Chemical Quantities Chapter 9 Chemical Stoichiometry The study

















- Slides: 31

Chemical Quantities Chapter 9

Chemical Stoichiometry - The study of quantities of materials consumed and produced in chemical reactions. Stoichiometry is used to determine how much stomach acid an antacid tablet can neutralize.

Chemical Equations A balanced chemical equation is like a recipe. One needs to know what the ingredients are and what relative amounts of ingredients are needed for both recipes and chemical equations.

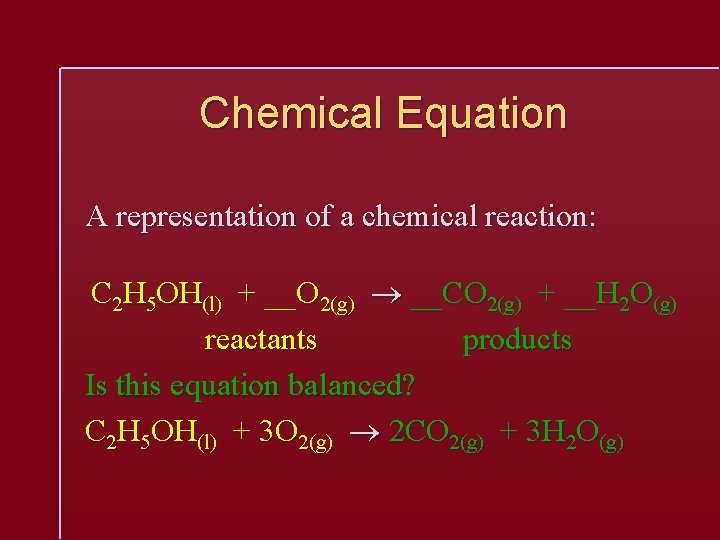
Chemical Equation A representation of a chemical reaction: C 2 H 5 OH(l) + __O 2(g) __CO 2(g) + __H 2 O(g) reactants products Is this equation balanced? C 2 H 5 OH(l) + 3 O 2(g) 2 CO 2(g) + 3 H 2 O(g)

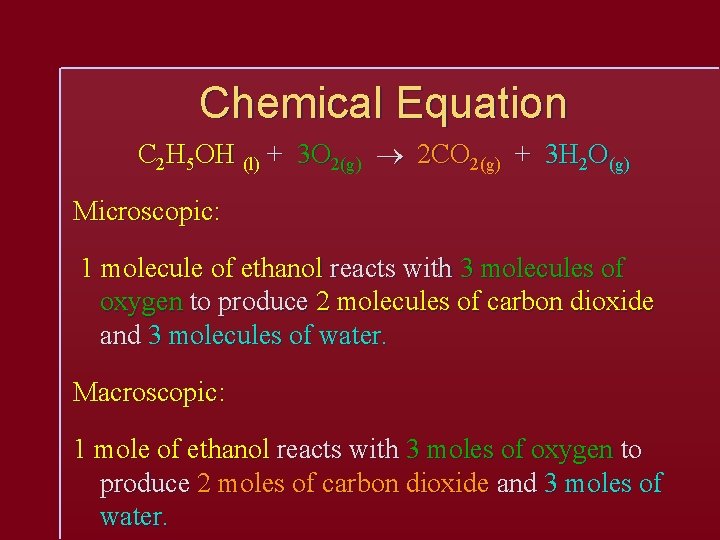
Chemical Equation C 2 H 5 OH (l) + 3 O 2(g) 2 CO 2(g) + 3 H 2 O(g) Microscopic: 1 molecule of ethanol reacts with 3 molecules of oxygen to produce 2 molecules of carbon dioxide and 3 molecules of water. Macroscopic: 1 mole of ethanol reacts with 3 moles of oxygen to produce 2 moles of carbon dioxide and 3 moles of water.

