Stoichiometry What is Stoichiometry Stoichiometry the study of







- Slides: 14

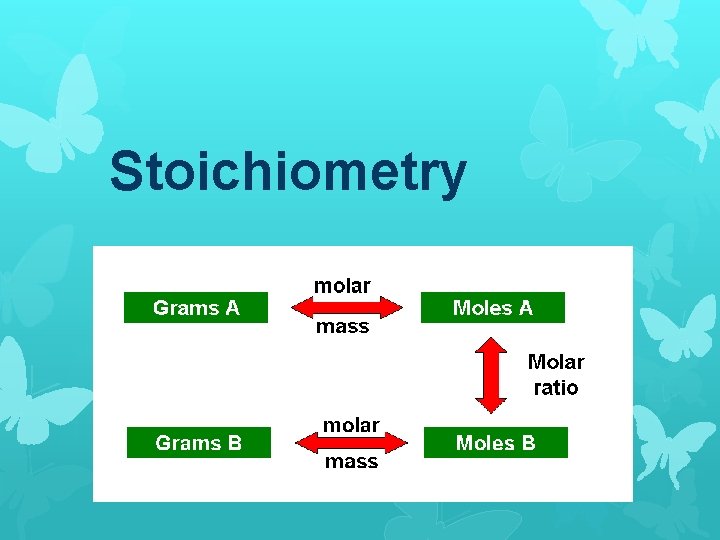
Stoichiometry

What is Stoichiometry? Stoichiometry: the study of quantitative relationships between amounts of reactants used and amounts of products formed. Stoichiometry is based on the Law of Conservation of Mass. Stoichiometry allows us to determine the amount of substance that is consumed or produced by a reaction. Ex: Consider the equation C 3 H 8 + 5 O 2 4 H 2 O + 3 CO 2 1: 5: 4: 3 We can “read” the molar ratios of the reactants and products. In this way, we can determine how many moles of O 2 are needed to produce a precise amount of CO 2.

How do we use Stoichiometry? How much cake do you need? Stoichiometry allows us to make the right amount of product.

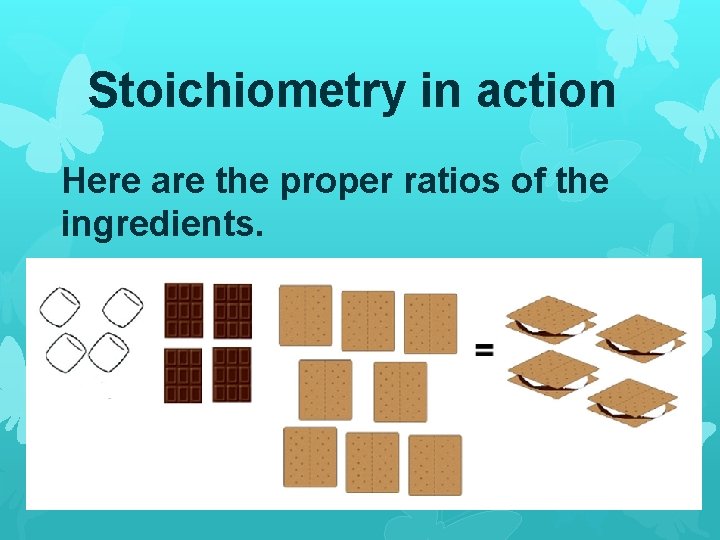
Does this seem right? If we want to make 4 s’mores, we will need to have the proper amounts of all ingredients.

Stoichiometry in action Here are the proper ratios of the ingredients.

Pair Work: Make a 1. With a partner make a list of at Poster least 4 ways you use stoichiometry. 2. Create an illustrated stoichiometry problem. (Use color, make it pop!) 3. Provide a complete answer to your stoichiometry problem. 4. Vote for the poster you think is best from the completed posters.

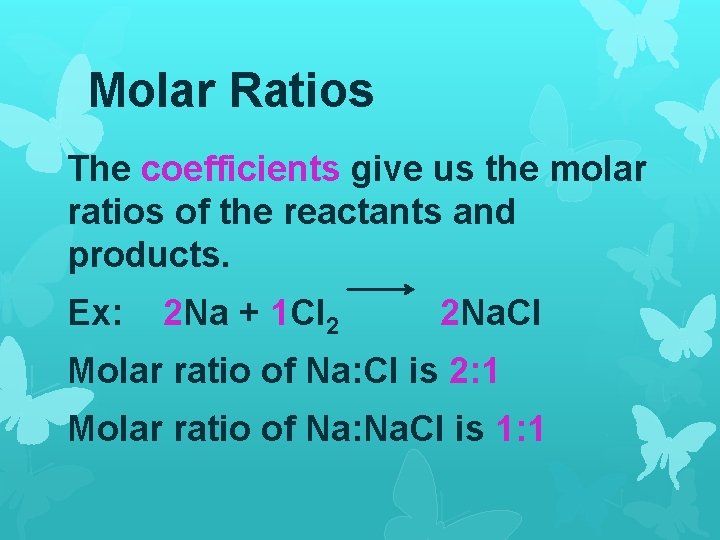
Molar Ratios The coefficients give us the molar ratios of the reactants and products. Ex: 2 Na + 1 Cl 2 2 Na. Cl Molar ratio of Na: Cl is 2: 1 Molar ratio of Na: Na. Cl is 1: 1

