Chemical Stoichiometry The study of quantities of materials









![Formulas molecular formula = (empirical formula)n [n = integer] molecular formula = C 6 Formulas molecular formula = (empirical formula)n [n = integer] molecular formula = C 6](https://slidetodoc.com/presentation_image_h/bd8e5200f42e8ba13c15208bb7ffa8a3/image-16.jpg)










- Slides: 32

Chemical Stoichiometry - The study of quantities of materials consumed and produced in chemical reactions. Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 1

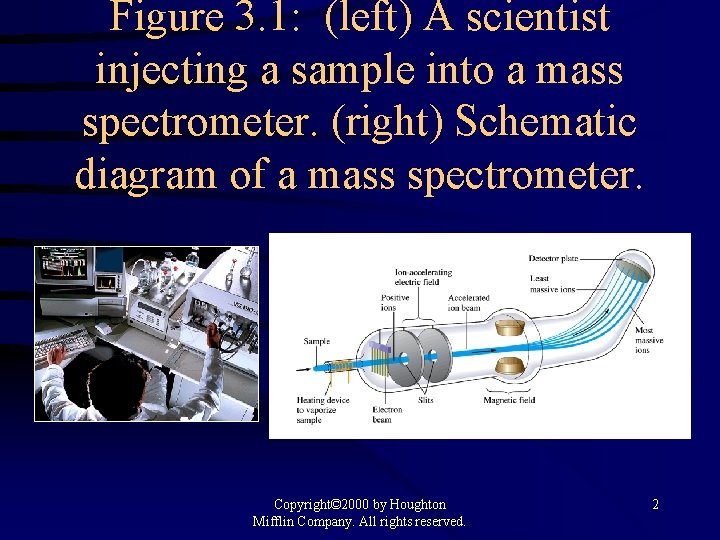
Figure 3. 1: (left) A scientist injecting a sample into a mass spectrometer. (right) Schematic diagram of a mass spectrometer. Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 2

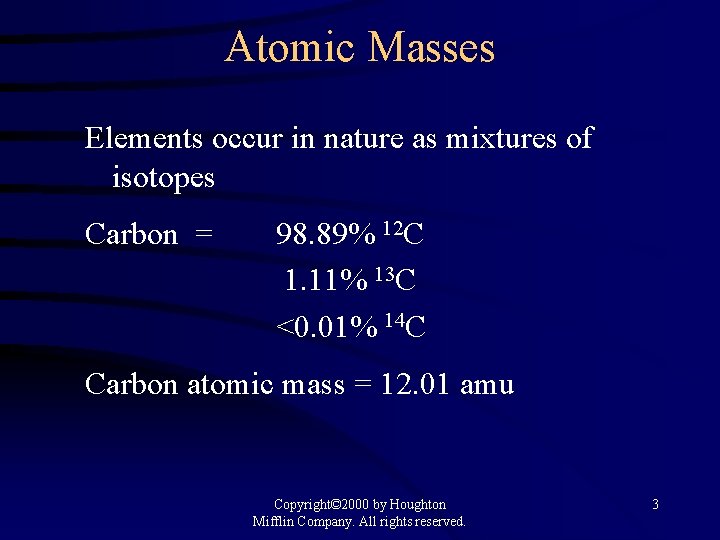
Atomic Masses Elements occur in nature as mixtures of isotopes Carbon = 98. 89% 12 C 1. 11% 13 C <0. 01% 14 C Carbon atomic mass = 12. 01 amu Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 3

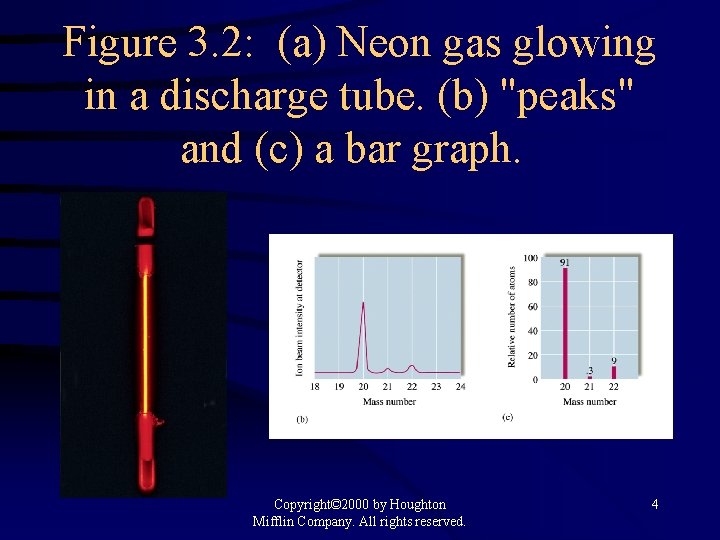
Figure 3. 2: (a) Neon gas glowing in a discharge tube. (b) "peaks" and (c) a bar graph. Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 4

