CHE30042 Inorganic Physical Solid State Chemistry Advanced Quantum
- Slides: 18

CHE-30042 Inorganic, Physical & Solid State Chemistry Advanced Quantum Chemistry: lecture 2 Rob Jackson LJ 1. 16, 01782 733042 r. a. jackson@keele. ac. uk www. facebook. com/robjteaching @robajackson

Lecture 2 contents 1. Trial wavefunctions and expectation values 2. The Variation Principle 3. Recap. on Molecular Orbitals: the hydrogen molecule ion 4. Molecular Orbitals for diatomic molecules 5. Secular Determinants che-30042 Advanced QC lecture 2 2

Trial wavefunctions and expectation values – (i) • In quantum chemistry, one of the main problems faced is determining the wavefunction of an atom or molecule. • If the ground state energy of a molecule is E 0, we expect the following to apply (if we know the wavefunction): H 0 = E 0 0 – Remember that this means that H acts on the wavefunction of the ground state to give the energy of the ground state. che-30042 Advanced QC lecture 2 3

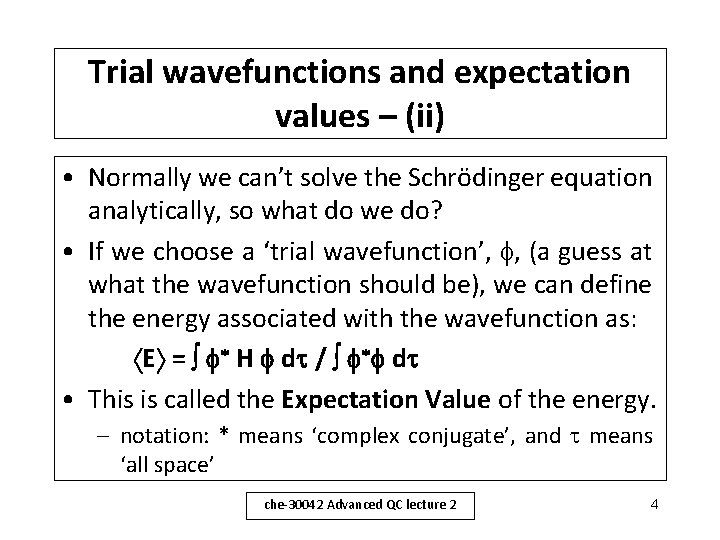
Trial wavefunctions and expectation values – (ii) • Normally we can’t solve the Schrödinger equation analytically, so what do we do? • If we choose a ‘trial wavefunction’, , (a guess at what the wavefunction should be), we can define the energy associated with the wavefunction as: E = H d / d • This is called the Expectation Value of the energy. – notation: * means ‘complex conjugate’, and means ‘all space’ che-30042 Advanced QC lecture 2 4

The Variation Principle • If we use a trial wavefunction, , and calculate the expectation value of the energy, how can we judge our choice of wavefunction? • The Variation Principle states that: – For any trial wavefunction, the expectation value of the energy can never be less than the true ground state energy. • This is expressed as E 0 H d / d – The expectation value is only E 0 if the trial wavefunction is equal to the true one. che-30042 Advanced QC lecture 2 5

Why is the Variation Principle useful? • It enables different trial wavefunctions to be tested and evaluated, since the wavefunction giving the lowest energy expectation value should be closest to the true wavefunction. • For the hydrogen atom we know the ground state energy so the variation principle can be tested for different wavefunctions. • A reference to an example calculation will be given and discussed. che-30042 Advanced QC lecture 2 6

