CHE20028 PHYSICAL INORGANIC CHEMISTRY QUANTUM CHEMISTRY LECTURE 3






- Slides: 31

CHE-20028: PHYSICAL & INORGANIC CHEMISTRY QUANTUM CHEMISTRY: LECTURE 3 Dr Rob Jackson Office: LJ 1. 16 r. a. jackson@keele. ac. uk http: //www. facebook. com/robjteaching

Use of the Schrödinger Equation in Chemistry • The Schrödinger equation introduced • What it means and what it does • Applications: – The particle in a box – The harmonic oscillator – The hydrogen atom CHE-20028 QC lecture 3 2

Learning objectives for lecture 3 • What the terms in the equation represent and what they do. • How the equation is applied to two general examples (particle in a box, harmonic oscillator) and one specific example (the hydrogen atom). CHE-20028 QC lecture 3 3

The Schrödinger Equation introduced • The equation relates the wave function to the energy of any ‘system’ (general system or specific atom or molecule). • In the last lecture we introduced the wave function, , and defined it as a function which contains all the available information about what it is describing, e. g. a 1 s electron in hydrogen. CHE-20028 QC lecture 3 4

What does the equation do? • It uses mathematical techniques to ‘operate’ on the wave function to give the energy of the system being studied, using mathematical functions called ‘operators’. • The energy is divided into potential and kinetic energy terms. CHE-20028 QC lecture 3 5

The equation itself • The simplest way to write the equation is: H = E • This means ‘an operator, H, acts on the wave function to give the energy E’. – Note – don’t read it like a normal algebraic equation! CHE-20028 QC lecture 3 6

More about the operator H Remember, energy is divided into potential and kinetic forms. H is called the Hamiltonian operator (after the Irish mathematician Hamilton). The Hamiltonian operator contains 2 terms, which are connected respectively with the kinetic and potential energies. William Rowan Hamilton (1805– 1865) CHE-20028 QC lecture 3 7

Obtaining the energy • So when H operates on the wave function we obtain the potential and kinetic energies of whatever is being described – e. g. a 1 s electron in hydrogen. • The PE will be associated with the attraction of the nucleus, and the KE with ‘movement’ of the electron. CHE-20028 QC lecture 3 8

What does H look like? • We can write H as: H = T + V, where ‘T’ is the kinetic energy operator, and ‘V’ is the potential energy operator. • The potential energy operator will depend on the system, but the kinetic energy operator has a common form: CHE-20028 QC lecture 3 9

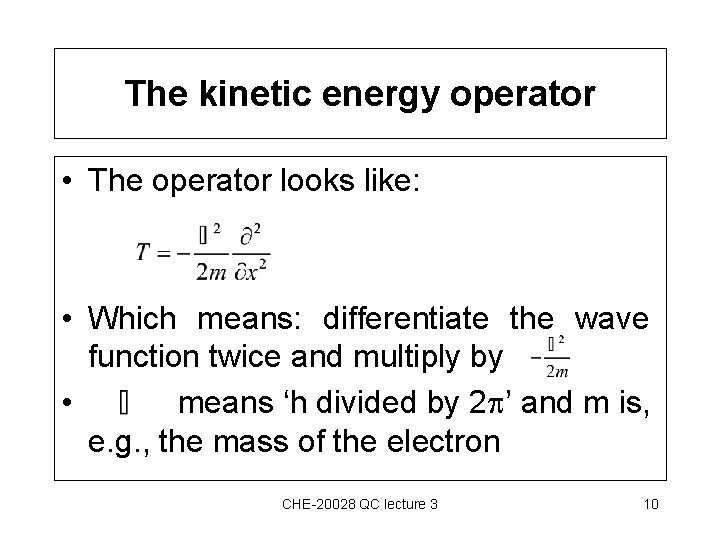
The kinetic energy operator • The operator looks like: • Which means: differentiate the wave function twice and multiply by • means ‘h divided by 2 ’ and m is, e. g. , the mass of the electron CHE-20028 QC lecture 3 10

Examples • Use of the Schrödinger equation is best illustrated through examples. • There are two types of example, generalised ones and specific ones, and we will consider three of these. • In each case we will work out the form of the Hamiltonian operator. CHE-20028 QC lecture 3 11