Chemical Equation C 2 H 5 OH (l) + 3 O 2(g) 2 CO 2(g) + 3 H 2 O(g) Microscopic: 1 dozen molecules of ethanol reacts with 3 dozen molecules of oxygen to produce 2 dozen molecules of carbon dioxide and 3 dozen molecules of water. Macroscopic: 6. 02 x 1023 molecules of ethanol reacts with 3(6. 02 x 1023) molecules of oxygen to produce 2(6. 02 x 1023) molecules of carbon dioxide and 3(6. 02 x 1023 ) molecules of water.

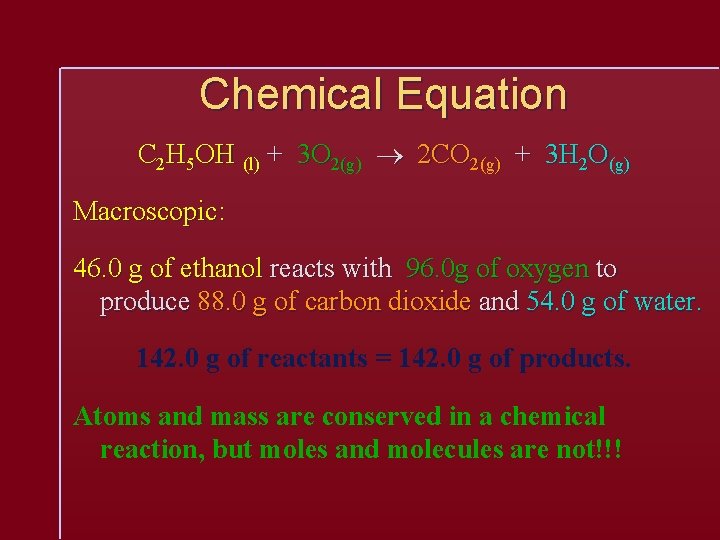
Chemical Equation C 2 H 5 OH (l) + 3 O 2(g) 2 CO 2(g) + 3 H 2 O(g) Macroscopic: 46. 0 g of ethanol reacts with 96. 0 g of oxygen to produce 88. 0 g of carbon dioxide and 54. 0 g of water. 142. 0 g of reactants = 142. 0 g of products. Atoms and mass are conserved in a chemical reaction, but moles and molecules are not!!!

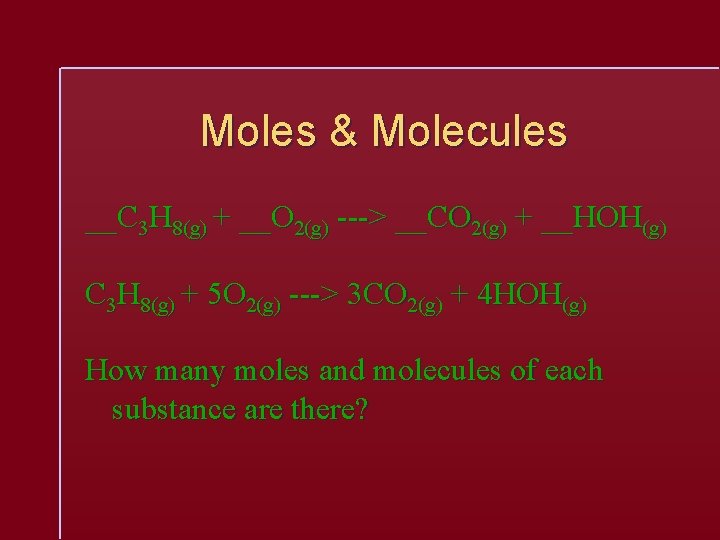
Moles & Molecules __C 3 H 8(g) + __O 2(g) ---> __CO 2(g) + __HOH(g) C 3 H 8(g) + 5 O 2(g) ---> 3 CO 2(g) + 4 HOH(g) How many moles and molecules of each substance are there?