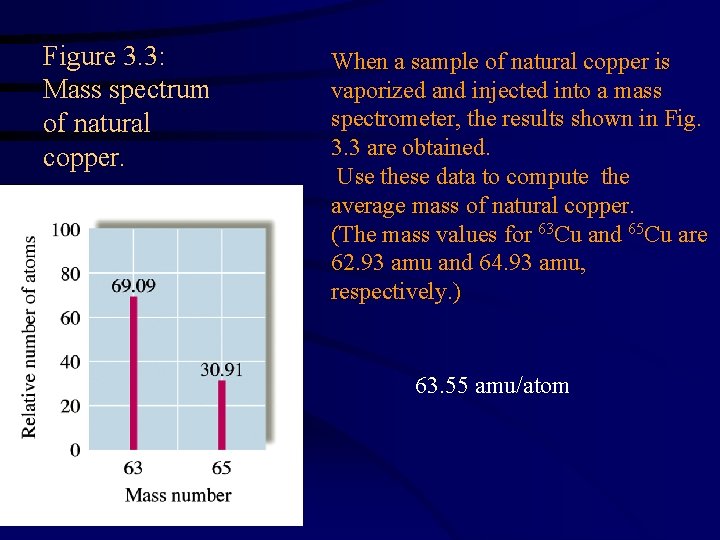
Figure 3. 3: Mass spectrum of natural copper. When a sample of natural copper is vaporized and injected into a mass spectrometer, the results shown in Fig. 3. 3 are obtained. Use these data to compute the average mass of natural copper. (The mass values for 63 Cu and 65 Cu are 62. 93 amu and 64. 93 amu, respectively. ) 63. 55 amu/atom

The Mole The number equal to the number of carbon atoms in exactly 12 grams of pure 12 C. 1 mole of anything = 6. 022 1023 units of that thing Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 6

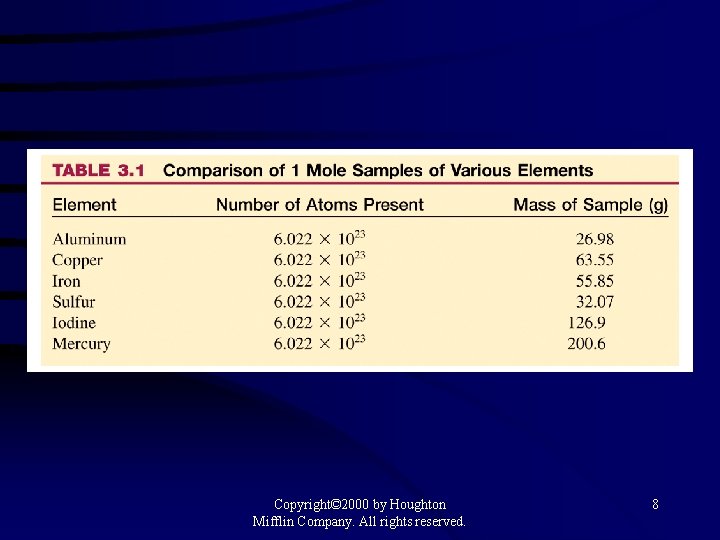
Figure 3. 4: Proceeding clockwise from the top samples containing one mole each of copper, aluminum, iron, sulfur, iodine, and (in the center) mercury. Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 7

Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 8

Avogadro’s number equals 23 6. 022 10 units Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 9

1 t eac R Now Try This… Calculate the number of copper atoms in a 63. 55 g sample of copper. Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 10

Molar Mass A substance’s molar mass (molecular weight) is the mass in grams of one mole of the compound. CO 2 = 44. 01 grams per mole Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 11