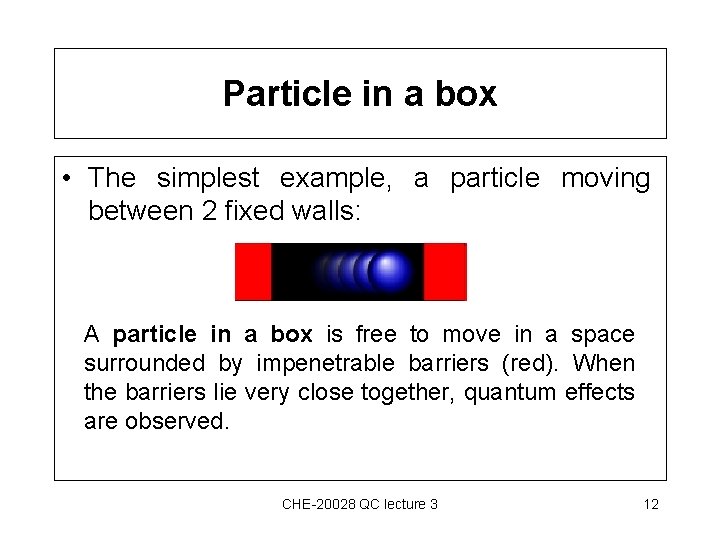
Particle in a box • The simplest example, a particle moving between 2 fixed walls: A particle in a box is free to move in a space surrounded by impenetrable barriers (red). When the barriers lie very close together, quantum effects are observed. CHE-20028 QC lecture 3 12

Particle in a box: relevance • 2 examples from Physics & Chemistry: • Semiconductor quantum wells, e. g. Ga. As between two layers of Alx. Ga 1 -x. As • electrons in conjugated molecules, e. g. butadiene, CH 2=CH-CH=CH 2 • References for more information will be given on the teaching pages. CHE-20028 QC lecture 3 13

Particle in a box – (i) • The derivation will be explained in the lecture, but the key equations are: (i) possible wavelengths are given by: = 2 L/n (L is length of the box), n = 1, 2, 3. . . See http: //www. chem. uci. edu/undergrad/applets/dwell. htm (ii) p = h/ = nh/2 L (from de Broglie equation) CHE-20028 QC lecture 3 14

Particle in a box – (ii) • (iii) the kinetic energy is related to p (momentum) by E = p 2/2 m • Permitted energies are therefore: En = n 2 h 2/8 m. L 2 (with n = 1, 2, 3. . . ) • So the particle is shown to only be able to have certain energies – this is an example of quantisation of energy. CHE-20028 QC lecture 3 15

The harmonic oscillator is a general example of solution of the Schrödinger equation with relevance in chemistry, especially in spectroscopy. ‘Classical’ examples include the pendulum in a clock, and the vibrating strings of a guitar or other stringed instrument. http: //en. wikipedia. org/wiki/Harmonic_oscillator CHE-20028 QC lecture 3 16

Example of a harmonic oscillator: a diatomic molecule H ------ H • If one of the atoms is displaced from its equilibrium position, it will experience a restoring force F, proportional to the displacement. F = - kx • where x is the displacement, and k is a force constant. • Note negative sign: force is in the opposite direction to the displacement CHE-20028 QC lecture 3 17

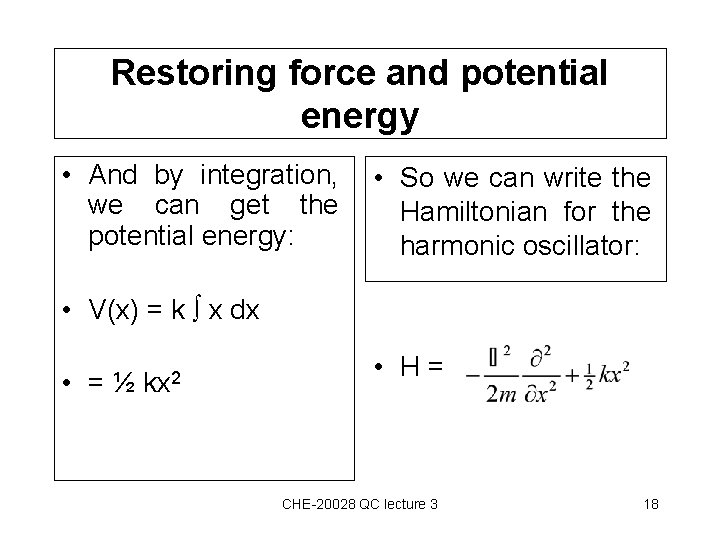
Restoring force and potential energy • And by integration, we can get the potential energy: • So we can write the Hamiltonian for the harmonic oscillator: • V(x) = k x dx • =½ kx 2 • H= CHE-20028 QC lecture 3 18

