CHAPTER 5 Water and Seawater The Water Planet













- Slides: 30

CHAPTER 5 Water and Seawater

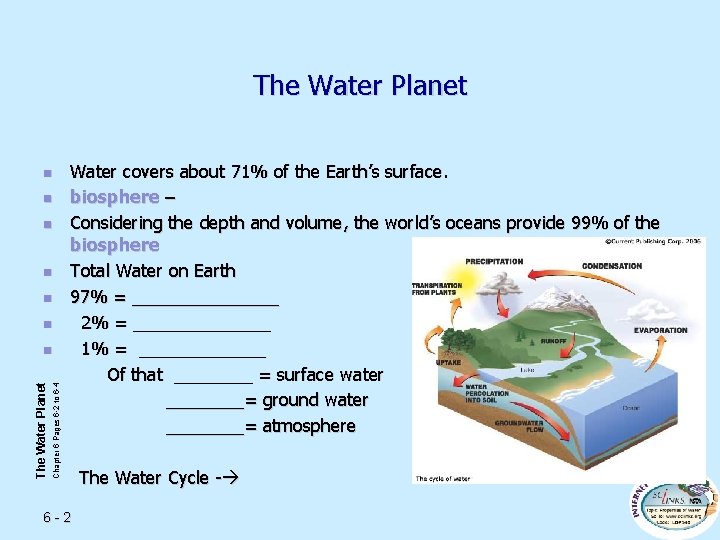
The Water Planet n n n Chapter 6 Pages 6 -2 to 6 -4 The Water Planet n Water covers about 71% of the Earth’s surface. biosphere – Considering the depth and volume, the world’s oceans provide 99% of the biosphere Total Water on Earth 97% = ________ 2% = _______ 1% = _______ Of that ____ = surface water ____= ground water ____= atmosphere 6 -2 The Water Cycle -

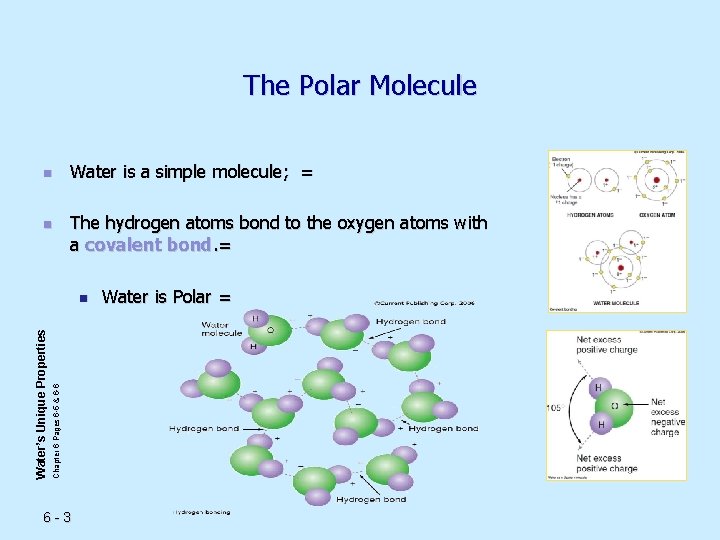
The Polar Molecule n n Water is a simple molecule; = The hydrogen atoms bond to the oxygen atoms with a covalent bond. = Chapter 6 Pages 6 -5 & 6 -6 Water’s Unique Properties n 6 -3 Water is Polar =