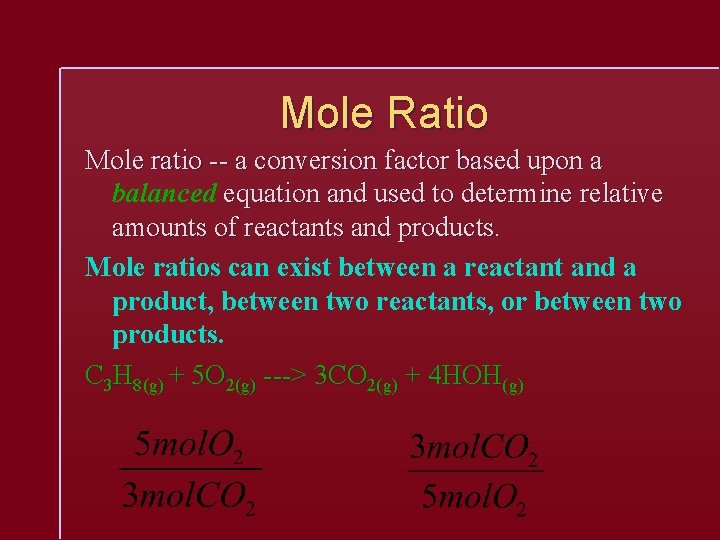
Mole Ratio Mole ratio -- a conversion factor based upon a balanced equation and used to determine relative amounts of reactants and products. Mole ratios can exist between a reactant and a product, between two reactants, or between two products. C 3 H 8(g) + 5 O 2(g) ---> 3 CO 2(g) + 4 HOH(g)

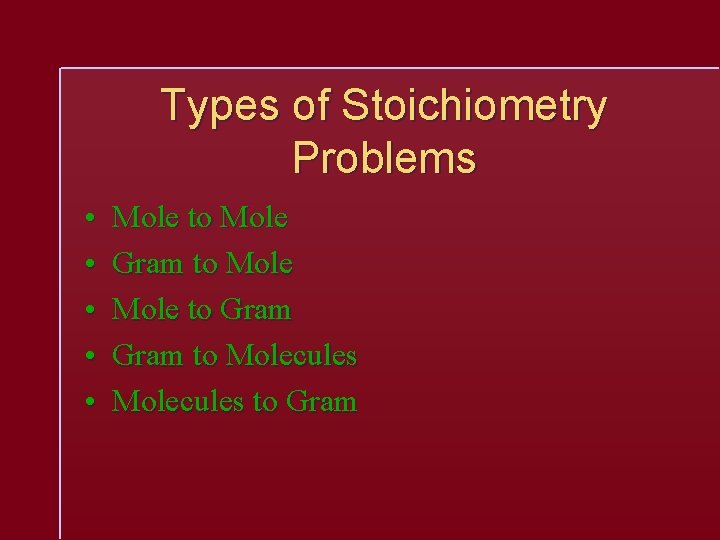
Types of Stoichiometry Problems • • • Mole to Mole Gram to Mole to Gram to Molecules to Gram

Calculating Masses of Reactants and Products 1. 2. 3. 4. Balance the equation. Convert mass to moles. Set up mole ratios. Use mole ratios to calculate moles of desired reactant or product. 5. Convert moles to grams, if necessary.