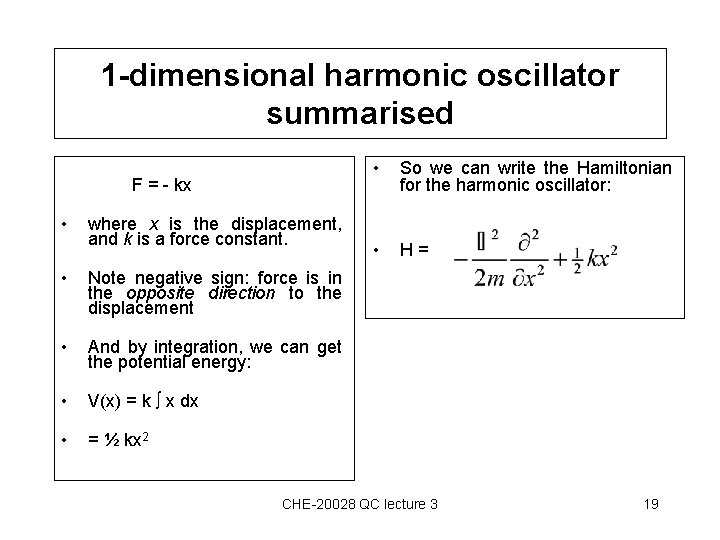
1 -dimensional harmonic oscillator summarised F = - kx • where x is the displacement, and k is a force constant. • Note negative sign: force is in the opposite direction to the displacement • And by integration, we can get the potential energy: • V(x) = k x dx • = ½ kx 2 • So we can write the Hamiltonian for the harmonic oscillator: • H= CHE-20028 QC lecture 3 19

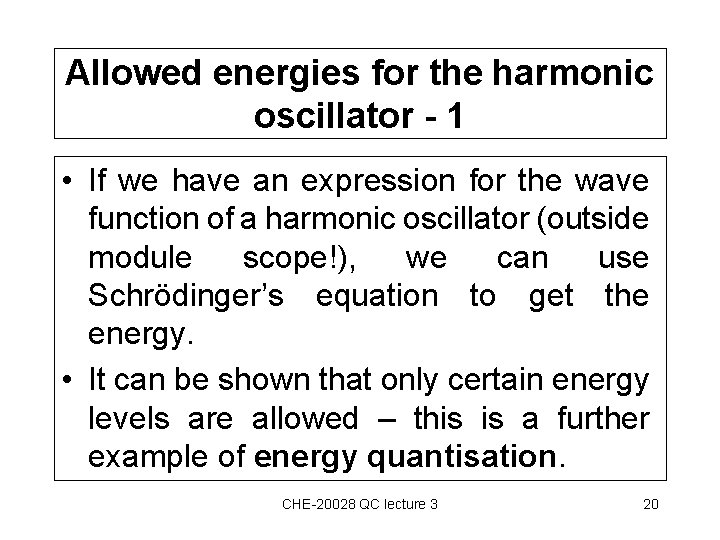
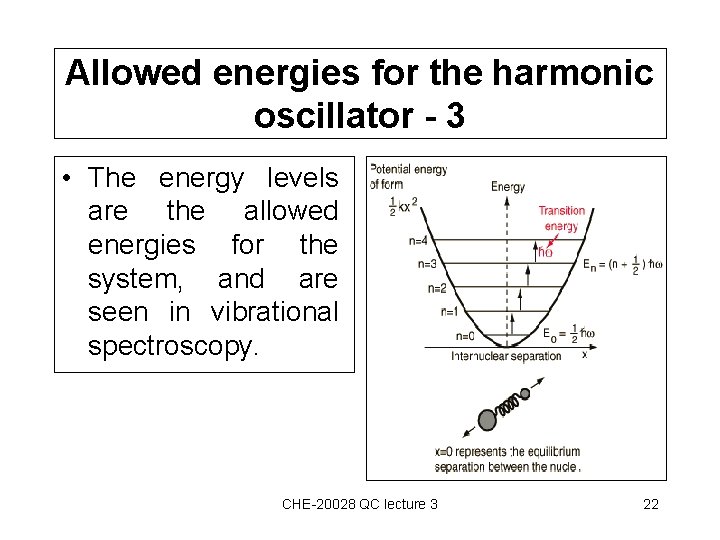
Allowed energies for the harmonic oscillator - 1 • If we have an expression for the wave function of a harmonic oscillator (outside module scope!), we can use Schrödinger’s equation to get the energy. • It can be shown that only certain energy levels are allowed – this is a further example of energy quantisation. CHE-20028 QC lecture 3 20