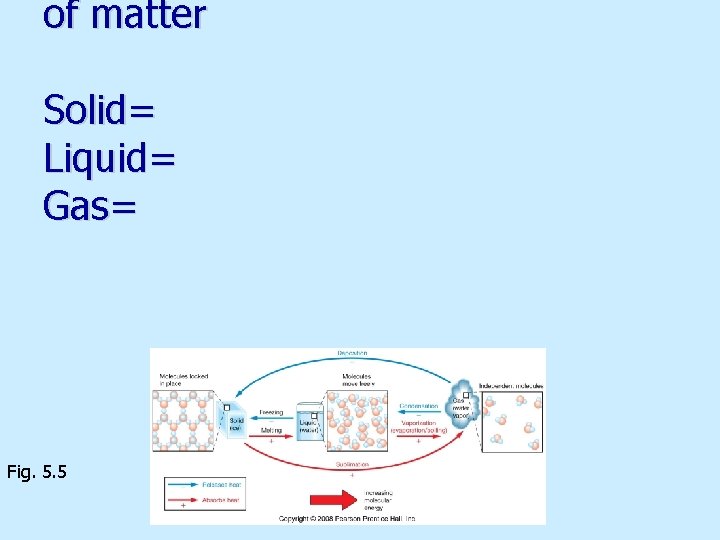
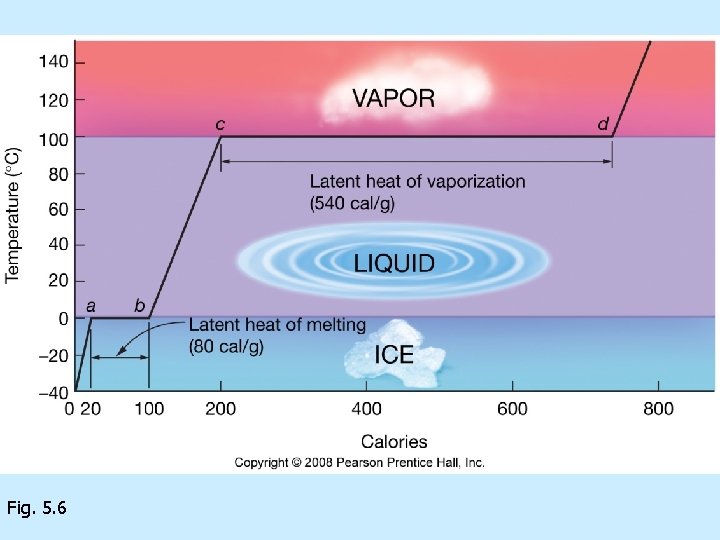
of matter Solid= Liquid= Gas= Fig. 5. 5

Changes of state due to adding or subtracting heat n n n Heat = Temperature= Calorie=

Unusual thermal properties of H 2 O n n n H 2 O has high boiling point = H 2 O has high freezing point = latent heats of n Vaporization/condensation= n Melting/freezing=

Fig. 5. 6

Unusual thermal properties of H 2 O n Water high heat capacity n Amount of heat required to raise the temperature of 1 gram of any substance 1 o C n Water can take in/lose lots of heat without changing temperature n Rocks low heat capacity n Rocks quickly change temperature as they gain/lose heat

Global thermostatic effects n Moderate temperature on Earth’s surface Equatorial oceans (hot) don’t boil n Polar oceans (cold) don’t freeze solid n n Marine effect n n Oceans moderate temperature changes day/night; different seasons Continental effect n Land areas have greater range of temperatures day/night and during different seasons

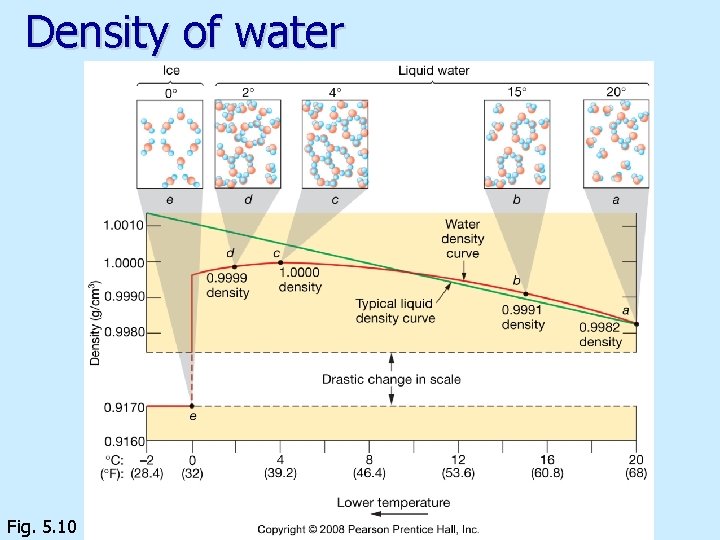
Density of water n n Water is the only substance that Density of water increases as temperature decreases Density of ice is less than density of water From 4 o. C to 0 o. C density of water decreases as temperature decreases