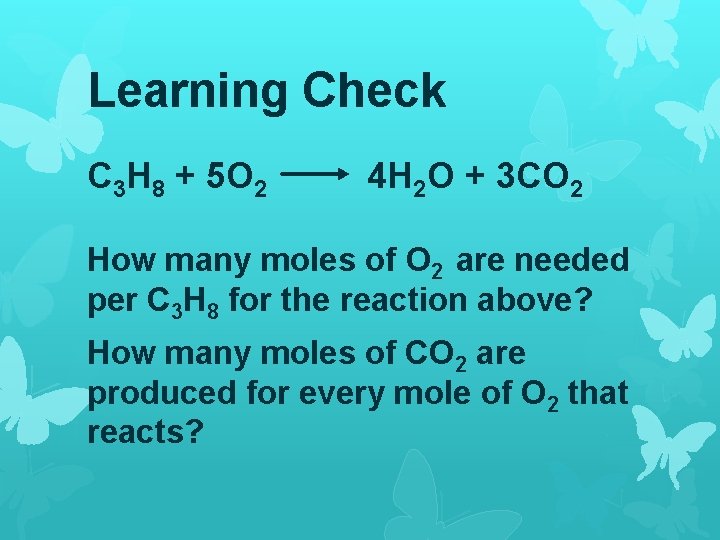
Learning Check C 3 H 8 + 5 O 2 4 H 2 O + 3 CO 2 How many moles of O 2 are needed per C 3 H 8 for the reaction above? How many moles of CO 2 are produced for every mole of O 2 that reacts?

Apply Stoichiometry to Recipes Use the M&M Cookie recipe for the following problems: 1) Double the recipe 2) Triple the recipe 3) Halve the recipe 4) Adjust the recipe to make enough cookies for 20 people to have 5 cookies each 5) Adjust the recipe to make 210 cookies 6) Predict how many cookies you could make if you had a dozen eggs

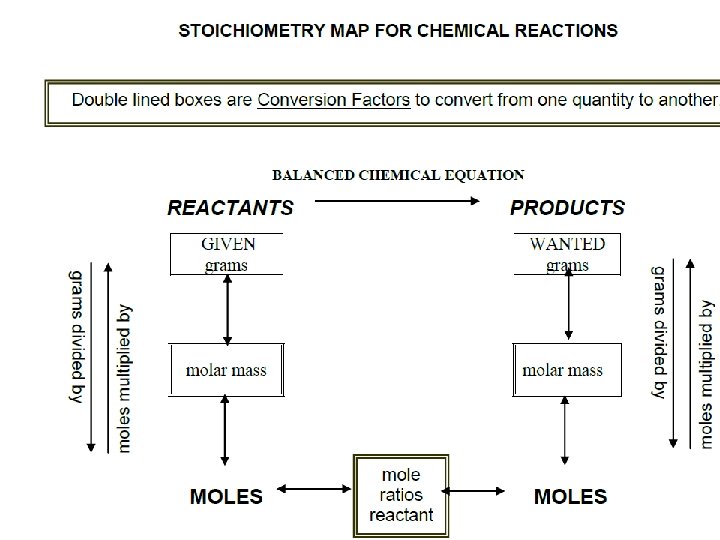
Stoichiometry Calculations We can convert grams to moles or moles to grams using molar masses. Ex: How many grams is 2 moles of H 2 O?

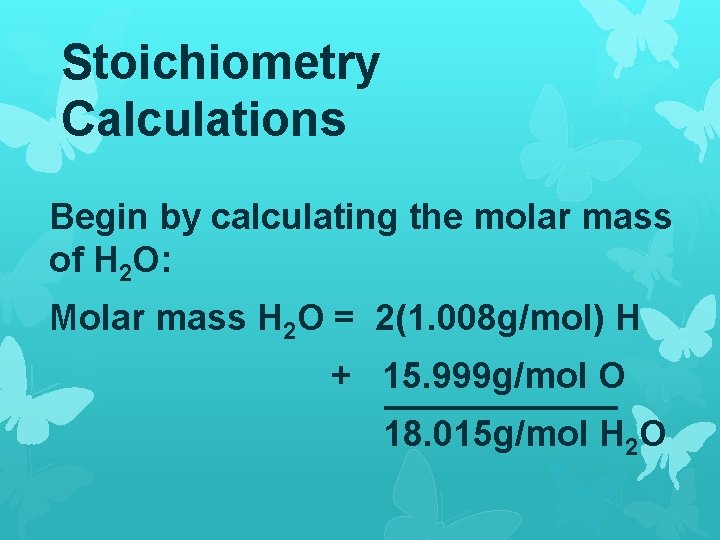
Stoichiometry Calculations Begin by calculating the molar mass of H 2 O: Molar mass H 2 O = 2(1. 008 g/mol) H + 15. 999 g/mol O 18. 015 g/mol H 2 O

Since we want to know the number of grams in 2 moles, we multiply the molar mass by 2 moles: (2 mol)(18. 015 g/mol) = 36. 03 g H 2 O So 2 moles of H 2 O has a mass of 36. 03 grams.

