CHE30042 Inorganic Physical Solid State Chemistry Advanced Quantum
- Slides: 15

CHE-30042 Inorganic, Physical & Solid State Chemistry Advanced Quantum Chemistry: lecture 4 Rob Jackson LJ 1. 16, 01782 733042 r. a. jackson@keele. ac. uk www. facebook. com/robjteaching @robajackson

Lecture 4 contents 1. 2. 3. 4. Representing atomic orbitals – basis sets Ab initio calculations Molecular geometry and geometry optimisation Improving SCF calculations – including Configuration Interaction (CI) 5. Alternatives to MO calculations – Density Functional Theory (DFT) che-30042 Advanced QC lecture 4 2

Representing atomic orbitals • If we are going to use atomic orbitals in calculations, we need to know how to represent them. • The usual expression for an atomic orbital (mentioned in che-20028) is: = exp (- r) Y ( , ) – Where (r, , ) are coordinates, and (e. g. ) an s-orbital only depends on ‘r’. • This is used for Slater-type orbitals (STOs) che-30042 Advanced QC lecture 4 3

STOs and basis sets – (i) • In molecular orbital calculations, atomic orbitals are represented by basis sets. – A minimal basis set has one function per orbital, but accuracy can be improved by having more than one function (‘double zeta’ basis sets, slide 6). • STOs are functions that have been fitted to numerical wavefunctions, and have a form similar to the one given in slide 3. che-30042 Advanced QC lecture 4 4

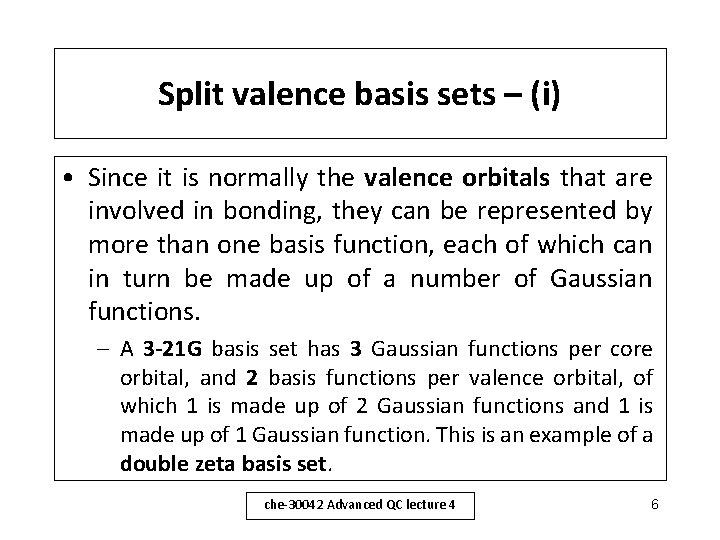
STOs and basis sets – (ii) • In the Gaussian workshop (lecture 6) you will see the effect of using different basis sets on calculation accuracy. – For example, STO-3 G means that 3 Gaussian functions are fitted to the numerical wavefunction, easier mathematically than fitting to the function on slide 3. – So an ‘s’ function is written as: = exp (- r) = c 1 1 + c 2 2 + c 3 3 Where 1= (2 1/ ) exp(- 1 r 2) ( 2, 3 are similarly defined). che-30042 Advanced QC lecture 4 5

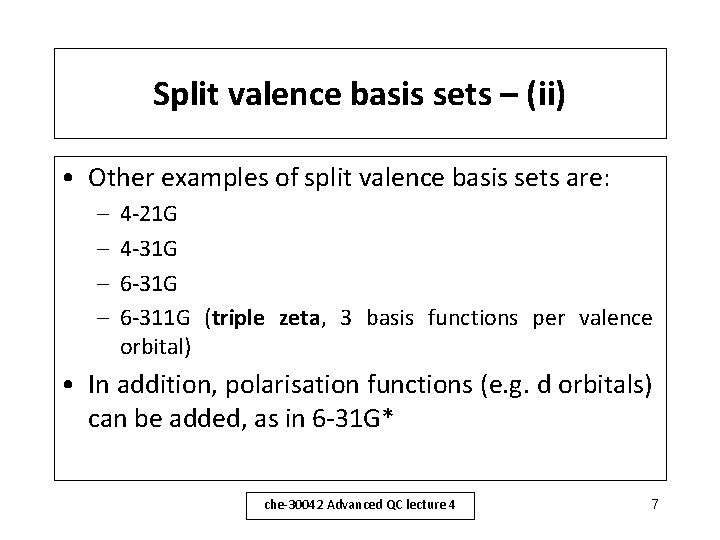
Split valence basis sets – (i) • Since it is normally the valence orbitals that are involved in bonding, they can be represented by more than one basis function, each of which can in turn be made up of a number of Gaussian functions. – A 3 -21 G basis set has 3 Gaussian functions per core orbital, and 2 basis functions per valence orbital, of which 1 is made up of 2 Gaussian functions and 1 is made up of 1 Gaussian function. This is an example of a double zeta basis set. che-30042 Advanced QC lecture 4 6