Density of water Fig. 5. 10

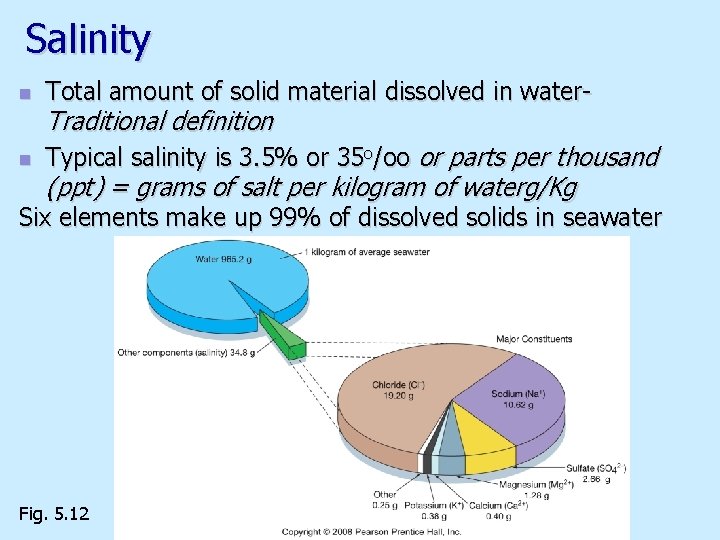
Salinity n Total amount of solid material dissolved in water- n Typical salinity is 3. 5% or 35 o/oo or parts per thousand Traditional definition (ppt) = grams of salt per kilogram of waterg/Kg Six elements make up 99% of dissolved solids in seawater Fig. 5. 12

The Colligative Properties of Seawater n Properties of Pure Water= 1. 2. 4. 5. n Colligative properties = n The Inorganic Chemistry of Water n 3. . Ability to conduct an electrical current n Chapter 6 Pages 6 -12 & 6 -13 an electrolyte. n n n A solution that can do this is called Decreased heat capacity. Takes less heat to raise the temperature of seawater. Raised boiling point. Seawater boils at a higher temperature than pure fresh water. Decreased freezing temperature. Seawater freezes at a lower temperature than fresh water due to increased salinity. n 6 - 14 Slowed evaporation. Seawater evaporates more slowly than fresh due to the attraction between ions and water molecules.

Salinity variations Open ocean salinity 33 to 38 o/oo n Coastal areas salinity varies more widely n n brackish conditions= n hypersaline conditions= n Salinity may vary with seasons (dry/rain)

