CHE30042 Inorganic Physical Solid State Chemistry Advanced Quantum




- Slides: 20

CHE-30042 Inorganic, Physical & Solid State Chemistry Advanced Quantum Chemistry: lecture 3 Rob Jackson LJ 1. 16, 01782 733042 r. a. jackson@keele. ac. uk www. facebook. com/robjteaching @robajackson

Lecture 3 contents 1. 2. 3. 4. Secular determinants revised Overlap, resonance and Coulomb integrals Introducing the Hückel approximation Applying the Hückel approximation conjugated hydrocarbons 5. Delocalisation energy 6. Aromatic systems che-30042 Advanced QC lecture 3 to 2

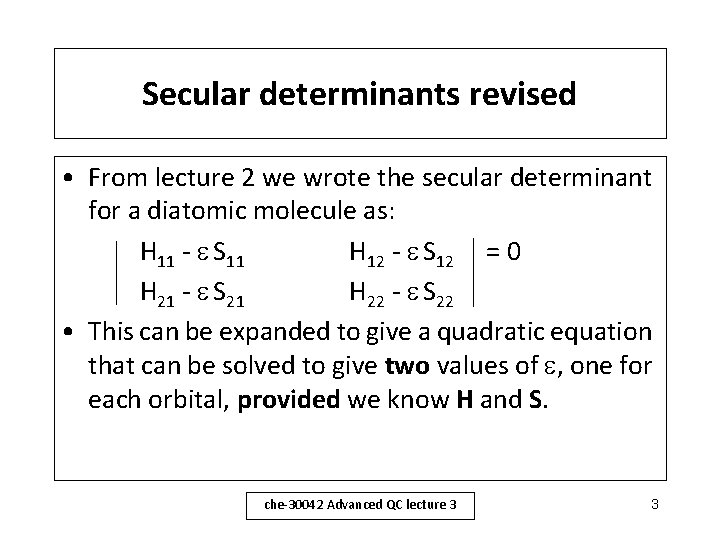
Secular determinants revised • From lecture 2 we wrote the secular determinant for a diatomic molecule as: H 11 - S 11 H 12 - S 12 = 0 H 21 - S 21 H 22 - S 22 • This can be expanded to give a quadratic equation that can be solved to give two values of , one for each orbital, provided we know H and S. che-30042 Advanced QC lecture 3 3

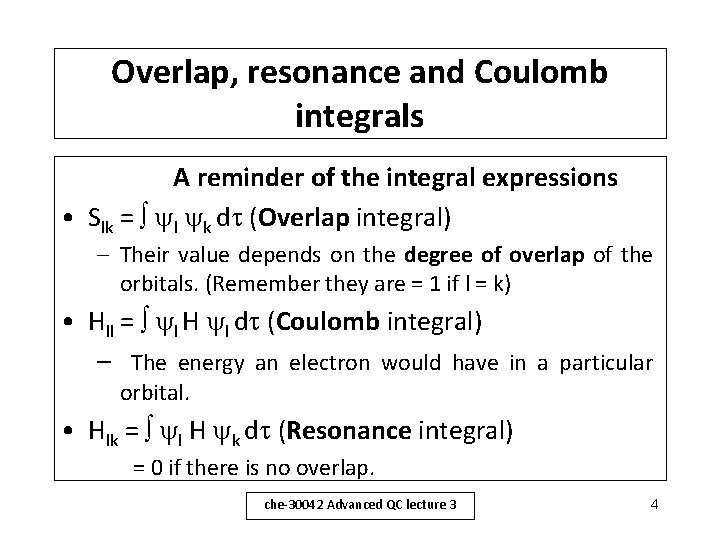
Overlap, resonance and Coulomb integrals A reminder of the integral expressions • Slk = l k d (Overlap integral) – Their value depends on the degree of overlap of the orbitals. (Remember they are = 1 if l = k) • Hll = l H l d (Coulomb integral) – The energy an electron would have in a particular orbital. • Hlk = l H k d (Resonance integral) = 0 if there is no overlap. che-30042 Advanced QC lecture 3 4

The Hückel approximation • The main problem with molecular orbital calculations is associated with calculating the integrals introduced in the last lecture, and revised on slide 4. . – Later we will see how to try to calculate them in full. • The Hückel approach neatly sidesteps the problem by making assumptions about the values of the integrals, which are listed on the next slide: che-30042 Advanced QC lecture 3 5

