Chemistry ACCURACY PRECISION SIGNIFICANT DIGITS Accuracy how closely




- Slides: 15

Chemistry ACCURACY, PRECISION, & SIGNIFICANT DIGITS

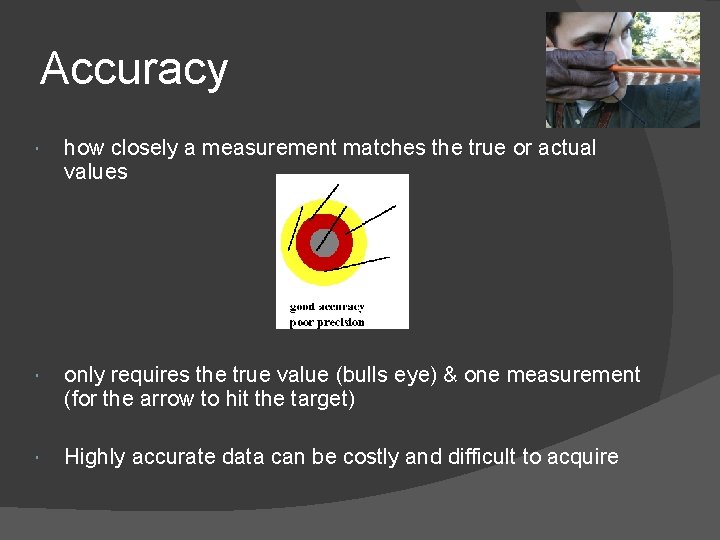
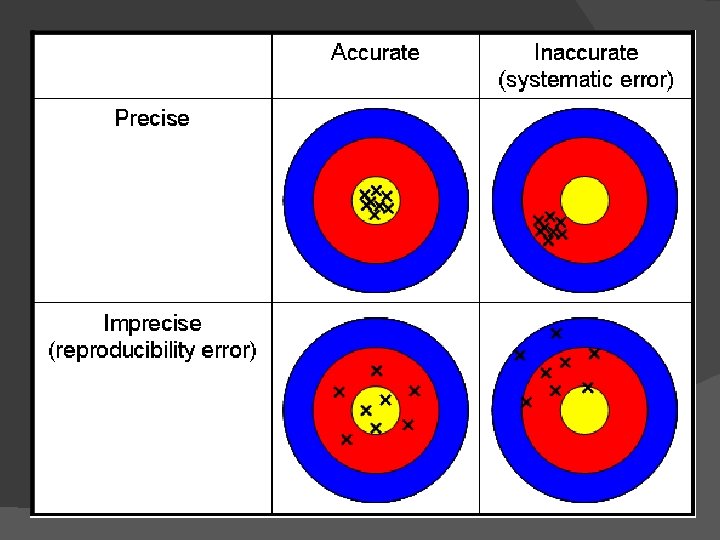
Accuracy how closely a measurement matches the true or actual values only requires the true value (bulls eye) & one measurement (for the arrow to hit the target) Highly accurate data can be costly and difficult to acquire

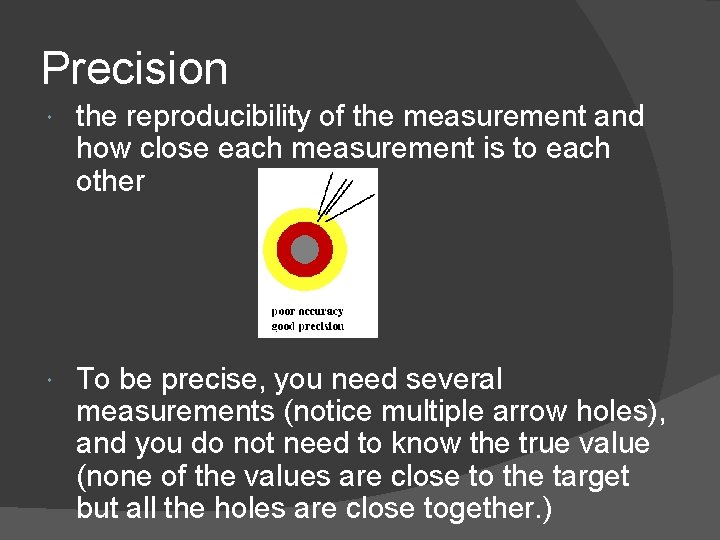
Precision the reproducibility of the measurement and how close each measurement is to each other To be precise, you need several measurements (notice multiple arrow holes), and you do not need to know the true value (none of the values are close to the target but all the holes are close together. )