QUESTION The compound Cr 2 O 3 (chromium (III) oxide) is one of the key components responsible for the red color of ruby gems. If you had 34. 8 grams of Cr 2 O 3, how many grams of Chromium (atomic number = 24) metal would be present? 1. 2. 3. 4. 11. 9 grams of Cr 5. 85 grams of Cr 23. 8 grams of Cr 69. 8 grams of Cr Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 12

ANSWER Choice 3 correctly predicts the grams of Cr found in 34. 8 grams of Cr 2 O 3. 34. 8 grams Cr 2 O 3 1 mol/152 g = 0. 229 moles of Cr 2 O 3 0. 229 Moles Cr 2 O 3 2 mol Cr/1 mol Cr 2 O 3 = 0. 458 moles of Cr 0. 458 moles Cr 52. 0 g/1 mol Cr = 23. 8 grams OR, 2 (52. 0)/152 100 = 68. 4 % Cr 0. 684 34. 8 = 23. 8 g Cr Section 3. 3: Molar Mass Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 13

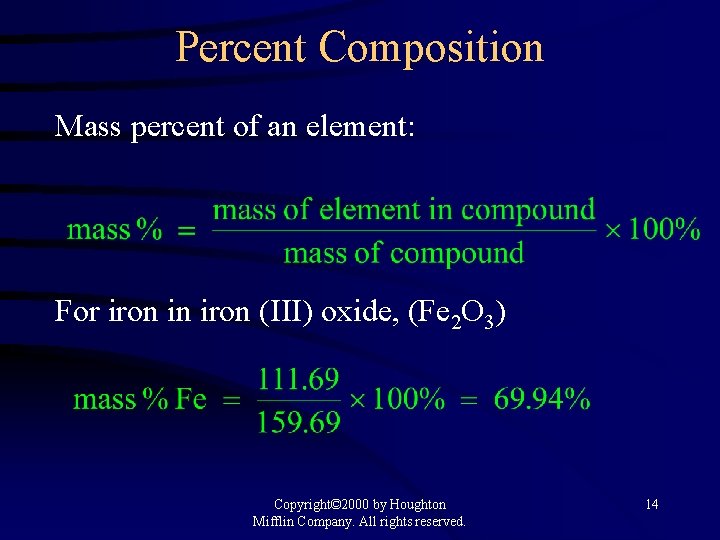
Percent Composition Mass percent of an element: For iron in iron (III) oxide, (Fe 2 O 3) Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 14

5 t eac R Consider separate 100. 0 -gram samples of each of the following: H 2 O N 2 O C 3 H 6 O 2 CO 2 Rank them from highest to lowest percent oxygen by mass. 15
![Formulas molecular formula empirical formulan n integer molecular formula C 6 Formulas molecular formula = (empirical formula)n [n = integer] molecular formula = C 6](https://slidetodoc.com/presentation_image_h/bd8e5200f42e8ba13c15208bb7ffa8a3/image-16.jpg)
Formulas molecular formula = (empirical formula)n [n = integer] molecular formula = C 6 H 6 = (CH)6 empirical formula = CH Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 16

QUESTION Without further information, which of the following is more likely to be both a molecular formula and an empirical formula for a compound? Briefly explain why. H 2 O 2; C 6 H 6; C 2 H 6 O 1. H 2 O 2. The whole numbers for each atom are reduced as far as possible and still retain the identity of the molecule. 2. C 6 H 6. The whole number of atoms are equal for each element. 3. C 2 H 6 O. One element shows only one atom so the formula ratio could not be further reduced, but the molecular formula could just have one atom. 4. None of these, it is not possible for a formula to be both empirical and molecular.

ANSWER Choice 3 shows the correct choice and proper justification. The whole numbers are reduced to their lowest value so C 2 H 6 O could be the empirical formula. The other choices could still be reduced to smaller whole numbers and maintain the same ratio. Section 3. 6: Determining the Formula of a Compound