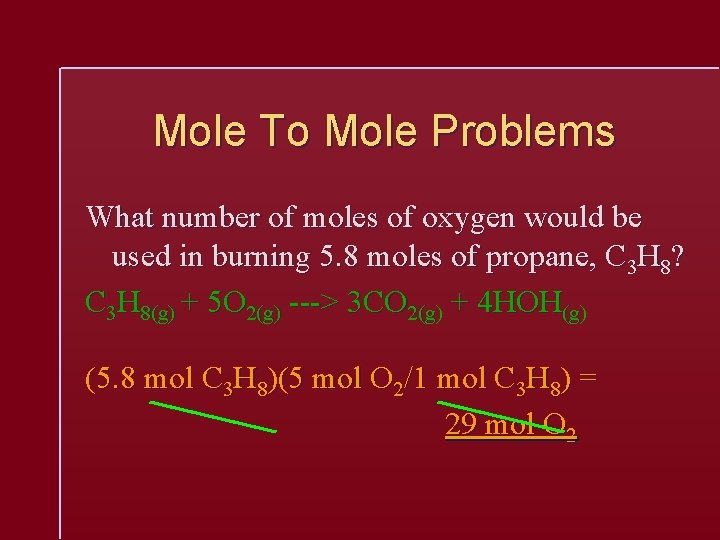
Mole To Mole Problems What number of moles of oxygen would be used in burning 5. 8 moles of propane, C 3 H 8? C 3 H 8(g) + 5 O 2(g) ---> 3 CO 2(g) + 4 HOH(g) (5. 8 mol C 3 H 8)(5 mol O 2/1 mol C 3 H 8) = 29 mol O 2

Gram to Mole & Gram to Gram __Al(s) + __I 2(s) ---> __Al. I 3(s) 2 Al(s) + 3 I 2(s) ---> 2 Al. I 3(s) How many moles and how many grams of aluminum iodide can be produce from 35. 0 g of aluminum?

Gram to Mole & Gram to Gram 2 Al(s) + 3 I 2(s) ---> 2 Al. I 3(s) (35. 0 g Al) (1 mol/26. 98 g)(2 mol Al. I 3/2 mol Al) = 1. 30 mol Al. I 3 (35. 0 g Al) (1 mol/26. 98 g)(2 mol Al. I 3/2 mol Al)(407. 68 g/1 mol) = 529 g Al. I 3

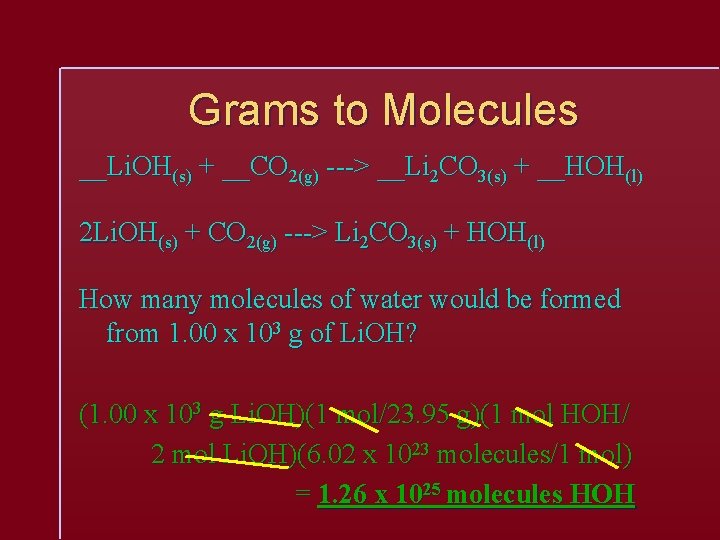
Grams to Molecules __Li. OH(s) + __CO 2(g) ---> __Li 2 CO 3(s) + __HOH(l) 2 Li. OH(s) + CO 2(g) ---> Li 2 CO 3(s) + HOH(l) How many molecules of water would be formed from 1. 00 x 103 g of Li. OH? (1. 00 x 103 g Li. OH)(1 mol/23. 95 g)(1 mol HOH/ 2 mol Li. OH)(6. 02 x 1023 molecules/1 mol) = 1. 26 x 1025 molecules HOH

Stoichiometric Quantities -- quantities of reactants mixed in exactly the amounts that result in their all being used up at the same time. How often do you think this occurs in reality? Almost never!!!!

Limiting Reactant The limiting reactant is the reactant that is consumed first, limiting the amounts of products formed. Almost all stoichiometric situations are of the limiting reactant type. The reactants that are left over and unreacted are said to be in excess.

