CHE30042 Inorganic Physical Solid State Chemistry Advanced Quantum

- Slides: 18

CHE-30042 Inorganic, Physical & Solid State Chemistry Advanced Quantum Chemistry: lecture 1 Rob Jackson LJ 1. 16, 01782 733042 r. a. jackson@keele. ac. uk www. facebook. com/robjteaching @robajackson

Background reading Recommended Atkins’ Physical Chemistry, 9 th edition Peter Atkins, Julio de Paula Supplementary (more detailed) Quantum Mechanics for Chemists David O Hayward (RSC Tutorial Chemistry Text no. 14) che-30042: Advanced QC lecture 1 2

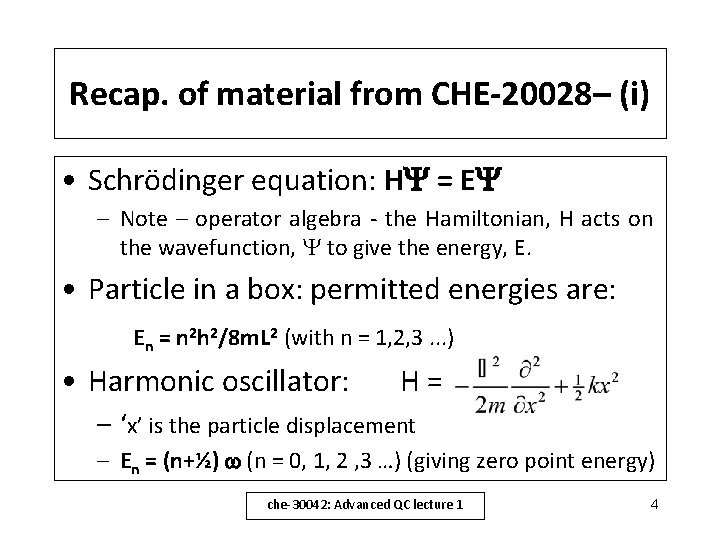
Lecture 1 contents 1. Recap from CHE-20028 – – – Schrödinger Equation: solution for model systems (particle in box, harmonic oscillator) Hydrogen atom – energies and orbitals Hydrogen-like orbitals 2. Wavefunctions for ‘many-electron’ atoms – – – Determinant notation introduced Wavefunctions for He – C The Self-Consistent Field Method che-30042: Advanced QC lecture 1 3

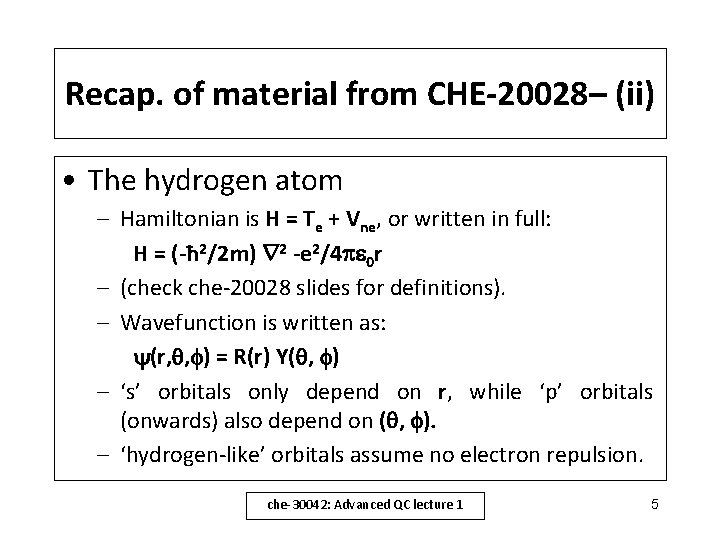
Recap. of material from CHE-20028– (i) • Schrödinger equation: H = E – Note – operator algebra - the Hamiltonian, H acts on the wavefunction, to give the energy, E. • Particle in a box: permitted energies are: En = n 2 h 2/8 m. L 2 (with n = 1, 2, 3. . . ) • Harmonic oscillator: H= – ‘x’ is the particle displacement – En = (n+½) (n = 0, 1, 2 , 3 …) (giving zero point energy) che-30042: Advanced QC lecture 1 4

Recap. of material from CHE-20028– (ii) • The hydrogen atom – Hamiltonian is H = Te + Vne, or written in full: H = (-ħ 2/2 m) 2 -e 2/4 0 r – (check che-20028 slides for definitions). – Wavefunction is written as: (r, , ) = R(r) Y( , ) – ‘s’ orbitals only depend on r, while ‘p’ orbitals (onwards) also depend on ( , ). – ‘hydrogen-like’ orbitals assume no electron repulsion. che-30042: Advanced QC lecture 1 5

