a measurement is to another measurement. Scientists often repeat their experiments to reduce uncertainty.

Accuracy and Precision Pretend you have a gold bar with a known mass of 100. 0 g. You weigh it three times. Your results are in the table. (Note: The weigh scale is certain to the ones place…thus the decimal number is uncertain. ) Trial Mass (g) 1 100. 0 2 100. 0 3 100. 0 Your results are accurate because they are exactly the expected value Your results are precise because they are all the same

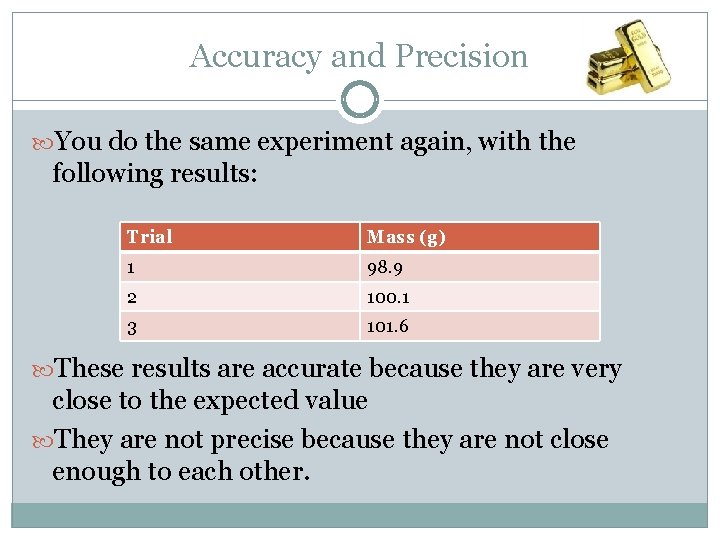
Accuracy and Precision You do the same experiment again, with the following results: Trial Mass (g) 1 98. 9 2 100. 1 3 101. 6 These results are accurate because they are very close to the expected value They are not precise because they are not close enough to each other.

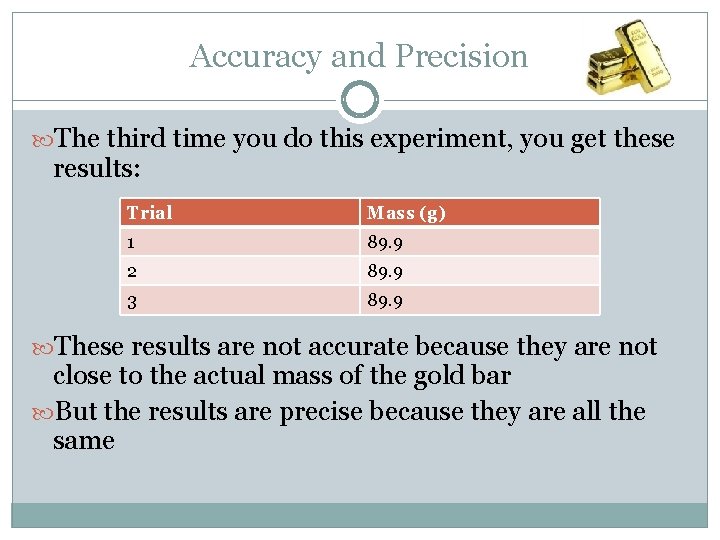
Accuracy and Precision The third time you do this experiment, you get these results: Trial Mass (g) 1 89. 9 2 89. 9 3 89. 9 These results are not accurate because they are not close to the actual mass of the gold bar But the results are precise because they are all the same