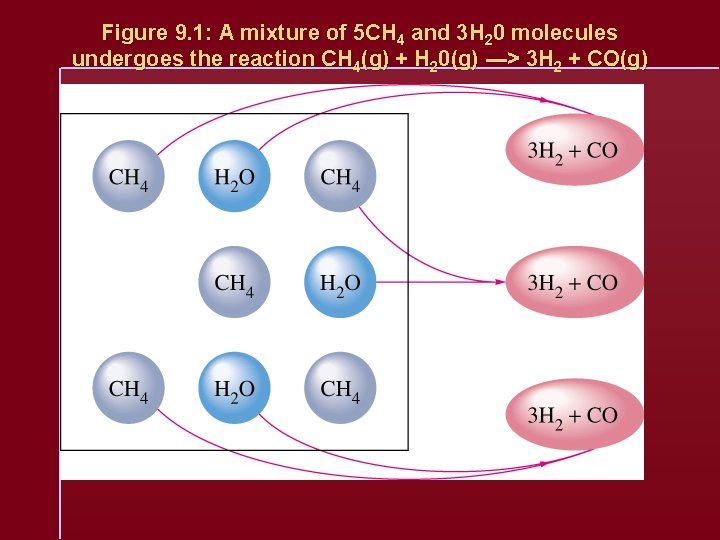
Figure 9. 1: A mixture of 5 CH 4 and 3 H 20 molecules undergoes the reaction CH 4(g) + H 20(g) ---> 3 H 2 + CO(g)

Double Cheeseburger Problem At the local “Burger Barn” a worker finds the following inventory: 22 hamburger patties 15 hamburger buns 7 slices of onion 18 slices of cheese How many double cheeseburgers with onion and cheese can be made to sell?

Double Cheeseburger Problem 2 h. b. patties + 1 h. b. bun + 2 slices cheese + 1 slice onion ---> 1 double cheeseburger What is the limiting reactant? Onion How many double cheeseburgers with onion and cheese can be made to sell? 7 double cheeseburgers

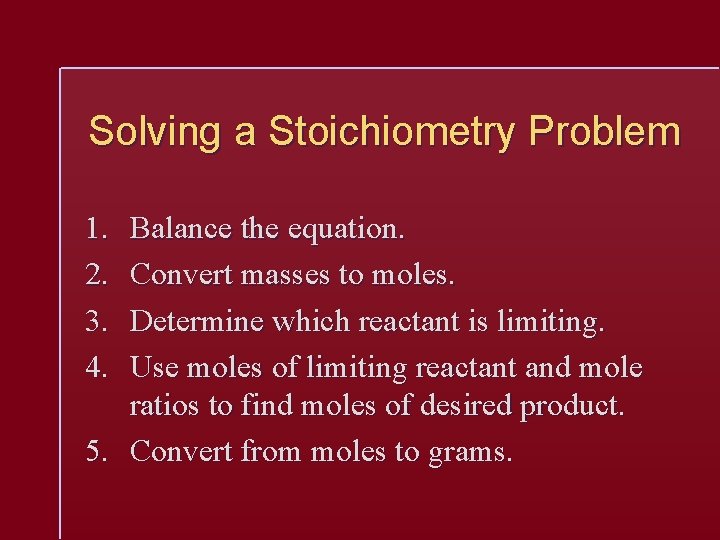
Solving a Stoichiometry Problem 1. 2. 3. 4. Balance the equation. Convert masses to moles. Determine which reactant is limiting. Use moles of limiting reactant and mole ratios to find moles of desired product. 5. Convert from moles to grams.