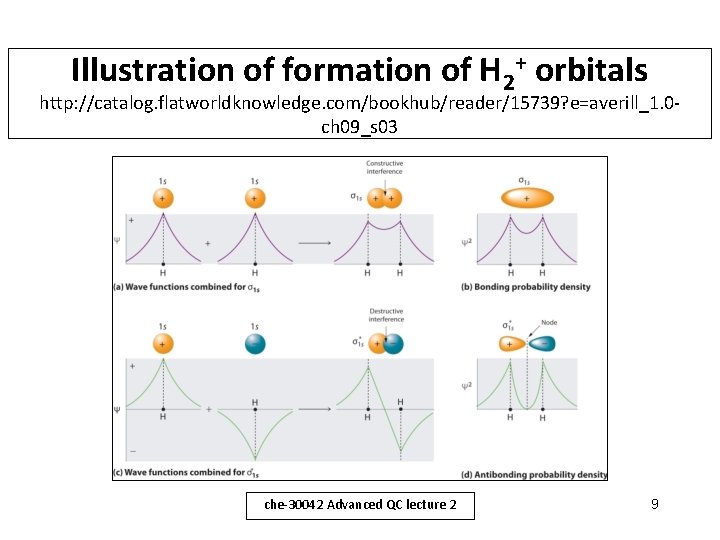
Molecular orbitals for H 2+ - (i) • For H 2+ we have 2 1 s atomic orbitals, each centred on a H atom. • We can form a trial wavefunction by combining the 1 s orbitals A and B. – Remember molecular orbital diagrams here! • This can be written as = N ( A B) – Here, N is a normalising constant. • The expectation value for the energy will be: E = (N )2 ( A B) H ( A B) d che-30042 Advanced QC lecture 2 7

Molecular orbitals for H 2+ - (ii) • The 2 molecular orbitals + , - are the bonding and antibonding orbitals in the H 2+ molecule. • In Hayward’s book there is a more detailed derivation, using expressions for the 1 s orbitals A and B (pp. 141 -145) • The shape of the wavefunction and the potential energy diagram will be shown and discussed in the lecture (see also Hayward figures 8. 2, 8. 3, pp. 142 -3). che-30042 Advanced QC lecture 2 8

Illustration of formation of H 2+ orbitals http: //catalog. flatworldknowledge. com/bookhub/reader/15739? e=averill_1. 0 ch 09_s 03 che-30042 Advanced QC lecture 2 9

Molecular orbitals for diatomic molecules – (i) • We can follow the same procedure for diatomic molecules as demonstrated for H 2+: • If the molecule is AB (i. e. different nuclei) we can suggest a trial wavefunction based on orbitals on atoms A and B: = c. A A + c. B B (where c. A, c. B are weighting coefficients) • The expectation value for the energy of this trial wavefunction will be (see definition on slide 5): E = H d / d che-30042 Advanced QC lecture 2 10

Molecular orbitals for diatomic molecules – (ii) • We will assume that the wavefunctions are not ‘complex’, so * = – Substituting for the wavefunction gives: E = (c. A A + c. B B) H (c. A A + c. B B) d / (c. A A + c. B B)2 d – On p. 150 of Hayward’s book, the expression is expanded to give: E = (c 2 AHAA + 2 c. Ac. BHAB + c 2 BHBB)/ (c 2 ASAA + 2 c. Ac. BSAB + c 2 BSBB ) – Here HAA, HAB, HBB, SAA, SAB and SBB are all integrals which are defined in the book and the next slide: che-30042 Advanced QC lecture 2 11

Overlap, Coulomb and Resonance integrals • The terms in the 2 nd equation, slide 10 are: SAA = A A d , SBB = B B d ( =1 for normalised orbitals) SAB = A B d = SBA = B A d (Overlap integrals) – The value depends on the amount of orbital overlap. HAA = A H A d , HBB = B H B d – Coulomb integral, the energy an electron would have in a particular orbital. HAB = A H B d = HBA = B H A d – Resonance integrals, = 0 if there is no overlap. che-30042 Advanced QC lecture 2 12