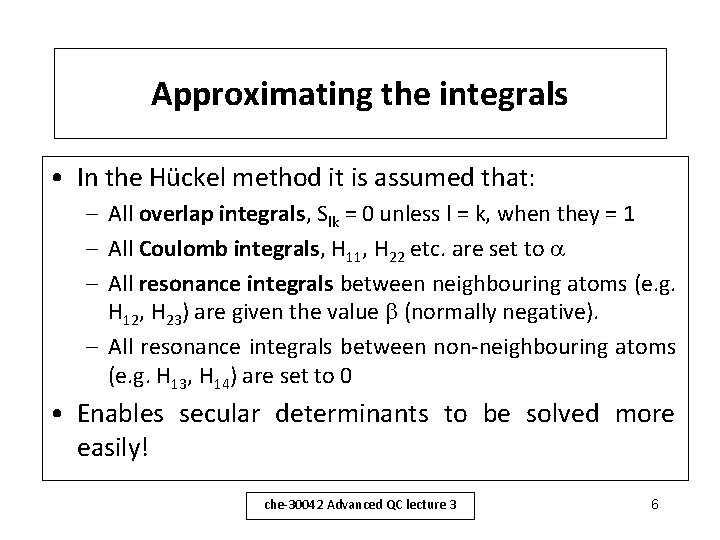
Approximating the integrals • In the Hückel method it is assumed that: – All overlap integrals, Slk = 0 unless l = k, when they = 1 – All Coulomb integrals, H 11, H 22 etc. are set to – All resonance integrals between neighbouring atoms (e. g. H 12, H 23) are given the value (normally negative). – All resonance integrals between non-neighbouring atoms (e. g. H 13, H 14) are set to 0 • Enables secular determinants to be solved more easily! che-30042 Advanced QC lecture 3 6

Background: molecular orbitals for conjugated hydrocarbons • Conjugated hydrocarbons are characterised by having a mix of and bonding; the bonds are responsible for holding the molecule together, while the bonding is delocalised, with the electrons free to move around the molecule. • The Hückel approach (Hückel, 1930) provides a more accurate description of bonding. – Hückel, E. (1930) Zeitschrift für Physik 60 (7– 8): 423– 456. doi: 10. 1007/BF 01341254. che-30042 Advanced QC lecture 3 7

Applying the Hückel approximation to simple hydrocarbons containing electrons • Although the Hückel approximation is most useful for conjugated and aromatic hydrocarbons, we will apply it to a simple example first to illustrate how it works for localised orbitals. • We will apply it to ethene, remembering that we are only looking at the molecular orbitals, which result from overlap of the pz orbitals on each C atom. che-30042 Advanced QC lecture 3 8

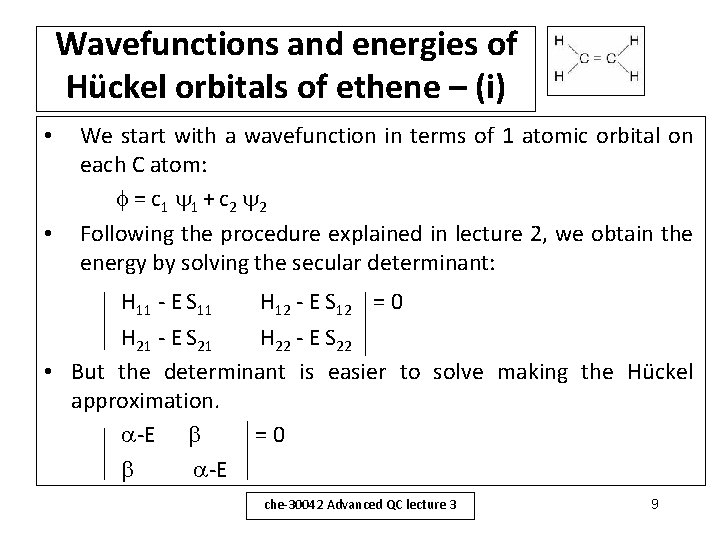
Wavefunctions and energies of Hückel orbitals of ethene – (i) • • We start with a wavefunction in terms of 1 atomic orbital on each C atom: = c 1 1 + c 2 2 Following the procedure explained in lecture 2, we obtain the energy by solving the secular determinant: H 11 - E S 11 H 12 - E S 12 = 0 H 21 - E S 21 H 22 - E S 22 • But the determinant is easier to solve making the Hückel approximation. -E =0 -E che-30042 Advanced QC lecture 3 9