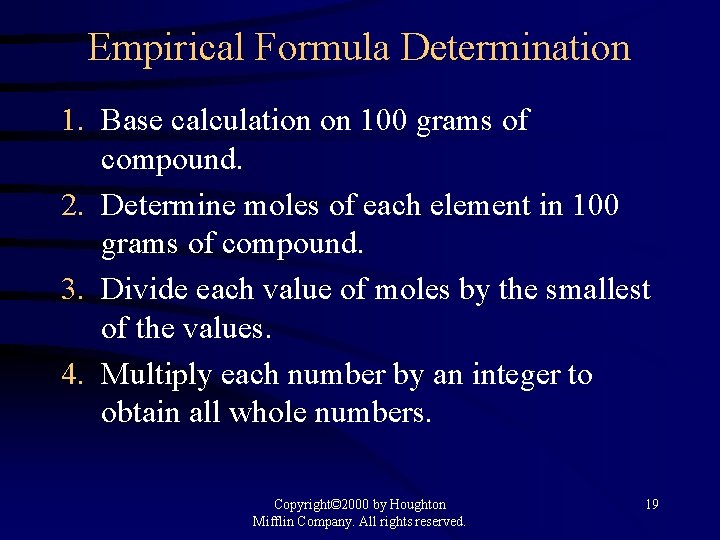
Empirical Formula Determination 1. Base calculation on 100 grams of compound. 2. Determine moles of each element in 100 grams of compound. 3. Divide each value of moles by the smallest of the values. 4. Multiply each number by an integer to obtain all whole numbers. Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 19

QUESTION The dye indigo is a compound with tremendous economic importance (blue jeans wouldn’t be blue without it. ) Indigo’s percent composition is: 73. 27% C; 3. 84% H; 10. 68%N and 12. 21% O. What is the empirical formula of indigo? 1. 2. 3. 4. C 6 H 4 NO C 8 H 3 NO C 8 H 5 NO I know this should be whole numbers for each atom, but I do not know how to accomplish that. Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 20

ANSWER Choice 3 is the smallest whole number ratio of the atoms that make up a molecule of indigo. The percentage must be converted to a mass, then the mass is converted to moles of the atoms and finally, the smallest is divided into the others to obtain the proper ratio. Section 3. 5: Determining the Formula of a Compound Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 21

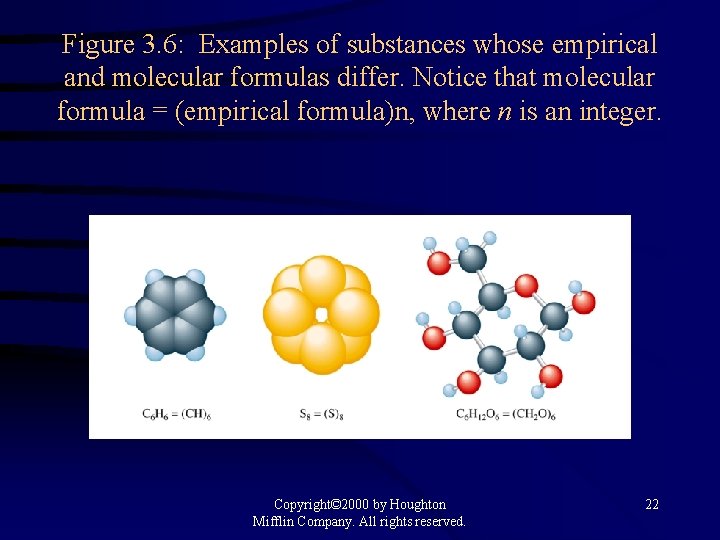
Figure 3. 6: Examples of substances whose empirical and molecular formulas differ. Notice that molecular formula = (empirical formula)n, where n is an integer. Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 22

Solve the following: A compound contains only carbon, hydrogen, and oxygen. Combustion of 10. 68 mg of the compound yield 16. 01 mg CO 2 and 4. 37 mg H 2 O. The molar mass of the compound is 176. 1 g/mol. What are the empirical and molecular formulas of the compound? C 3 H 4 O 3 C 6 H 8 O 6 23

Chemical Equations Chemical change involves a reorganization of the atoms in one or more substances. Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 24