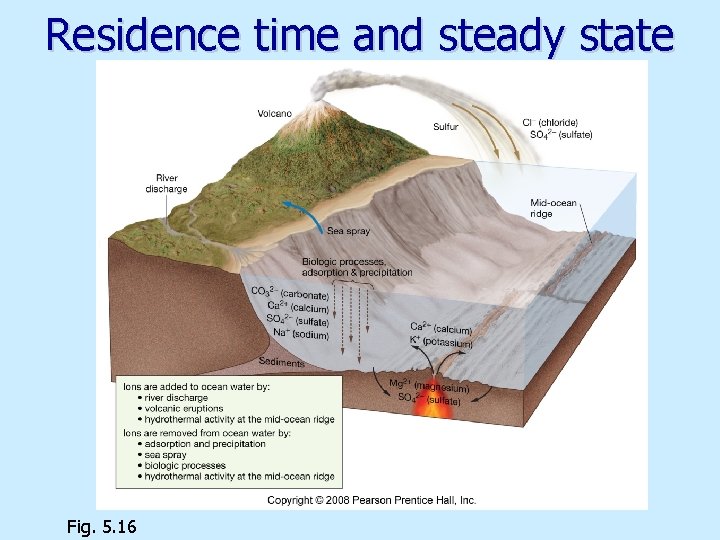
Why the Seas Are Salty n n The Inorganic Chemistry of Water n n Chapter 6 Page 6 -17 n Source of sea salts = minerals and chemicals on land eroding and dissolving into fresh water flowing into the ocean. Waves and surf appear to contribute by eroding coastal rock. Hydrothermal vents change seawater by adding some materials while removing others. Scientists believe these processes all counterbalance so the average salinity of seawater remains constant. The ocean is said to be in chemical equilibrium. 6 - 16

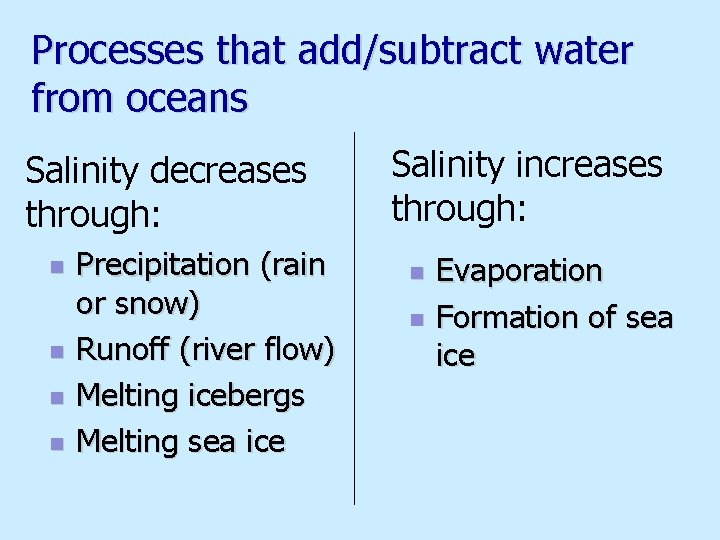
Processes that add/subtract water from oceans Salinity decreases through: n n Precipitation (rain or snow) Runoff (river flow) Melting icebergs Melting sea ice Salinity increases through: n n Evaporation Formation of sea ice

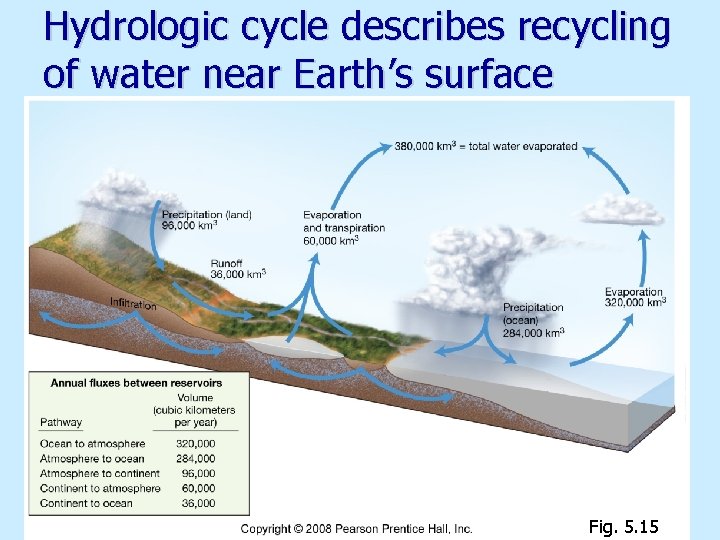
Hydrologic cycle describes recycling of water near Earth’s surface Fig. 5. 15