Wavefunctions and energies of Hückel orbitals of ethene – (ii) • Expanding the determinant gives a quadratic equation: ( - E)2 = 2 • The solutions to this (obtained by expanding the equation and applying the quadratic formula) are: E = • These are the energies of the orbitals. What about the wavefunctions? • The secular equations are (slide 15 lecture 2): c 1 ( - E) + c 2 = 0, c 1 + c 2 ( - E) = 0 che-30042 Advanced QC lecture 3 10

Wavefunctions and energies of Hückel orbitals of ethene – (iii) • If we substitute the two possible values for E in either of these equations, we find the following: – When E = + , c 1 = c 2, so b = c 1 1 + c 1 2 – When E = - , c 1 = -c 2, so a = c 1 1 - c 2 2 • Here ‘b’ and ‘a’ denote bonding and antibonding orbitals. • They will be sketched in the lecture, or you can find a better diagram in Hayward p 172. • However, the Hückel method was really devised for larger molecules, particularly with alternating double and single bonds. che-30042 Advanced QC lecture 3 11

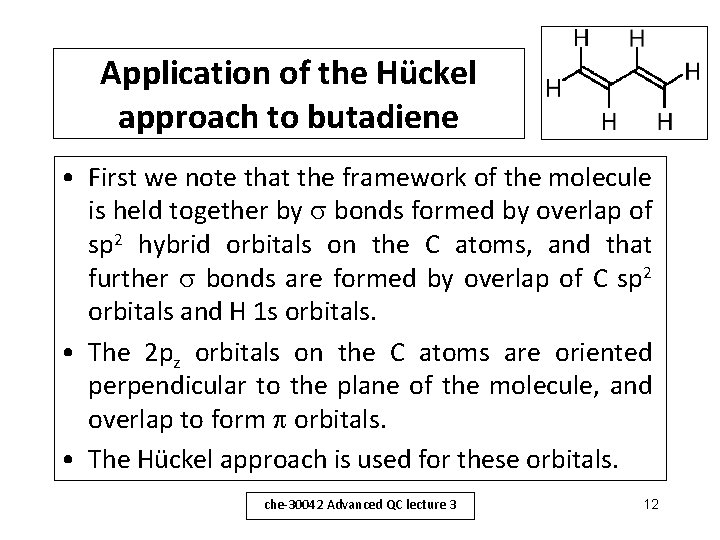
Application of the Hückel approach to butadiene • First we note that the framework of the molecule is held together by bonds formed by overlap of sp 2 hybrid orbitals on the C atoms, and that further bonds are formed by overlap of C sp 2 orbitals and H 1 s orbitals. • The 2 pz orbitals on the C atoms are oriented perpendicular to the plane of the molecule, and overlap to form orbitals. • The Hückel approach is used for these orbitals. che-30042 Advanced QC lecture 3 12

The Hückel approach to -bonding in butadiene – (i) • We form a molecular orbital from the 2 pz orbitals on the 4 C atoms: = c 1 1 + c 2 2 + c 3 3 + c 4 4 • This leads to 4 secular equations: c 1(H 11 - ES 11) + c 2(H 12 - ES 12) + c 3(H 13 - ES 13) + c 4(H 14 - ES 14) = 0 c 1(H 21 - ES 21) + c 2(H 22 - ES 22) + c 3(H 23 - ES 23) + c 4(H 24 - ES 24) = 0 c 1(H 31 - ES 31) + c 2(H 32 - ES 32) + c 3(H 33 - ES 33) + c 4(H 34 - ES 34) = 0 c 1(H 41 - ES 41) + c 2(H 42 - ES 42) + c 3(H 43 - ES 43) + c 4(H 44 - ES 44) = 0 • Now form the secular determinant! che-30042 Advanced QC lecture 3 13