Hydrogen-like orbitals • The orbitals widely used in Chemistry (1 s, 2 p, 3 s, 3 p, 3 d etc. ) are known as hydrogen-like orbitals because in their ‘ideal’ form they only apply to hydrogen (with no electron repulsion), or to other 1 electron ‘atoms’ like He+, Li 2+ etc. • A link to a good diagram will be given (also see teaching pages). Also see next slide. che-30042: Advanced QC lecture 1 6

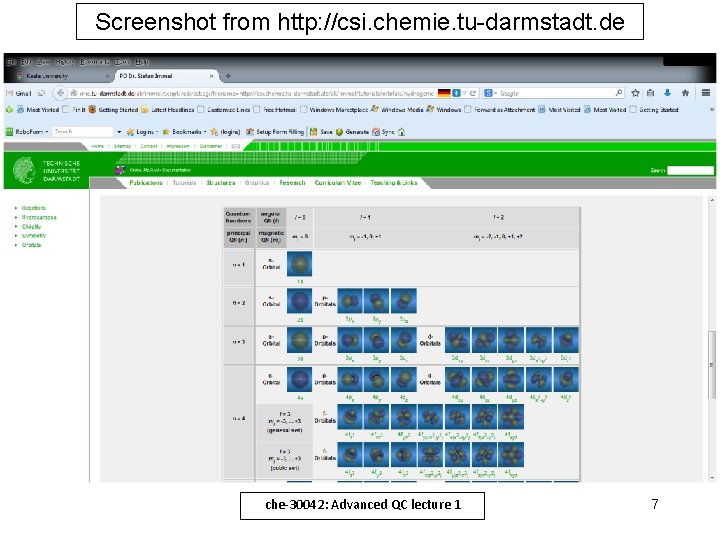
Screenshot from http: //csi. chemie. tu-darmstadt. de che-30042: Advanced QC lecture 1 7

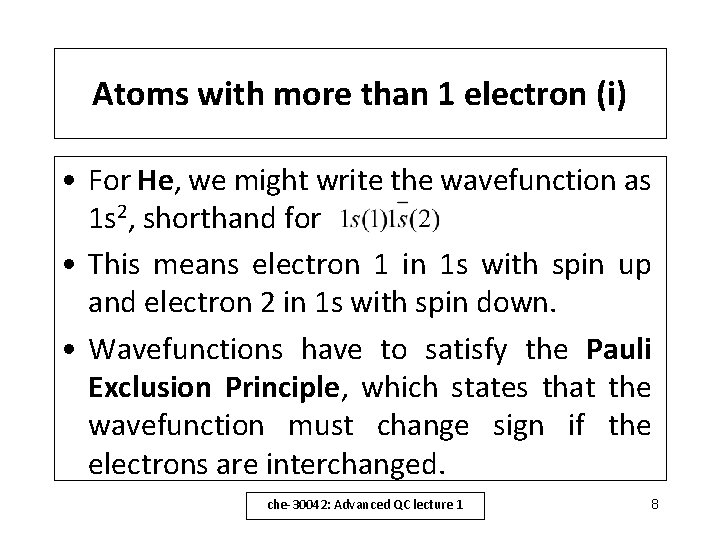
Atoms with more than 1 electron (i) • For He, we might write the wavefunction as 1 s 2, shorthand for • This means electron 1 in 1 s with spin up and electron 2 in 1 s with spin down. • Wavefunctions have to satisfy the Pauli Exclusion Principle, which states that the wavefunction must change sign if the electrons are interchanged. che-30042: Advanced QC lecture 1 8