So how do we get the energy? • We need to evaluate the integrals, and get the values of c. A and c. B that give the lowest energy (Variation Principle). • The general approach to this will be explained (not restricted to diatomics), and applied to specific cases. • We start by writing the trial wavefunction as a linear combination of atomic orbitals: i = k cik k • So a trial wavefunction for a triatomic molecule would look like: i = ci 1 1 + ci 2 2 + ci 3 3 che-30042 Advanced QC lecture 2 13

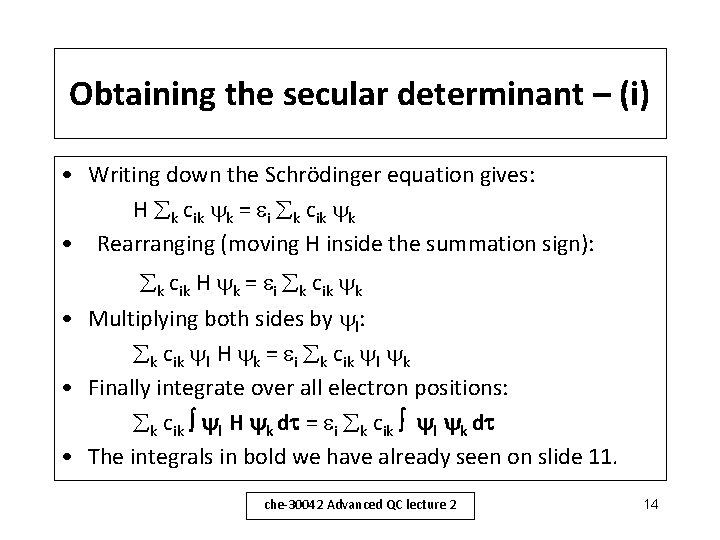
Obtaining the secular determinant – (i) • Writing down the Schrödinger equation gives: H k cik k = i k cik k • Rearranging (moving H inside the summation sign): k cik H k = i k cik k • Multiplying both sides by l: k cik l H k = i k cik l k • Finally integrate over all electron positions: k cik l H k d = i k cik l k d • The integrals in bold we have already seen on slide 11. che-30042 Advanced QC lecture 2 14

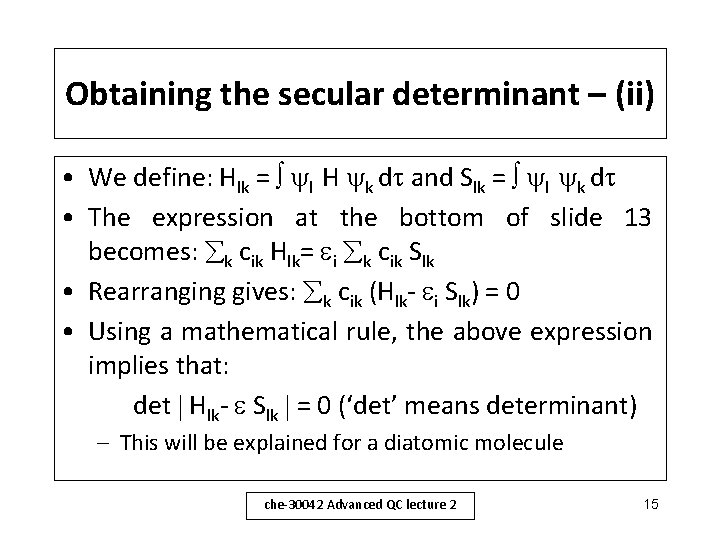
Obtaining the secular determinant – (ii) • We define: Hlk = l H k d and Slk = l k d • The expression at the bottom of slide 13 becomes: k cik Hlk= i k cik Slk • Rearranging gives: k cik (Hlk- i Slk) = 0 • Using a mathematical rule, the above expression implies that: det Hlk- Slk = 0 (‘det’ means determinant) – This will be explained for a diatomic molecule che-30042 Advanced QC lecture 2 15