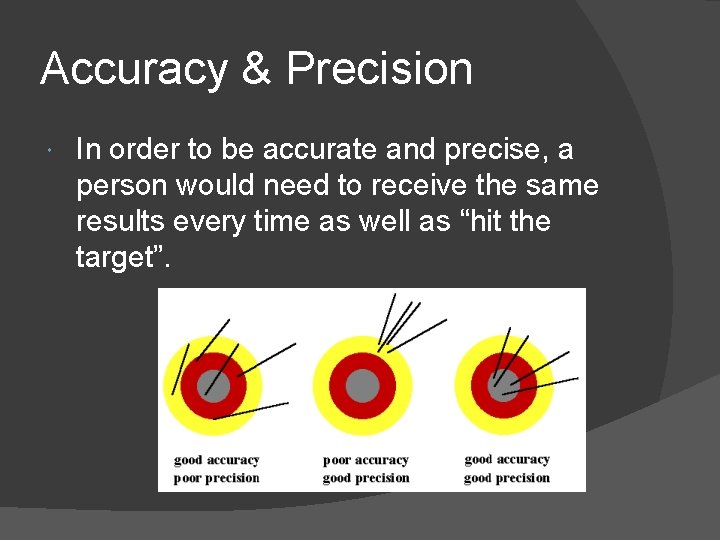
Accuracy & Precision In order to be accurate and precise, a person would need to receive the same results every time as well as “hit the target”.

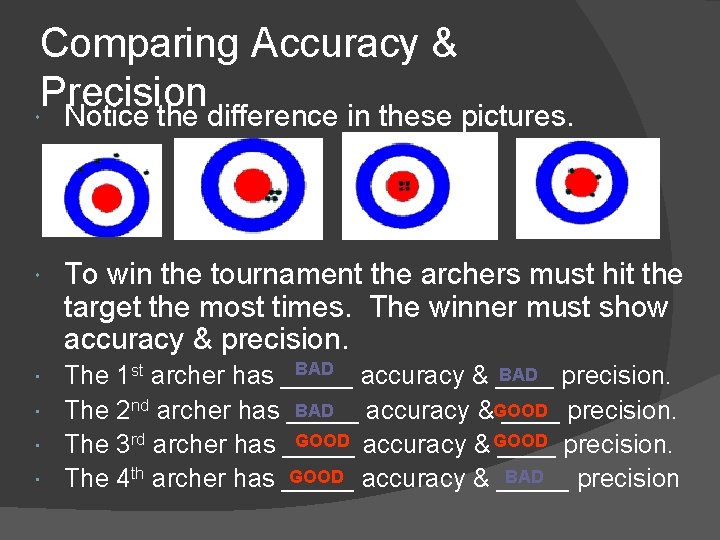
Comparing Accuracy & Precision Notice the difference in these pictures. To win the tournament the archers must hit the target the most times. The winner must show accuracy & precision. BAD precision. The 1 st archer has _____ accuracy & ____ BAD The 2 nd archer has _____ accuracy &GOOD ____ precision. GOOD accuracy & GOOD The 3 rd archer has _____ precision. GOOD accuracy & _____ BAD The 4 th archer has _____ precision

Example 1 A sample is known to weigh 3. 182 g. Jane weighed the sample five different times with the resulting data. Which measurement was the most accurate? 3. 200 g 3. 180 g 3. 152 g 3. 189 g