Double Cheeseburger Problem 2 h. b. patties + 1 h. b. bun + 2 slices cheese + 1 slice onion ---> 1 double cheeseburger What materials are in excess and by how much? 8 hamburger patties 8 hamburger buns 4 slices cheese

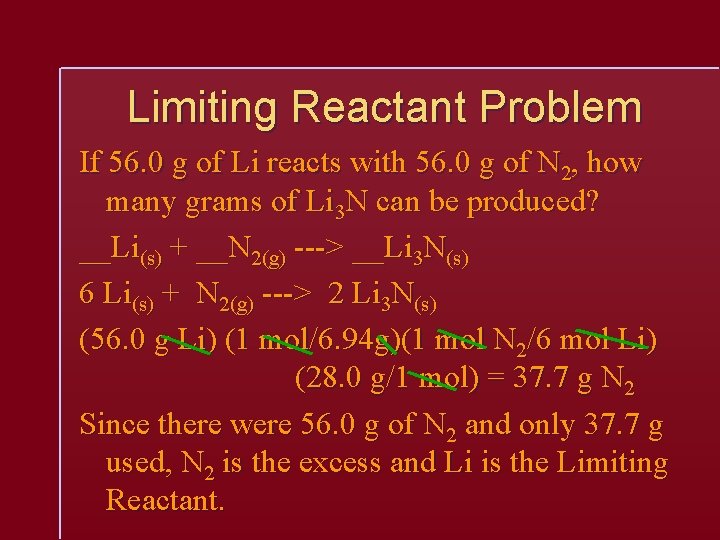
Limiting Reactant Problem If 56. 0 g of Li reacts with 56. 0 g of N 2, how many grams of Li 3 N can be produced? __Li(s) + __N 2(g) ---> __Li 3 N(s) 6 Li(s) + N 2(g) ---> 2 Li 3 N(s) (56. 0 g Li) (1 mol/6. 94 g)(1 mol N 2/6 mol Li) (28. 0 g/1 mol) = 37. 7 g N 2 Since there were 56. 0 g of N 2 and only 37. 7 g used, N 2 is the excess and Li is the Limiting Reactant.

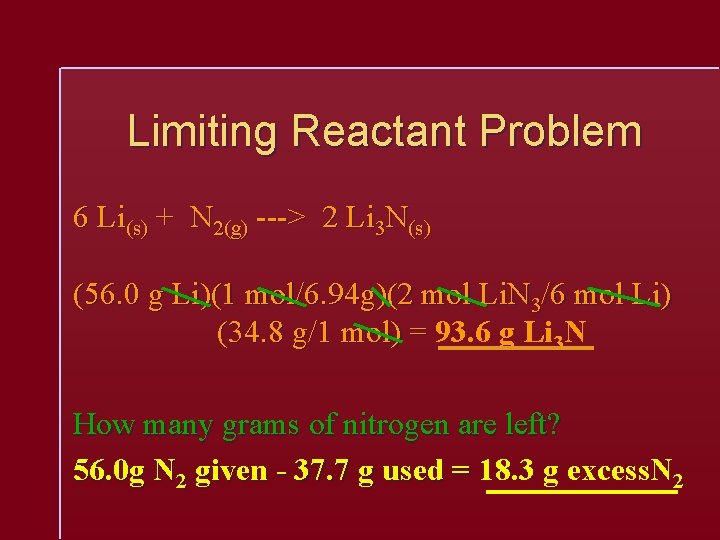
Limiting Reactant Problem 6 Li(s) + N 2(g) ---> 2 Li 3 N(s) (56. 0 g Li)(1 mol/6. 94 g)(2 mol Li. N 3/6 mol Li) (34. 8 g/1 mol) = 93. 6 g Li 3 N How many grams of nitrogen are left? 56. 0 g N 2 given - 37. 7 g used = 18. 3 g excess. N 2

Double Cheeseburger Yield At the local “Burger Barn” a worker finds the following inventory: 22 hamburger patties 15 hamburger buns 7 slices of onion 18 slices of cheese We found that seven double cheeseburgers could be made from these ingredients.

Double Cheeseburger Yield If a worker eats one slice of onion, how many double cheeseburgers can actually be made? 6 double cheeseburgers The number of cheeseburgers that could have been made (7) is theoretical yield. The number of cheeseburgers that actually were made (6) is the actual yield.