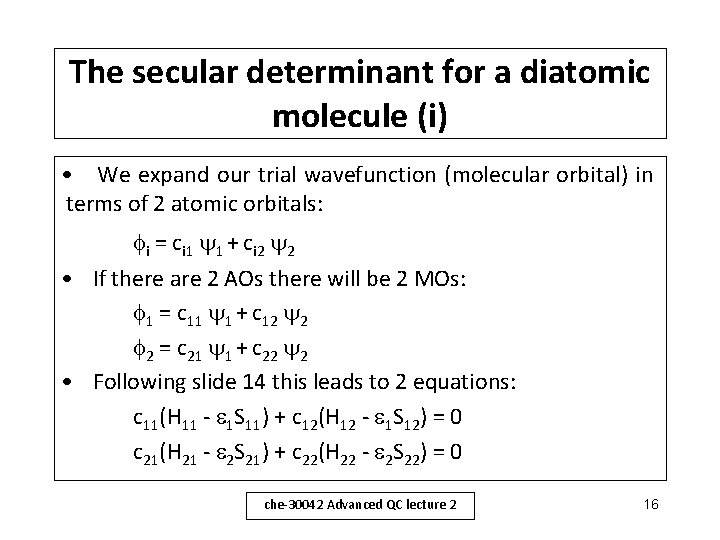
The secular determinant for a diatomic molecule (i) • We expand our trial wavefunction (molecular orbital) in terms of 2 atomic orbitals: i = ci 1 1 + ci 2 2 • If there are 2 AOs there will be 2 MOs: 1 = c 11 1 + c 12 2 2 = c 21 1 + c 22 2 • Following slide 14 this leads to 2 equations: c 11(H 11 - 1 S 11) + c 12(H 12 - 1 S 12) = 0 c 21(H 21 - 2 S 21) + c 22(H 22 - 2 S 22) = 0 che-30042 Advanced QC lecture 2 16

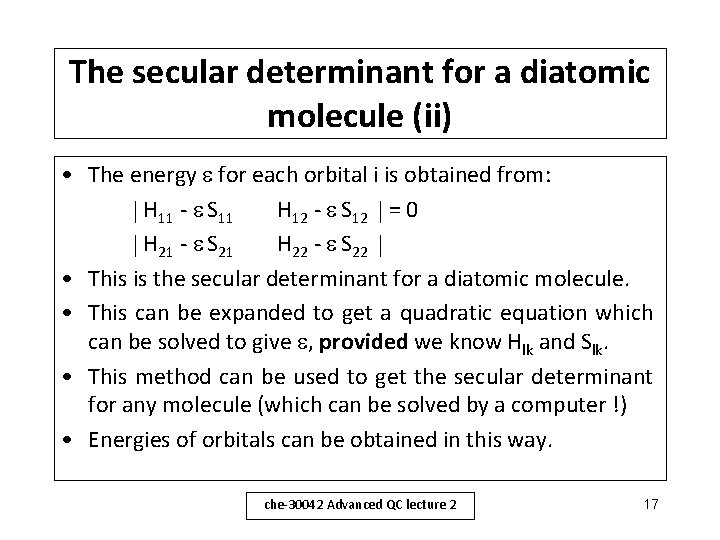
The secular determinant for a diatomic molecule (ii) • The energy for each orbital i is obtained from: H 11 - S 11 H 12 - S 12 = 0 H 21 - S 21 H 22 - S 22 • This is the secular determinant for a diatomic molecule. • This can be expanded to get a quadratic equation which can be solved to give , provided we know Hlk and Slk. • This method can be used to get the secular determinant for any molecule (which can be solved by a computer !) • Energies of orbitals can be obtained in this way. che-30042 Advanced QC lecture 2 17

Lecture summary • The Expectation Value of a property has been introduced and defined. • The Variation Principle has been introduced and explained. • Molecular Orbitals for H 2+ and diatomic molecules in general have been calculated. • Coulomb, Overlap and Resonance integrals have been defined • Secular Determinants have been introduced. che-30042 Advanced QC lecture 2 18