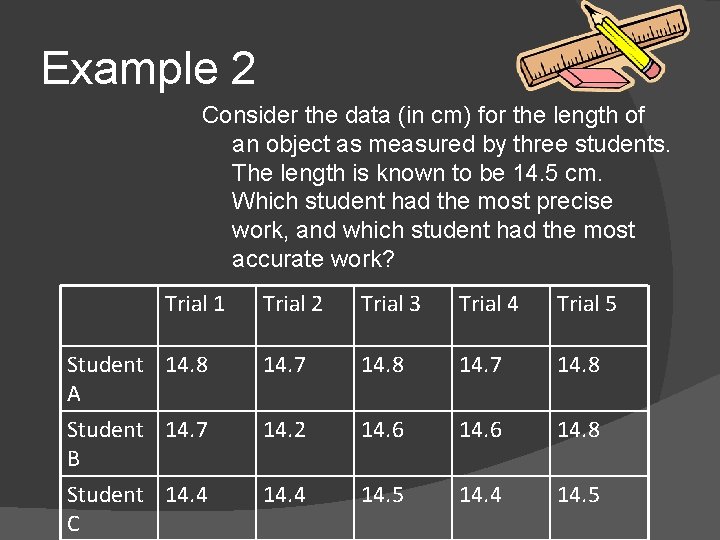
Example 2 Consider the data (in cm) for the length of an object as measured by three students. The length is known to be 14. 5 cm. Which student had the most precise work, and which student had the most accurate work? Trial 1 Student 14. 8 A Student 14. 7 B Student 14. 4 C Trial 2 Trial 3 Trial 4 Trial 5 14. 7 14. 8 14. 2 14. 6 14. 8 14. 4 14. 5

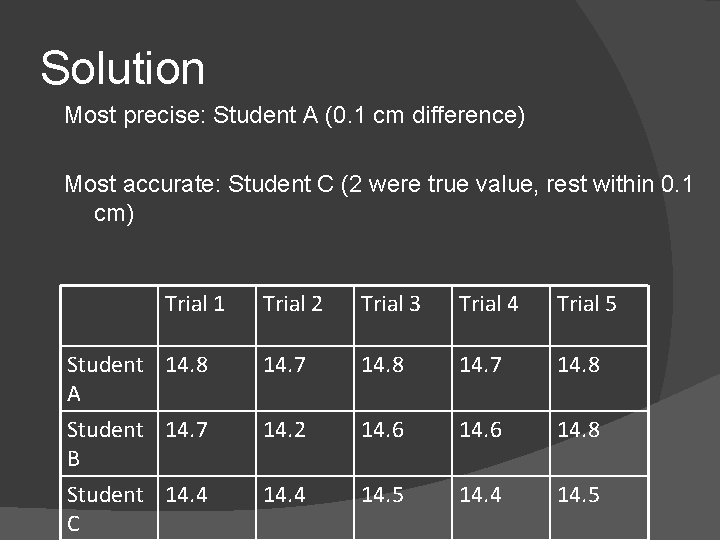
Solution Most precise: Student A (0. 1 cm difference) Most accurate: Student C (2 were true value, rest within 0. 1 cm) Trial 1 Student 14. 8 A Student 14. 7 B Student 14. 4 C Trial 2 Trial 3 Trial 4 Trial 5 14. 7 14. 8 14. 2 14. 6 14. 8 14. 4 14. 5


Significant Figures Why are significant figures necessary? True accuracy is no better than the measurement obtained by the least precise method. We use significant digits so we are not exaggerating our precision.

Why are S. F. s Important? When reporting a measurement the number of digits indicates the precision of an instrument. 100 ml Estimated to the tens place 99. 9 m. L Estimated to the tenths place

Example 1: How would you record this measurement?