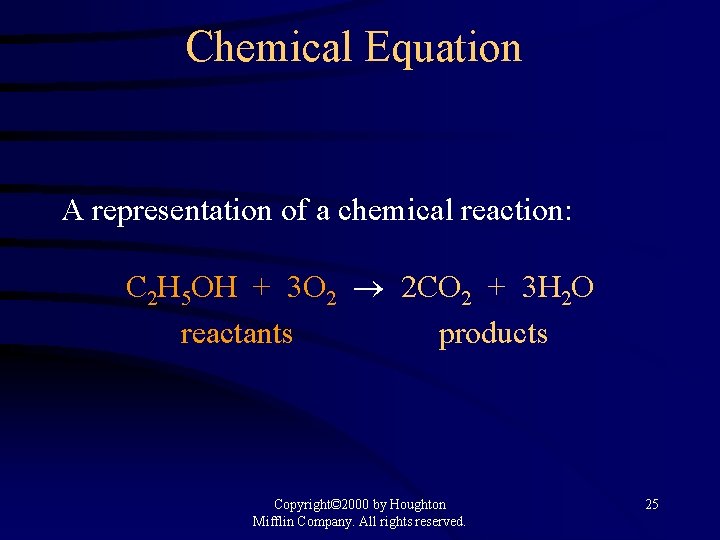
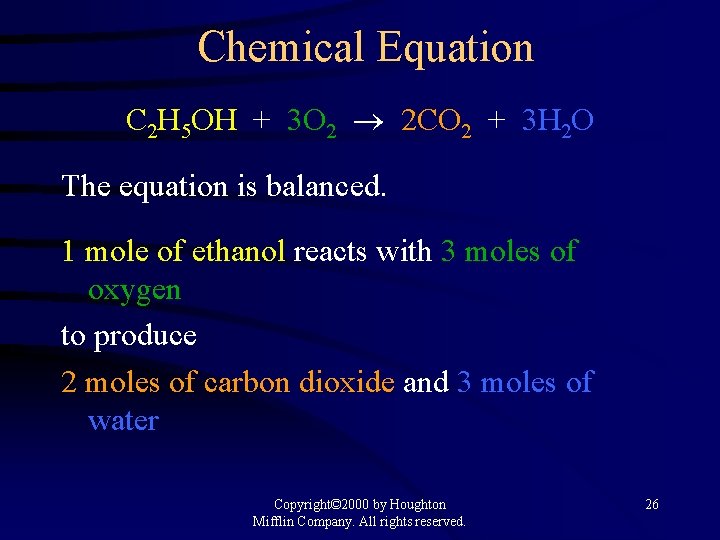
Chemical Equation A representation of a chemical reaction: C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O reactants products Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 25

Chemical Equation C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O The equation is balanced. 1 mole of ethanol reacts with 3 moles of oxygen to produce 2 moles of carbon dioxide and 3 moles of water Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 26

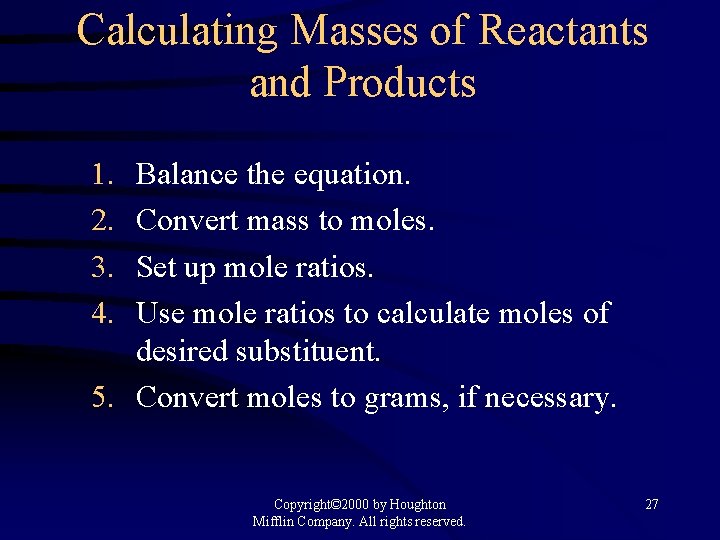
Calculating Masses of Reactants and Products 1. 2. 3. 4. Balance the equation. Convert mass to moles. Set up mole ratios. Use mole ratios to calculate moles of desired substituent. 5. Convert moles to grams, if necessary. Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 27

Limiting Reactant The limiting reactant is the reactant that is consumed first, limiting the amounts of products formed. Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 28