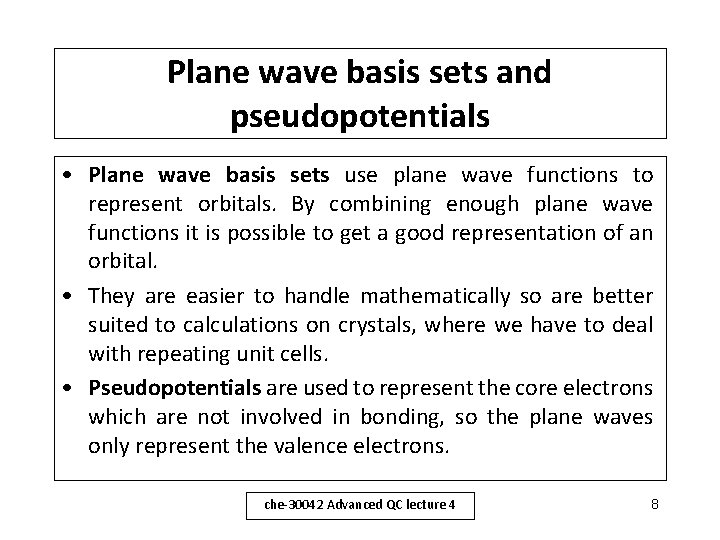
Split valence basis sets – (ii) • Other examples of split valence basis sets are: – – 4 -21 G 4 -31 G 6 -311 G (triple zeta, 3 basis functions per valence orbital) • In addition, polarisation functions (e. g. d orbitals) can be added, as in 6 -31 G* che-30042 Advanced QC lecture 4 7

Plane wave basis sets and pseudopotentials • Plane wave basis sets use plane wave functions to represent orbitals. By combining enough plane wave functions it is possible to get a good representation of an orbital. • They are easier to handle mathematically so are better suited to calculations on crystals, where we have to deal with repeating unit cells. • Pseudopotentials are used to represent the core electrons which are not involved in bonding, so the plane waves only represent the valence electrons. che-30042 Advanced QC lecture 4 8

Ab initio calculations • The Hückel method, described in lecture 3, avoided the problem of calculating the integrals Hlk and Slk by setting them equal to numerical constants. • In ‘ab initio’ calculations (meaning ‘from the beginning’), no such drastic assumptions are made, and the calculations are carried out in full. • The procedure used will now be explained. che-30042 Advanced QC lecture 4 9

Ab initio SCF calculations – (i) i. Start with a trial molecular orbital as a sum of weighted atomic orbitals (see lec. 2 slide 10). – e. g. = c. A A + c. B B (use estimates for c. A, c. B) ii. Set up the secular determinant (lec. 2 slides 14 -15) iii. Solve, having calculated all the integrals Hlk & Slk. iv. Determine orbital energy, and substitute into the secular equations to get coefficients c. A, c. B. v. This gives a new orbital, . che-30042 Advanced QC lecture 4 10

Ab initio SCF calculations – (ii) vi. Set up the secular determinant again (Hlk and Slk have to be recalculated because has changed). vii. Calculate new values of the energy, and then the weighting coefficients. viii. Repeat until no change (self consistency). ix. The final result gives the energy and orbital coefficients. che-30042 Advanced QC lecture 4 11

Molecular geometry and geometry optimisation • If we are carrying out an ab initio calculation on a molecule, we will need to specify the distance between the atoms, and the molecular geometry. • The bond distances etc. can be varied, and the energy calculated. • It is then possible to find the structure corresponding to the lowest energy – geometry optimisation. • This will be done in the workshop for H 2. che-30042 Advanced QC lecture 4 12

Configuration Interaction (CI) calculations • In lecture 1 when we introduced the SCF method, it was mentioned that the method doesn’t treat electron correlation (repulsion) very well. • The CI method is one way of correcting this, by including all possible electron configurations in the calculation (i. e. not just the ground state). So for H 2, the antibonding orbital could be included in the calculation. – However the method is computationally expensive! che-30042 Advanced QC lecture 4 13

Density Functional Theory (DFT) • DFT is widely used in place of molecular orbital calculations, especially for solids. • The main difference with MO methods is that it works with overall electron density rather than by representing each orbital by a function. • This makes the calculations quicker, but there are still problems in dealing with electron exchange and correlation, and approximations have to be made here. che-30042 Advanced QC lecture 4 14

Lecture summary • The representation of orbitals has been revised. • Basis sets have been introduced and explained. • The procedure for ab initio SCF calculations has been explained. • Geometry optimisation has been mentioned – to be looked at in the workshop. • Density Functional Theory has been introduced. che-30042 Advanced QC lecture 4 15