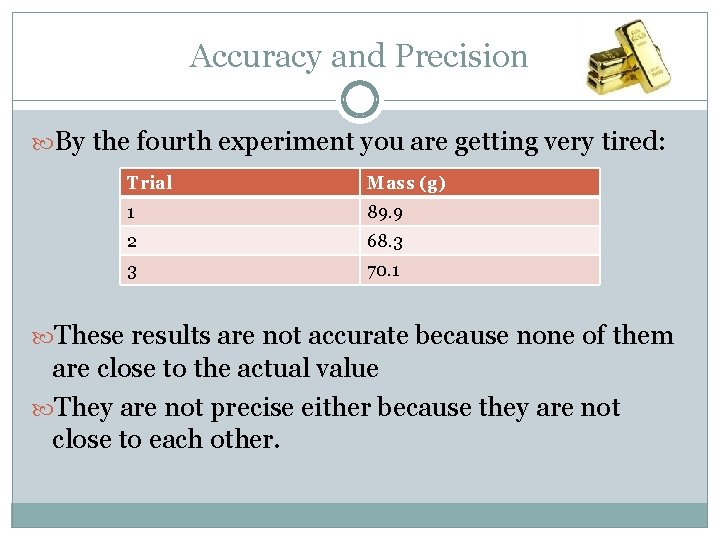
Accuracy and Precision By the fourth experiment you are getting very tired: Trial Mass (g) 1 89. 9 2 68. 3 3 70. 1 These results are not accurate because none of them are close to the actual value They are not precise either because they are not close to each other.

Accuracy and Precision Depends upon the measuring tool, but generally measurements are accurate and/or precise if they are a ½ unit on either side of the unit of measure. Accuracy…. how close the repeated measurements are to the ACTUAL value Precision…how close the repeated measurements are to EACH OTHER http: //www. mathsisfun. com/accuracy-precision. html

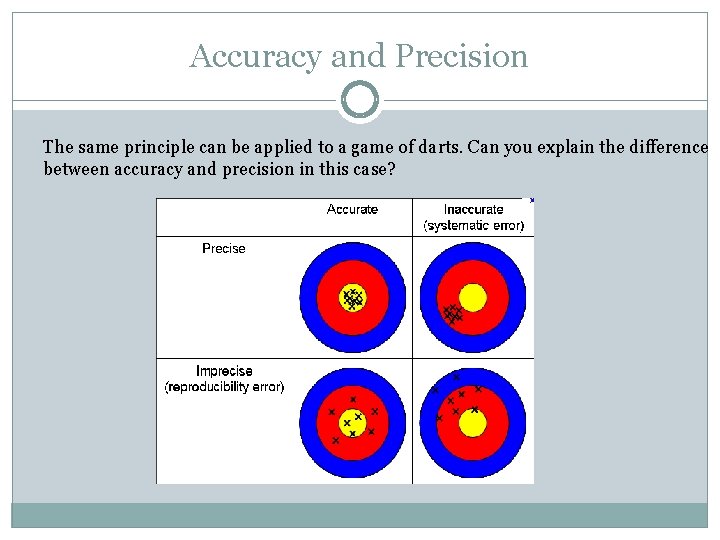
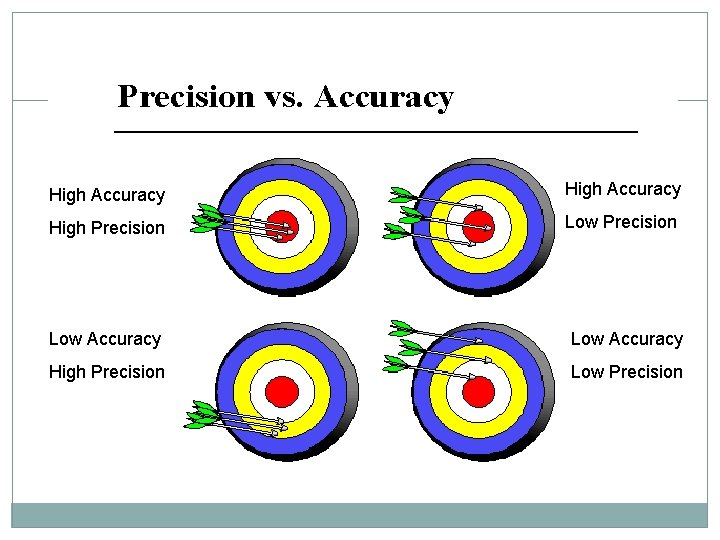
Accuracy and Precision The same principle can be applied to a game of darts. Can you explain the difference between accuracy and precision in this case?

High Accuracy High Precision Low Accuracy High Precision Low Precision

Thinking back to your experiment… In an experiment, what are some possible causes for not obtaining accurate results? What are some possible causes for not obtaining precise results? Why is it important to think about accuracy and precision?