The Hückel approach to -bonding in butadiene – (ii) • This leads to a secular determinant: H 11 - ES 11 H 12 - ES 12 H 13 - ES 13 H 14 - ES 14 = 0 H 21 - ES 21 H 22 - ES 22 H 23 - ES 23 H 24 - ES 24 H 31 - ES 31 H 32 - ES 32 H 33 - ES 33 H 34 - ES 34 H 41 - ES 41 H 42 - ES 42 H 43 - ES 43 H 44 - ES 44 • Simplify the determinant, making the approximations on slide 6. Pay particular attention to resonance integrals. • Hayward (p 168) then shows how the determinant is solved, and energies and weighting coefficients determined. che-30042 Advanced QC lecture 3 14

Delocalisation energy in butadiene • The total electron energy in butadiene (Hayward p 169 fig. 8. 26) is: 2 x ( + 1. 62 ) + 2 x ( + 0. 62 ) = 4 + 4. 48 • Compare this with the electron energy of 2 ethene molecules (slide 8): 2 x 2 ( + ) = 4 + 4 • Because is negative (slide 6), this means that the electron energy in butadiene is lower than the 2 localised bonds in ethene by 0. 48. • This is called the delocalisation energy. che-30042 Advanced QC lecture 3 15

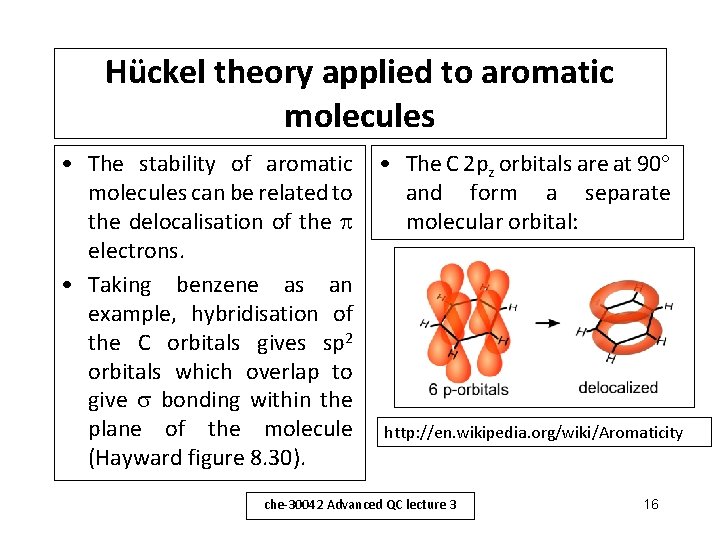
Hückel theory applied to aromatic molecules • The stability of aromatic molecules can be related to the delocalisation of the electrons. • Taking benzene as an example, hybridisation of the C orbitals gives sp 2 orbitals which overlap to give bonding within the plane of the molecule (Hayward figure 8. 30). • The C 2 pz orbitals are at 90 and form a separate molecular orbital: http: //en. wikipedia. org/wiki/Aromaticity che-30042 Advanced QC lecture 3 16

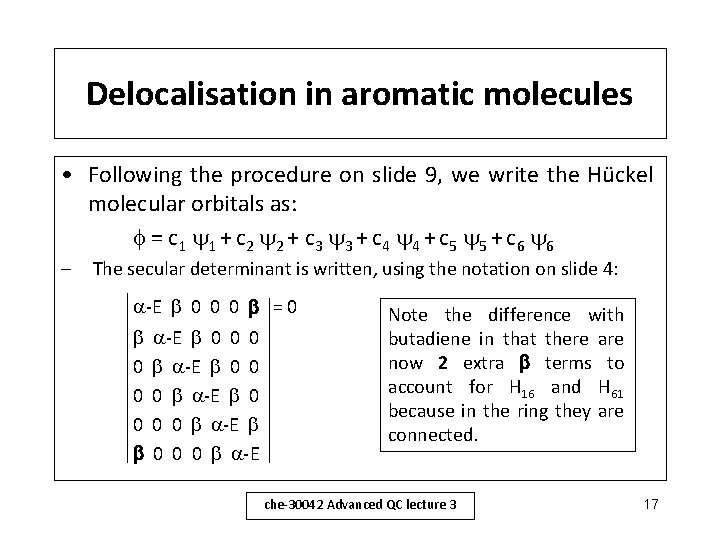
Delocalisation in aromatic molecules • Following the procedure on slide 9, we write the Hückel molecular orbitals as: = c 1 1 + c 2 2 + c 3 3 + c 4 4 + c 5 5 + c 6 6 – The secular determinant is written, using the notation on slide 4: -E 0 0 0 = 0 0 0 0 -E 0 0 0 -E Note the difference with butadiene in that there are now 2 extra terms to account for H 16 and H 61 because in the ring they are connected. che-30042 Advanced QC lecture 3 17