Processes that add/subtract dissolved substances Salinity increases through: n n River flow Volcanic eruptions Atmosphere Biologic interactions Salinity decreases through: n n n Salt spray Chemical reactions at seawater-sea floor interface Biologic interactions Evaporite formation Adsorption

Residence time and steady state Fig. 5. 16

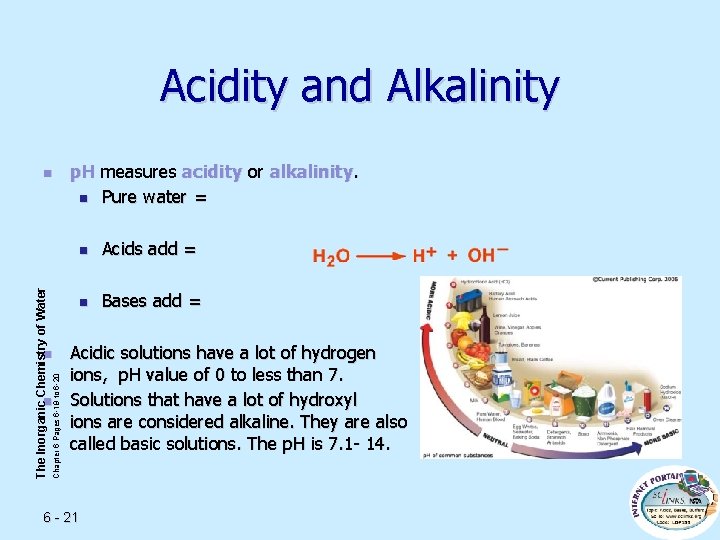
Acidity and Alkalinity The Inorganic Chemistry of Water n Chapter 6 Pages 6 -19 to 6 -20 n n p. H measures acidity or alkalinity. n Pure water = n Acids add = n Bases add = Acidic solutions have a lot of hydrogen ions, p. H value of 0 to less than 7. Solutions that have a lot of hydroxyl ions are considered alkaline. They are also called basic solutions. The p. H is 7. 1 - 14. 6 - 21

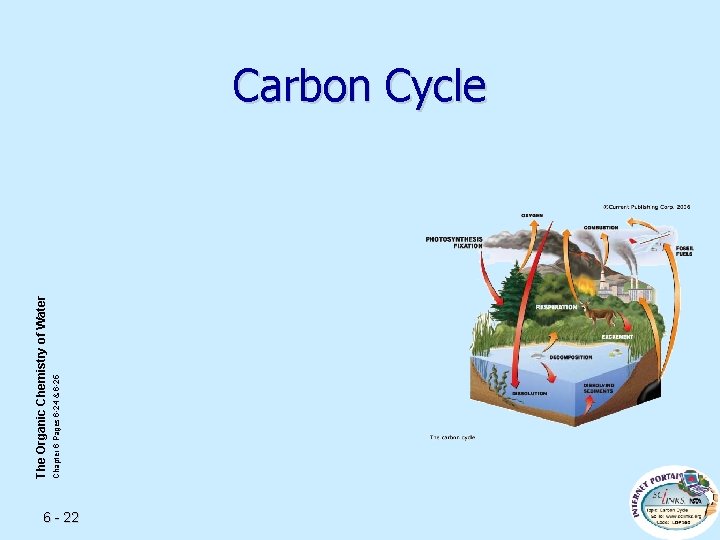
Chapter 6 Pages 6 -24 & 6 -25 The Organic Chemistry of Water Carbon Cycle 6 - 22