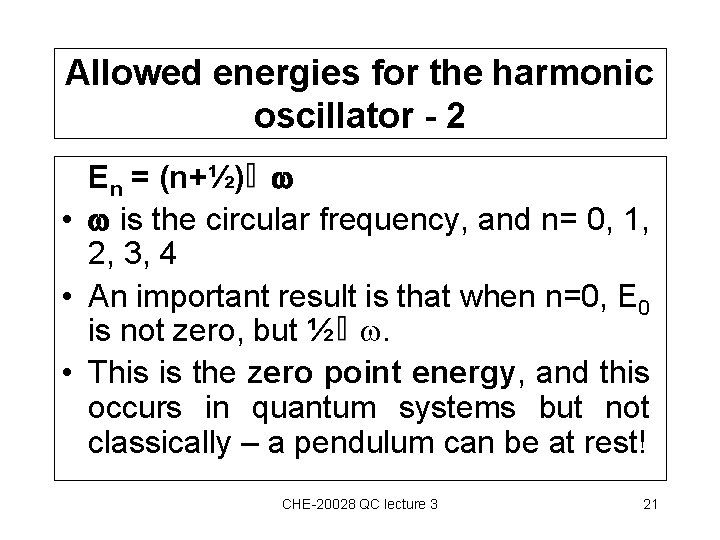
Allowed energies for the harmonic oscillator - 2 En = (n+½) • is the circular frequency, and n= 0, 1, 2, 3, 4 • An important result is that when n=0, E 0 is not zero, but ½ . • This is the zero point energy, and this occurs in quantum systems but not classically – a pendulum can be at rest! CHE-20028 QC lecture 3 21

Allowed energies for the harmonic oscillator - 3 • The energy levels are the allowed energies for the system, and are seen in vibrational spectroscopy. CHE-20028 QC lecture 3 22

Quantum and classical behaviour • Quantum behaviour (atomic systems) characterised by zero point energy, and quantisation of energy. • Classical behaviour (pendulum, swings etc) – systems can be at rest, and can accept energy continuously. • We now look at a specific chemical system and apply the same principles. CHE-20028 QC lecture 3 23

The hydrogen atom • Contains 1 proton and 1 electron. • So there will be: – potential energy of attraction between the electron and the proton – kinetic energy of the electron • (we ignore kinetic energy of the proton Born-Oppenheimer approximation). CHE-20028 QC lecture 3 24

The Hamiltonian operator for hydrogen - 1 • H will have 2 terms, for the electron kinetic energy and the proton-electron potential energy H = Te + Vne • Writing the terms in full, the most straightforward is Vne : Vne = -e 2/4 0 r (Coulomb’s Law) • Note negative sign - attraction CHE-20028 QC lecture 3 25

The Hamiltonian operator for hydrogen - 2 • The kinetic energy operator will be as before but in 3 dimensions: • A shorthand version of the term in brackets is 2. • We can now re-write Te and the full expression for H. CHE-20028 QC lecture 3 26

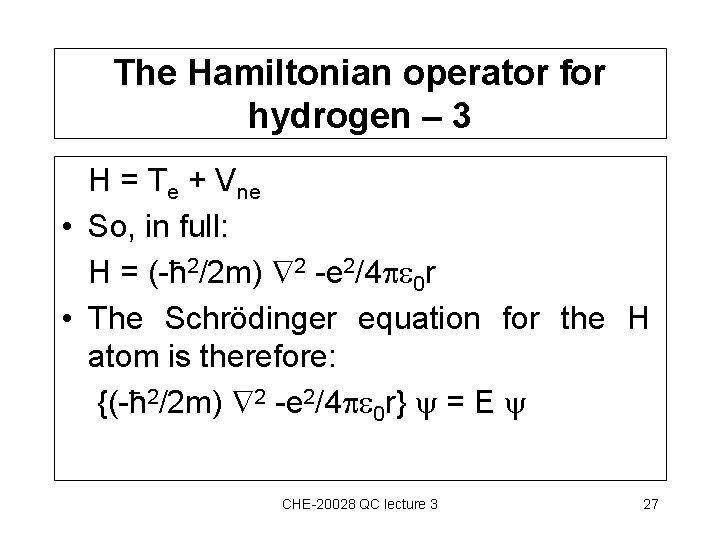
The Hamiltonian operator for hydrogen – 3 H = Te + Vne • So, in full: H = (-ħ 2/2 m) 2 -e 2/4 0 r • The Schrödinger equation for the H atom is therefore: {(-ħ 2/2 m) 2 -e 2/4 0 r} = E CHE-20028 QC lecture 3 27