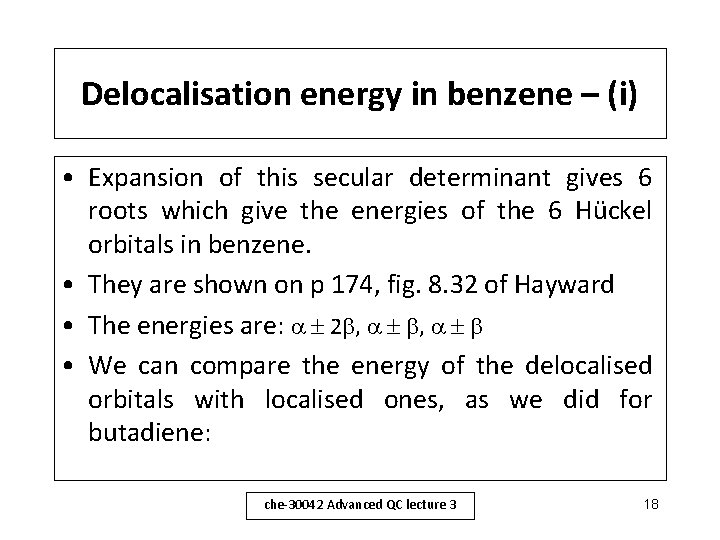
Delocalisation energy in benzene – (i) • Expansion of this secular determinant gives 6 roots which give the energies of the 6 Hückel orbitals in benzene. • They are shown on p 174, fig. 8. 32 of Hayward • The energies are: 2 , , • We can compare the energy of the delocalised orbitals with localised ones, as we did for butadiene: che-30042 Advanced QC lecture 3 18

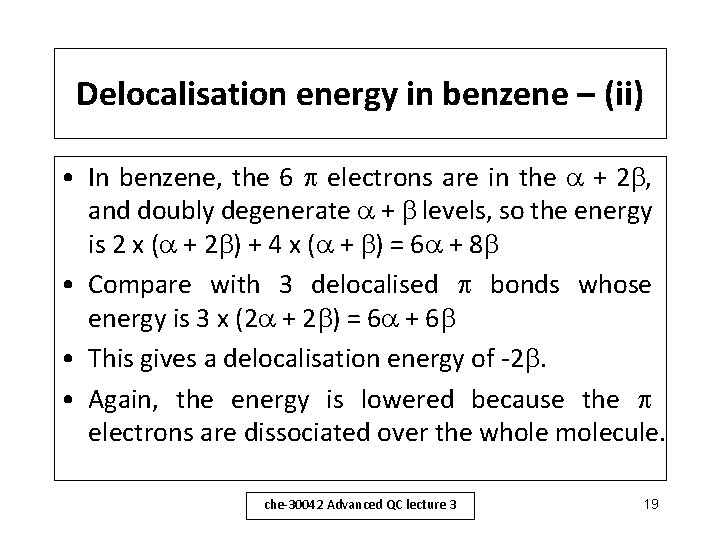
Delocalisation energy in benzene – (ii) • In benzene, the 6 electrons are in the + 2 , and doubly degenerate + levels, so the energy is 2 x ( + 2 ) + 4 x ( + ) = 6 + 8 • Compare with 3 delocalised bonds whose energy is 3 x (2 + 2 ) = 6 + 6 • This gives a delocalisation energy of -2. • Again, the energy is lowered because the electrons are dissociated over the whole molecule. che-30042 Advanced QC lecture 3 19

Lecture summary • The Hückel approximation has been introduced and applied to: – Simple hydrocarbons – Conjugated hydrocarbons – Aromatic hydrocarbons • Delocalisation energy has been defined and calculated for conjugated and aromatic hydrocarbons. che-30042 Advanced QC lecture 3 20