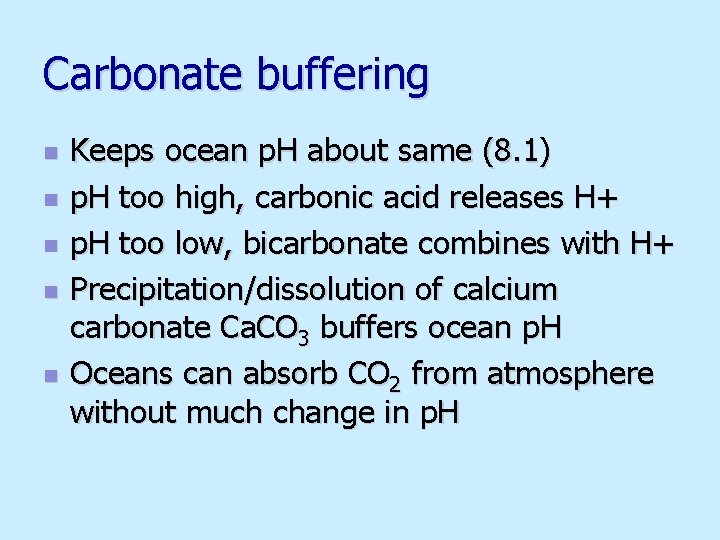
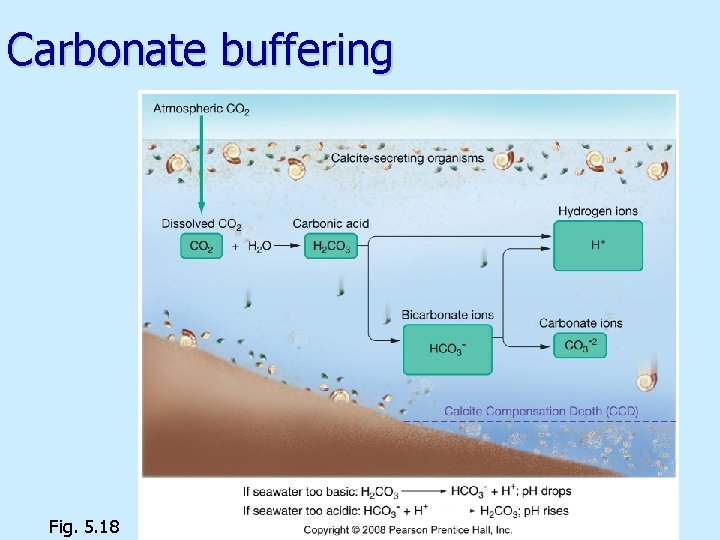
Carbonate buffering n n n Keeps ocean p. H about same (8. 1) p. H too high, carbonic acid releases H+ p. H too low, bicarbonate combines with H+ Precipitation/dissolution of calcium carbonate Ca. CO 3 buffers ocean p. H Oceans can absorb CO 2 from atmosphere without much change in p. H

Carbonate buffering Fig. 5. 18

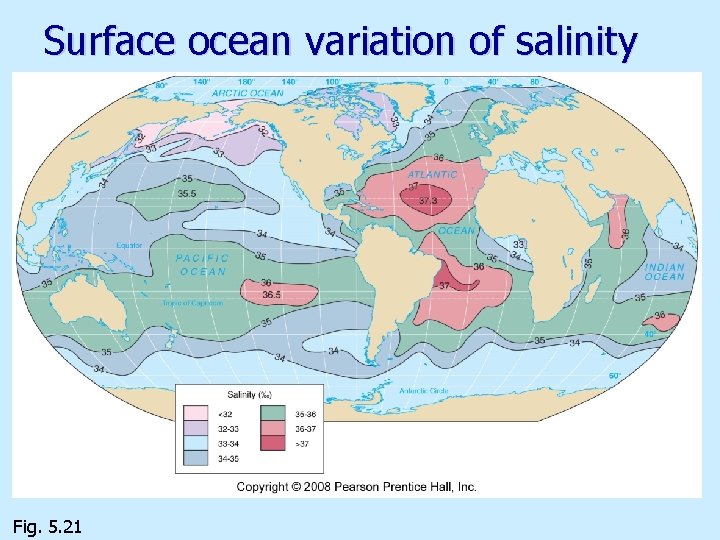
Surface ocean variation of salinity n Polar regions: salinity lower, lots of rain/snow and runoff n Mid-latitudes: salinity higher, high rate of evaporation Equator: salinity lower, lots of rain n Thus, salinity at surface varies primarily with latitude n

