CHE30042 Inorganic Physical Solid State Chemistry Advanced Quantum

- Slides: 15

CHE-30042 Inorganic, Physical & Solid State Chemistry Advanced Quantum Chemistry: lecture 5 Rob Jackson LJ 1. 16, 01782 733042 r. a. jackson@keele. ac. uk www. facebook. com/robjteaching @robajackson

Lecture 5 contents 1. An example of molecular orbital calculations: HF • Showing how MO diagrams and calculation results relate to each other. 2. Using the Gaussian program: • • • Setting up the calculation Extracting the results Comparison with Coulson’s results from 1937. che-30042 Advanced QC lecture 5 2

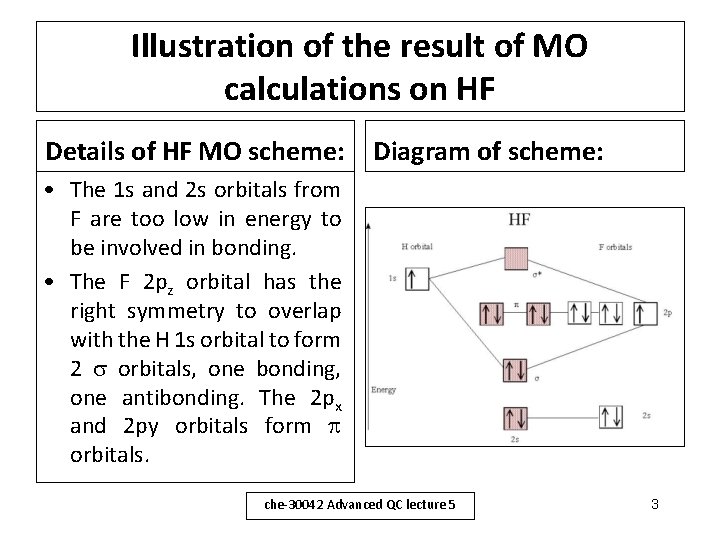
Illustration of the result of MO calculations on HF Details of HF MO scheme: Diagram of scheme: • The 1 s and 2 s orbitals from F are too low in energy to be involved in bonding. • The F 2 pz orbital has the right symmetry to overlap with the H 1 s orbital to form 2 orbitals, one bonding, one antibonding. The 2 px and 2 py orbitals form orbitals. che-30042 Advanced QC lecture 5 3

How are the MOs in HF written? • In lecture 4, we used expressions for the MOs: = c A A + c B B • If we carry out an SCF calculation on HF we get, for the orbitals: and ( ) = 0. 33 H, 1 s + 0. 94 F, 2 pz ( ) = 0. 94 H, 1 s + 0. 33 F, 2 pz – This shows that the bonding orbital is mainly from the F 2 pz, and the antibonding orbital is mainly from H 1 s. che-30042 Advanced QC lecture 5 4

Using the Gaussian program • Gaussian is a computer program for computational chemistry initially released in 1970 by John Pople and his research group at Carnegie-Mellon University, USA. It has been continuously updated since then. The name originates from Pople's use of Gaussian orbitals to speed up calculations compared to those using Slater-type orbitals, a choice made to improve performance considering the limited computing capacities of then available computer hardware. • We will use the Windows version of Gaussian 03, available on the PCs in the Faculty Lab. che-30042 Advanced QC lecture 5 5

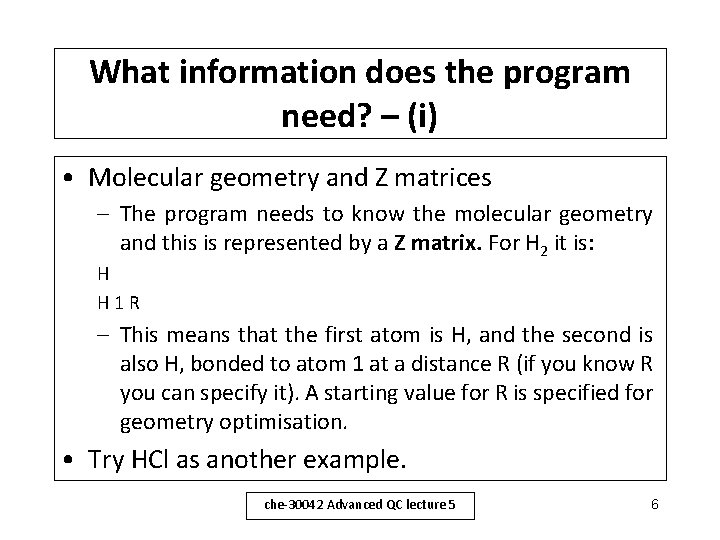
What information does the program need? – (i) • Molecular geometry and Z matrices – The program needs to know the molecular geometry and this is represented by a Z matrix. For H 2 it is: H H 1 R – This means that the first atom is H, and the second is also H, bonded to atom 1 at a distance R (if you know R you can specify it). A starting value for R is specified for geometry optimisation. • Try HCl as another example. che-30042 Advanced QC lecture 5 6