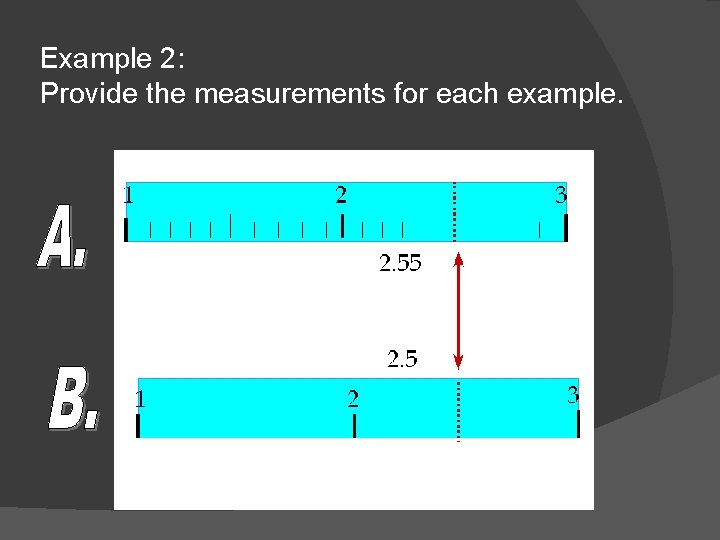
Example 2: Provide the measurements for each example.

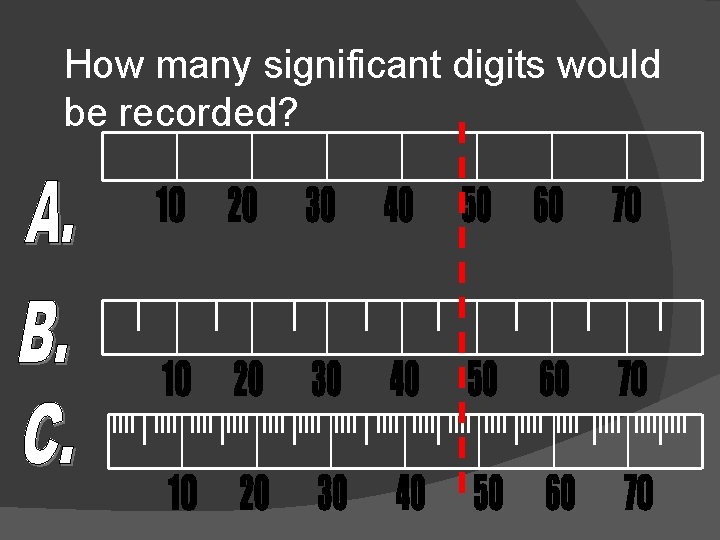
How many significant digits would be recorded?

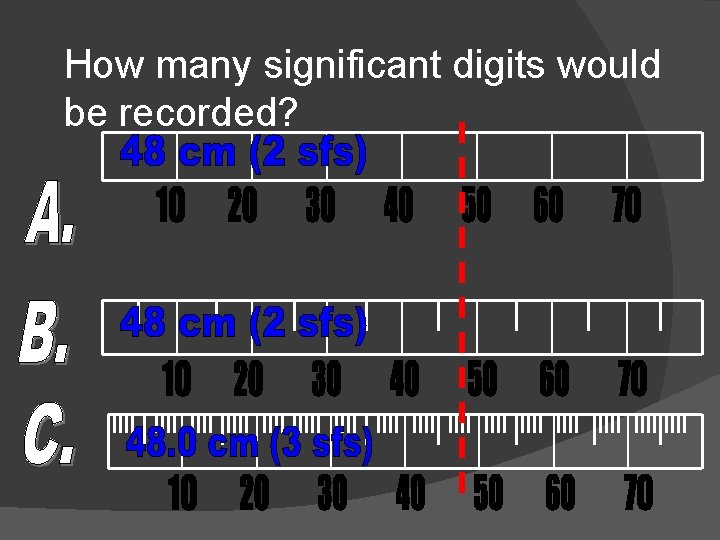
How many significant digits would be recorded?