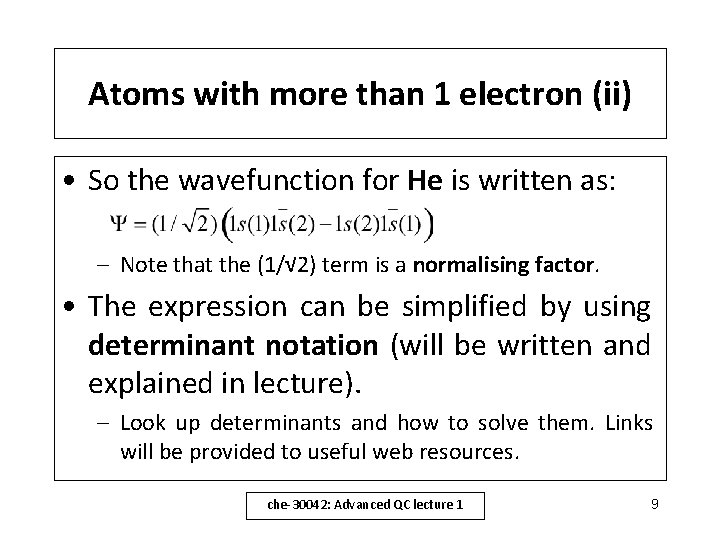
Atoms with more than 1 electron (ii) • So the wavefunction for He is written as: – Note that the (1/√ 2) term is a normalising factor. • The expression can be simplified by using determinant notation (will be written and explained in lecture). – Look up determinants and how to solve them. Links will be provided to useful web resources. che-30042: Advanced QC lecture 1 9

Atoms with more than 1 electron (iii) • The determinant notation can be used to simplify the expressions for wavefunctions of atoms with more electrons. – Li will be done in the lecture – Self-test: try Be, B & C • Using this procedure we can (in principle) write out the wavefunction of any atom in terms of orbitals. – Note: expressions for the orbitals are still needed. che-30042: Advanced QC lecture 1 10

Correcting hydrogen like orbitals: the Self. Consistent Field method • We can write the wavefunction of an atom in terms of orbitals, e. g. for Li: = 1 s 2 2 s 1 (or in determinant notation to take into account the Pauli Exclusion Principle). • Each term is a 1 -electron orbital, so how is electron repulsion taken into account? • This is done using the Self-Consistent Field method. che-30042: Advanced QC lecture 1 11

The Self-Consistent Field (SCF) method – (i) • The s, p, d, f … orbitals that we are familiar with are 1 -electron orbitals – i. e. they don’t take into account the presence of other electrons. • The SCF method provides a way of correcting wavefunctions for the presence of more than one electron. • This can explain, e. g. , why electrons in 4 s orbitals have lower energy than those in 3 d orbitals in K and Ca • How has this been explained up to now? che-30042: Advanced QC lecture 1 12

The Self-Consistent Field (SCF) method – (ii) • What is the Hamiltonian for a 1 -electron atom? • It will include 2 terms: electron kinetic energy (Te) and electron-nucleus potential energy (Vne). H = Te + Vne • If we have more than one electron, there will be an additional term due to electron-electron repulsion (Vee): H = Te + Vne + Vee • (Note there will generally be more than one Te and Vne term). che-30042: Advanced QC lecture 1 13

The Self-Consistent Field (SCF) method – (iii) • There is a problem in calculating the electron repulsion energy because we are using 1 -electron orbital wave functions – i. e. each orbital only contains one electron, so how can we explain how they interact? • The SCF method provides a way of correcting orbitals for the effect of other electrons. • It starts by calculating the average potential energy of interaction between the first electron and the others (i. e. Vee in the previous equation). This is done assuming 1 -electron orbitals. che-30042: Advanced QC lecture 1 14

Application of the SCF method – (i) • If we now consider a particular example, Be, which has electronic structure 1 s 22 s 2 (ignoring spin and antisymmetry for now), we follow this procedure: – (i) calculate the Vee term assuming 1 -electron orbitals. – (ii) solve the Schrödinger equation for the 2 s orbital using this Vee term. – (iii) This gives a new updated 2 s orbital. che-30042: Advanced QC lecture 1 15

Application of the SCF method – (ii) – (iv) using the new 2 s orbital obtained, the Vee term is recalculated, and the Schrödinger equation solved for the 1 s orbital. – (v) An updated 1 s orbital is obtained, and this is then used to amend the value of Vee, which is then used to recalculate the 2 s orbital. – (vi) The procedure is then repeated until there is no change in the orbitals and their energies in successive calculations. che-30042: Advanced QC lecture 1 16

Application of the SCF method – (iii) • For Be, the ordering of the orbitals will not be affected, but in the case of K and Ca, the energy of the 4 s orbital will be found to be lower than 3 d at the end of the calculation, explaining the orbital occupancies. • A problem with the SCF method is that it does not treat electron correlation (i. e. electrostatic repulsion) properly, and this has to be corrected for, using Configuration Interaction calculations. che-30042: Advanced QC lecture 1 17

Summary of Lecture • Material from che-20028 has been revisited. • Wavefunctions that obey the Pauli Exclusion Principle have been introduced. • The determinant notation has been introduced for atoms beyond hydrogen. • The SCF method for atoms has been introduced and explained. che-30042: Advanced QC lecture 1 18