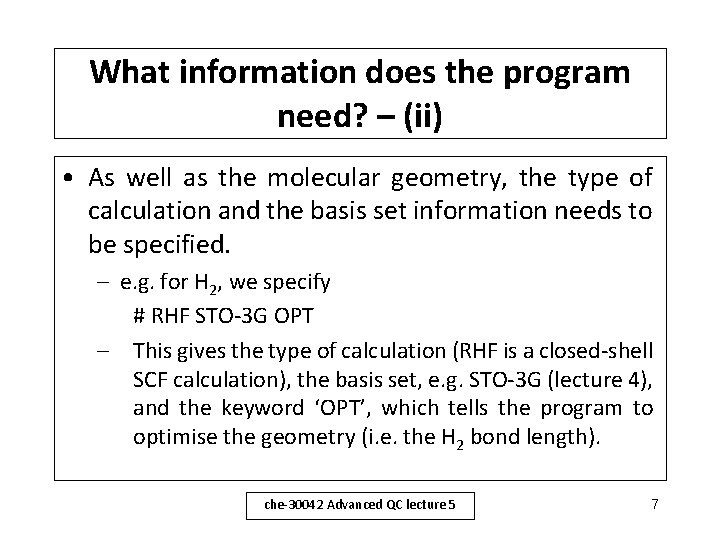
What information does the program need? – (ii) • As well as the molecular geometry, the type of calculation and the basis set information needs to be specified. – e. g. for H 2, we specify # RHF STO-3 G OPT – This gives the type of calculation (RHF is a closed-shell SCF calculation), the basis set, e. g. STO-3 G (lecture 4), and the keyword ‘OPT’, which tells the program to optimise the geometry (i. e. the H 2 bond length). che-30042 Advanced QC lecture 5 7

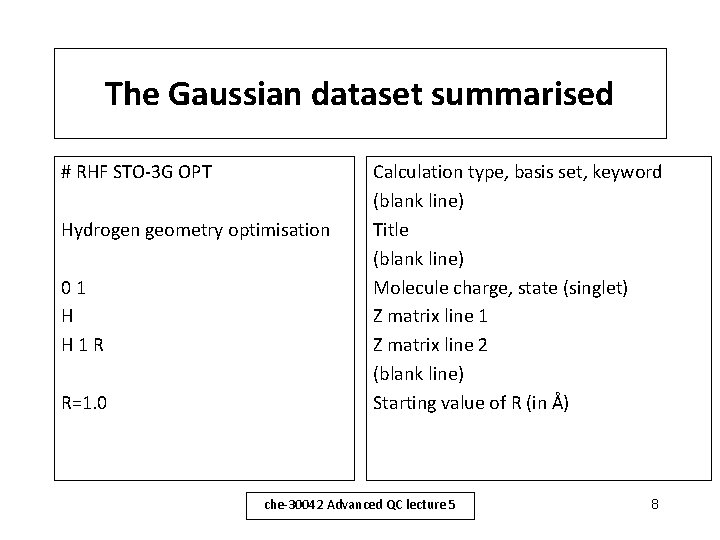
The Gaussian dataset summarised # RHF STO-3 G OPT Hydrogen geometry optimisation 01 H H 1 R R=1. 0 Calculation type, basis set, keyword (blank line) Title (blank line) Molecule charge, state (singlet) Z matrix line 1 Z matrix line 2 (blank line) Starting value of R (in Å) che-30042 Advanced QC lecture 5 8

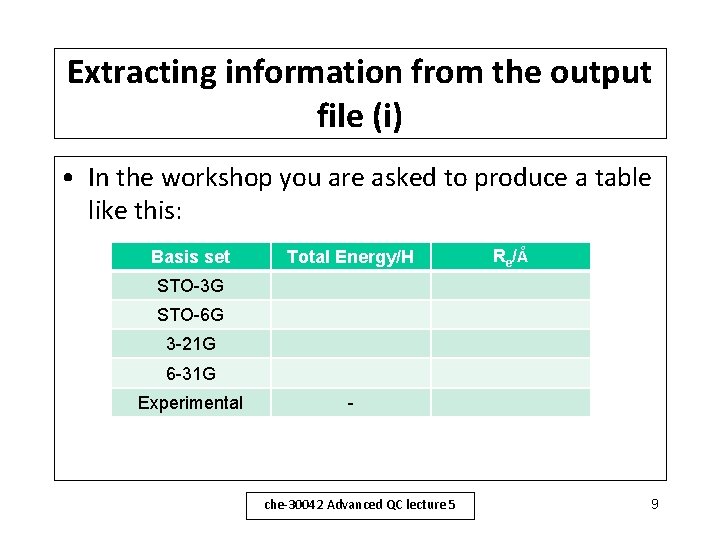
Extracting information from the output file (i) • In the workshop you are asked to produce a table like this: Basis set Total Energy/H Re/Å STO-3 G STO-6 G 3 -21 G 6 -31 G Experimental - che-30042 Advanced QC lecture 5 9