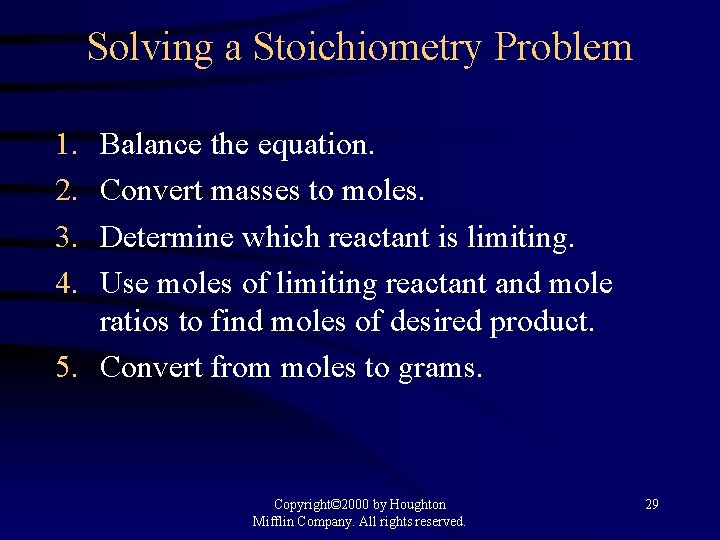
Solving a Stoichiometry Problem 1. 2. 3. 4. Balance the equation. Convert masses to moles. Determine which reactant is limiting. Use moles of limiting reactant and mole ratios to find moles of desired product. 5. Convert from moles to grams. Copyright© 2000 by Houghton Mifflin Company. All rights reserved. 29

Solve DDT, an insecticide harmful to fish, birds, and humans, is produced by the following reaction: 2 C 6 H 5 Cl + C 2 HOCl 3 C 14 H 9 Cl 5 + H 2 O In a government lab, 1142 g of chlorobenzene is reacted with 485 g of chloral. a. What mass of DDT is formed? 1170 g b. What is the mass of excess reactant left over? 401 g c. If the actual yield of DDT is 200. 0 g, what is the percent yield? 17. 1% 30

QUESTION Tungsten metal is used to make many light bulb filaments. 18. 0 grams of tungsten was made in the following reaction from 25. 0 grams of WO 3. What percent yield does this represent? WO 3 + 3 H 2 W + 3 H 2 O 1. 2. 3. 4. 25. 8% 110% 90. 8% I think I may have confused one of the steps in this calculation.

ANSWER Choice 3 has properly converted the initial grams of WO 3 to moles and used the mole ratio of the equation to determine the maximum moles of W that would be produced if all the WO 3 reacted (theoretical yield – 19. 8 g). The division of the actual yield by theoretical yield 100 produces the answer. Section 3. 10: Calculations Involving a Limiting Reactant
 Angular acceleration formula
Angular acceleration formula The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Label an atom
Label an atom Chemistry chapter 10 chemical quantities
Chemistry chapter 10 chemical quantities Chapter 7 chemical quantities answer key
Chapter 7 chemical quantities answer key The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Chemical quantities calculator
Chemical quantities calculator Calculate it
Calculate it Chapter 10 chemical quantities practice problems answer key
Chapter 10 chemical quantities practice problems answer key Unit chemical quantities the mole 1 step
Unit chemical quantities the mole 1 step Chapter 9 chemical quantities
Chapter 9 chemical quantities Chemical rxns/balancing equ./stoichiometry
Chemical rxns/balancing equ./stoichiometry Stoichiometry refers to
Stoichiometry refers to Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chemical accounting stoichiometry
Chemical accounting stoichiometry Chemical accounting: stoichiometry
Chemical accounting: stoichiometry Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Quantitative study of reactants and products
Quantitative study of reactants and products Stoichiometry is defined as the quantitative study of
Stoichiometry is defined as the quantitative study of Chapter 11 study guide chemistry stoichiometry answer key
Chapter 11 study guide chemistry stoichiometry answer key Natural materials
Natural materials A material is useful when
A material is useful when Natural materials and man made materials
Natural materials and man made materials Adapting and adopting materials
Adapting and adopting materials Direct materials budget with multiple materials
Direct materials budget with multiple materials Thermal properties of dental materials
Thermal properties of dental materials Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chapter 7 review chemical formulas and chemical compounds
Chapter 7 review chemical formulas and chemical compounds Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Love formula
Love formula Are kc and kp equal
Are kc and kp equal