Double Cheeseburger Yield % Yield = 85. 7 %

% Yield Values calculated using stoichiometry are always theoretical yields! Values determined experimentally in the laboratory are actual yields!

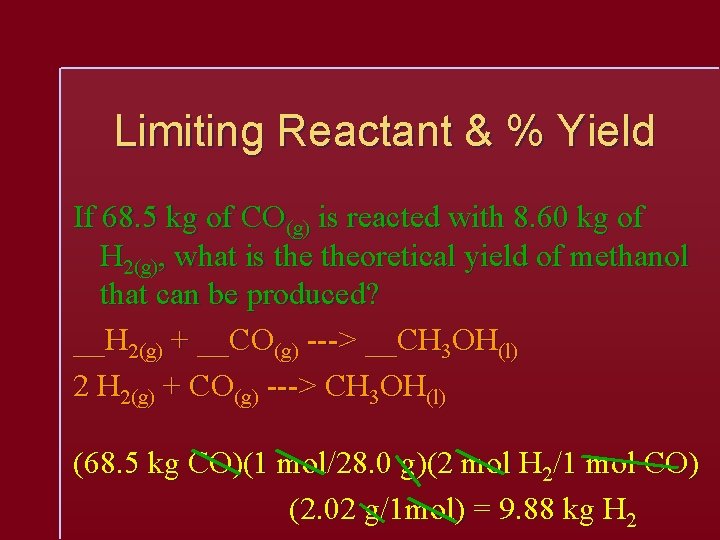
Limiting Reactant & % Yield If 68. 5 kg of CO(g) is reacted with 8. 60 kg of H 2(g), what is theoretical yield of methanol that can be produced? __H 2(g) + __CO(g) ---> __CH 3 OH(l) 2 H 2(g) + CO(g) ---> CH 3 OH(l) (68. 5 kg CO)(1 mol/28. 0 g)(2 mol H 2/1 mol CO) (2. 02 g/1 mol) = 9. 88 kg H 2

Limiting Reactant & % Yield 2 H 2(g) + CO(g) ---> CH 3 OH(l) Since only 8. 60 kg of H 2 were provided, the H 2 is the limiting reactant, and the CO is in excess. (8. 60 kg H 2)(1000 g/1 kg)(1 mol/2. 02 g)(1 mol CH 3 OH/2 mol H 2)(32. 0 g/1 mol) = 6. 85 x 104 g CH 3 OH

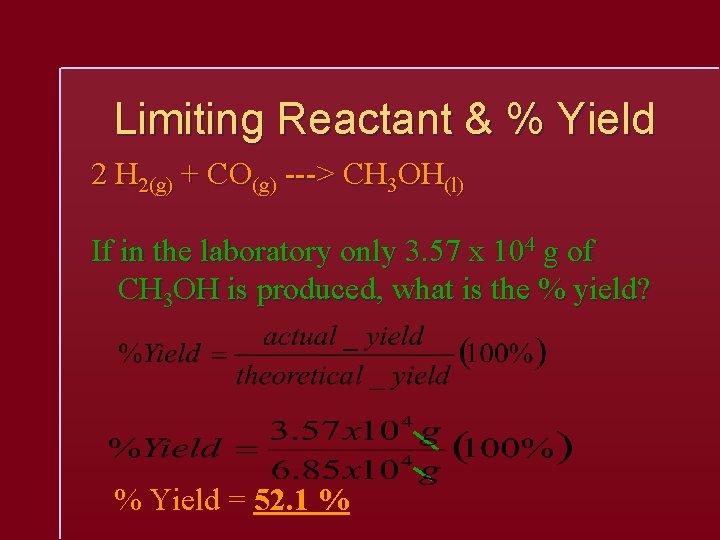
Limiting Reactant & % Yield 2 H 2(g) + CO(g) ---> CH 3 OH(l) If in the laboratory only 3. 57 x 104 g of CH 3 OH is produced, what is the % yield? % Yield = 52. 1 %