Surface ocean variation of salinity Fig. 5. 21

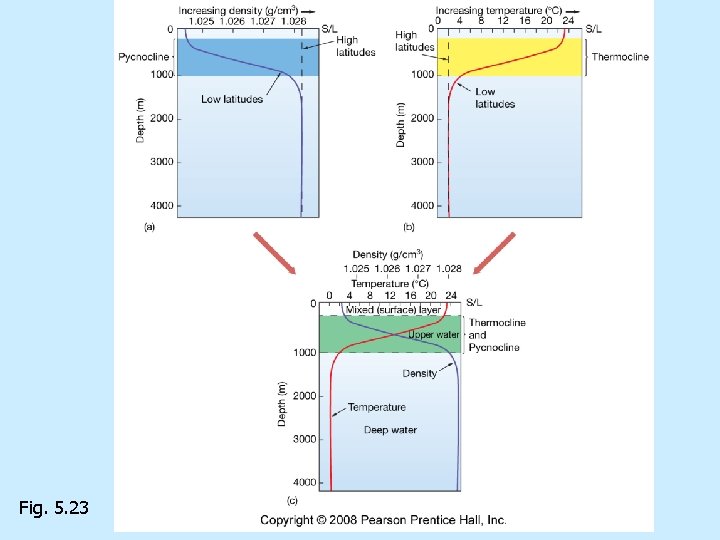
Density versus depth n Density differences cause a layered ocean Halocline= n Thermocline= n Pycnocline= n

Fig. 5. 23

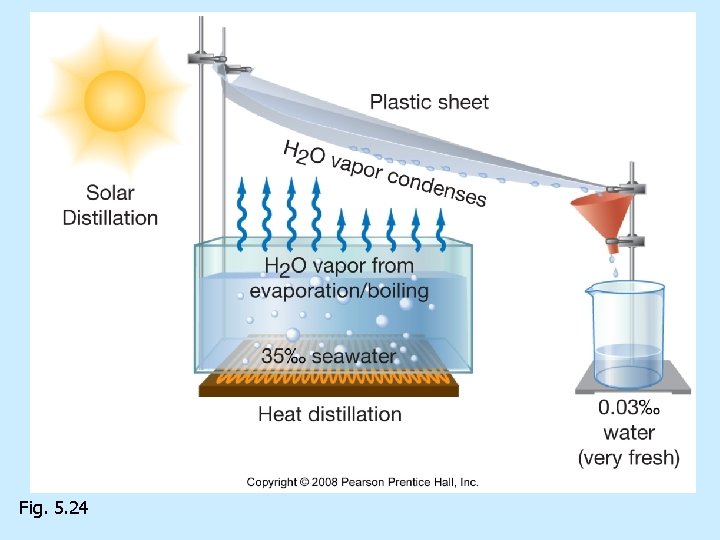
Desalination processes n n n Remove salt from seawater Distillation--most common process Electrolysis Reverse osmosis Freeze separation

Fig. 5. 24
 Water and water and water water
Water and water and water water Why is earth called the blue planet
Why is earth called the blue planet Boiling point of seawater
Boiling point of seawater Chemical properties of seawater
Chemical properties of seawater Bromine from sea water
Bromine from sea water Composition seawater
Composition seawater Characteristics of pure water
Characteristics of pure water Trace elements in seawater
Trace elements in seawater Sit on the planet chapter 1
Sit on the planet chapter 1 Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Thang điểm glasgow
Thang điểm glasgow Chúa sống lại
Chúa sống lại Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Phép trừ bù
Phép trừ bù Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