Hamiltonians for molecules • When there are more nuclei and electrons the expressions for H get longer. • H 2+ and H 2 will be written as examples. • Note that H 2 has an electron repulsion term: +e 2/4 0 r CHE-20028 QC lecture 3 28

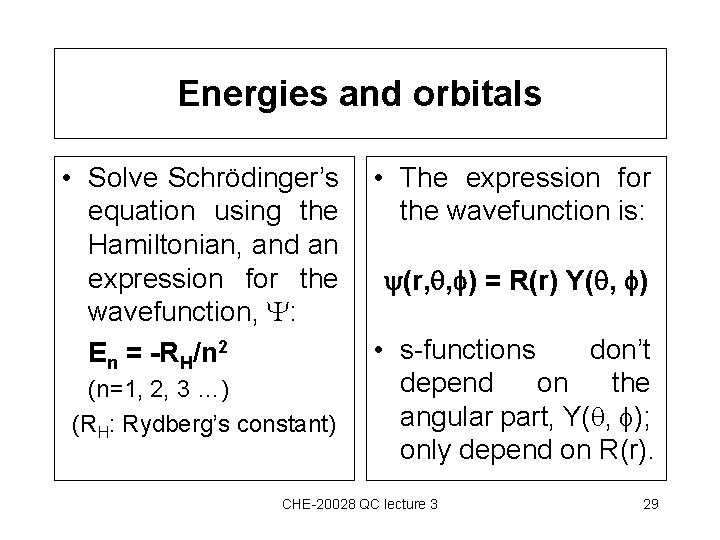
Energies and orbitals • Solve Schrödinger’s equation using the Hamiltonian, and an expression for the wavefunction, : En = -RH/n 2 (n=1, 2, 3 …) (RH: Rydberg’s constant) • The expression for the wavefunction is: (r, , ) = R(r) Y( , ) • s-functions don’t depend on the angular part, Y( , ); only depend on R(r). CHE-20028 QC lecture 3 29

Conclusions on lecture • The Schrödinger equation has been introduced (and the Hamiltonian operator defined), and applied to: – The particle in a box – The harmonic oscillator – The hydrogen atom • In all cases, the allowed energies are found to be quantised. CHE-20028 QC lecture 3 30

Final conclusions from the Quantum Chemistry lectures • Two important concepts have been introduced: wave-particle duality, and quantisation of energy. • In each case, experiments and examples have been given to illustrate the development of the concepts. CHE-20028 QC lecture 3 31
 Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes Organic vs inorganic chemistry
Organic vs inorganic chemistry Quantum physics vs quantum mechanics
Quantum physics vs quantum mechanics Quantum physics vs quantum mechanics
Quantum physics vs quantum mechanics Define pharmaceutical inorganic chemistry
Define pharmaceutical inorganic chemistry Organic vs inorganic molecules
Organic vs inorganic molecules Inert pair effect
Inert pair effect Is inorganic chemistry hard
Is inorganic chemistry hard 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Quantum mechanical model definition chemistry
Quantum mechanical model definition chemistry Quantum mechanics postulate
Quantum mechanics postulate Quantum chemistry
Quantum chemistry Physical science lecture notes
Physical science lecture notes Atmospheric chemistry lecture notes
Atmospheric chemistry lecture notes Smear layer adalah
Smear layer adalah Organic and inorganic compounds experiment
Organic and inorganic compounds experiment Heterotrophic plants
Heterotrophic plants Organic vs inorganic
Organic vs inorganic Organic chemistry chapter 1
Organic chemistry chapter 1 Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Organic versus inorganic compounds
Organic versus inorganic compounds Veins are often formed from hot water solutions
Veins are often formed from hot water solutions Organic and inorganic cofactors
Organic and inorganic cofactors What is the difference between organic and inorganic
What is the difference between organic and inorganic Six classes of enzymes
Six classes of enzymes Bottle method of emulsion
Bottle method of emulsion Organic and inorganic cofactors
Organic and inorganic cofactors Inorganic catalyst vs enzyme
Inorganic catalyst vs enzyme Phospoholipid
Phospoholipid Binomial nomenclature worksheet
Binomial nomenclature worksheet Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Inorganic mineral definition
Inorganic mineral definition