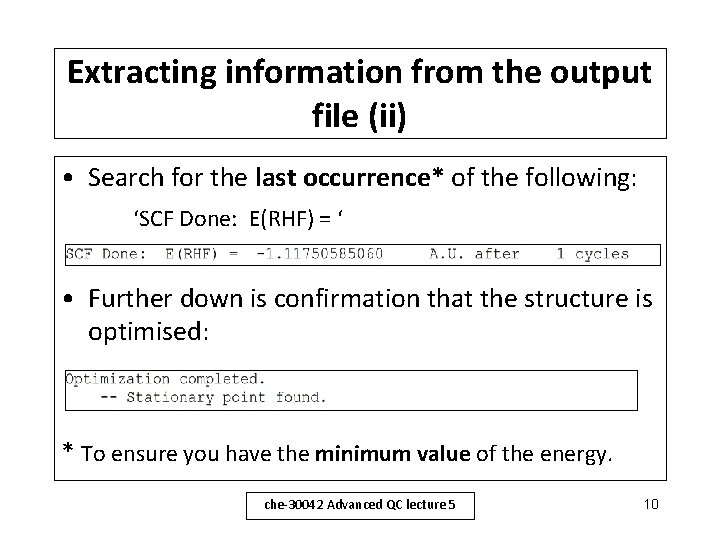
Extracting information from the output file (ii) • Search for the last occurrence* of the following: ‘SCF Done: E(RHF) = ‘ • Further down is confirmation that the structure is optimised: * To ensure you have the minimum value of the energy. che-30042 Advanced QC lecture 5 10

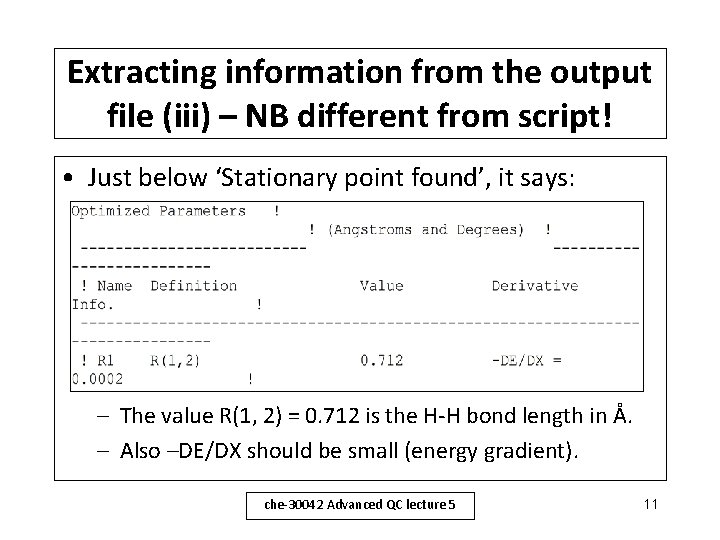
Extracting information from the output file (iii) – NB different from script! • Just below ‘Stationary point found’, it says: – The value R(1, 2) = 0. 712 is the H-H bond length in Å. – Also –DE/DX should be small (energy gradient). che-30042 Advanced QC lecture 5 11

Coulson: the first MO calculations (1937) • This paper (available from the KLE) describes Coulson’s first calculations on H 2. • Keele trivia point – he did his Ph. D with Lennard-Jones in Cambridge, who later became one of the first Principals of UCNS, which in turn became Keele. che-30042 Advanced QC lecture 5 12

Comparing results with those of Coulson • Coulson carried out one of the first MO calculations on H 2 in 1937 – Note: these calculations were probably largely done by hand! • He initially used a MO based on free atomic orbitals, 1 s. A + 1 s. B – This gives Re= 0. 850 Å, which becomes 0. 732 Å if the orbitals are scaled to account for the molecular environment. che-30042 Advanced QC lecture 5 13

Further comparison • See ‘The Gaussian Programs as a Teaching Tool: A Case Study on Molecular Hydrogen Calculations’ (copy on KLE). • It is seen that the minimal basis sets STO-3 G & STO-6 G give better results than Coulson’s free atom orbitals, but not as good as his scaled result. • This paper also shows you how to set up a calculation that closely emulates what Coulson did. Try it and see if you can get it to work! che-30042 Advanced QC lecture 5 14

Lecture summary • An example of MO calculations has been given for HF, relating MO diagrams to calculation results. • The Gaussian program has been introduced, and the procedure for running calculations and extracting results explained. • Results for molecular hydrogen have been compared with those obtained by Coulson, and the differences have been discussed. che-30042 Advanced QC lecture 5 15