Seawater Properties Seawater How did the water get





- Slides: 11

Seawater Properties

Seawater • How did the water get salty? 1. Chemical weathering of rocks on land 2. From the Earth’s interior

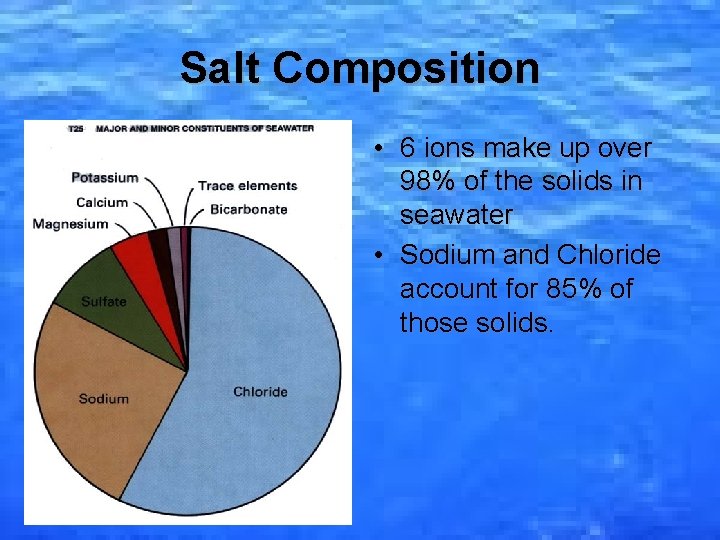
Salt Composition • 6 ions make up over 98% of the solids in seawater • Sodium and Chloride account for 85% of those solids.

Salinity • Is defined as: the total amount of salt dissolved in seawater. • Average salinity of the ocean is 35 ‰ or 35 parts per thousand. • Salinity is also expressed in grams/liter. • Changes in salinity are controlled by the addition (rain or snow) or removal (evaporation or freezing) of pure water.

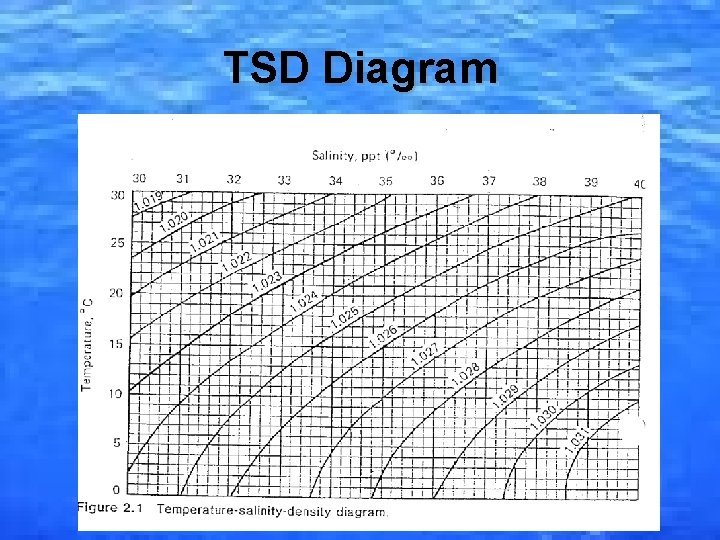
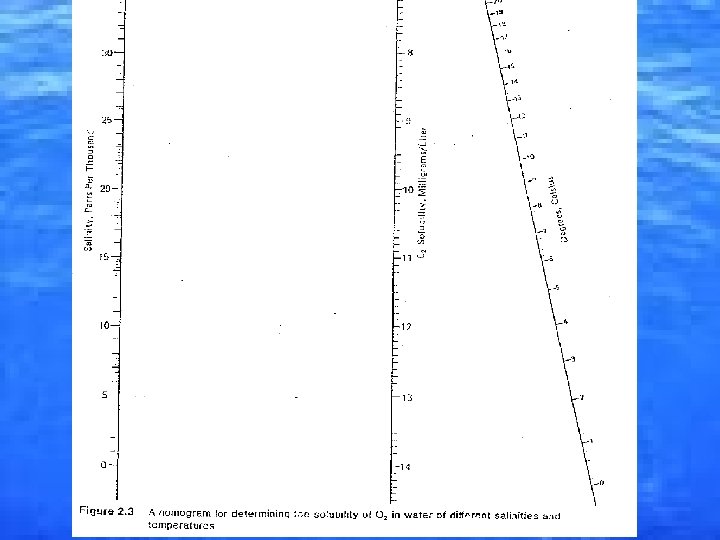
Temperature (T), Salinity (S) and Density (D) • Temperature varies greatly in the ocean (between -2 ºC to 30 ºC). This has a strong influence on density. • Density is mass/volume. It is measured in g/cm 3, g/ml or g/L. • A Hydrometer is the instrument used to determine density. • Salinity and temperature affect the density of water (as salinity and temperature decrease, density increases).

TSD Diagram

Ideal Aquarium Conditions • Temperature: 25 -26. 6ºC • Salinity: 35 parts per thousand (ppt), ‰ • Density: 1. 020 -1. 024 g/cm 3

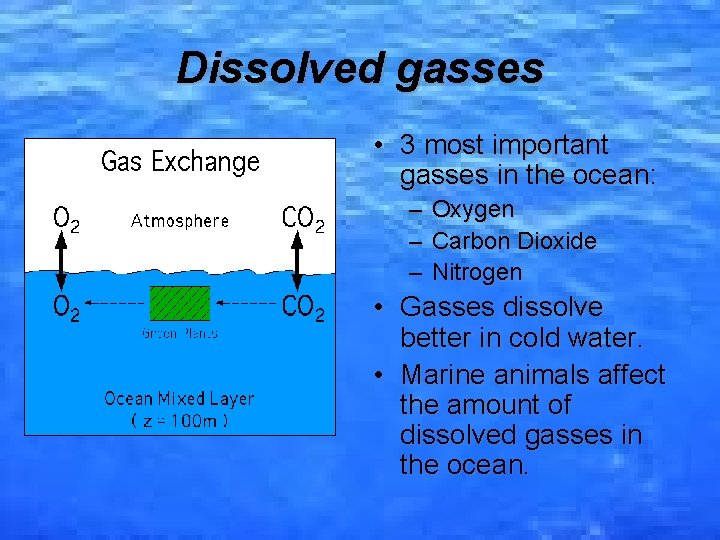
Dissolved gasses • 3 most important gasses in the ocean: – Oxygen – Carbon Dioxide – Nitrogen • Gasses dissolve better in cold water. • Marine animals affect the amount of dissolved gasses in the ocean.

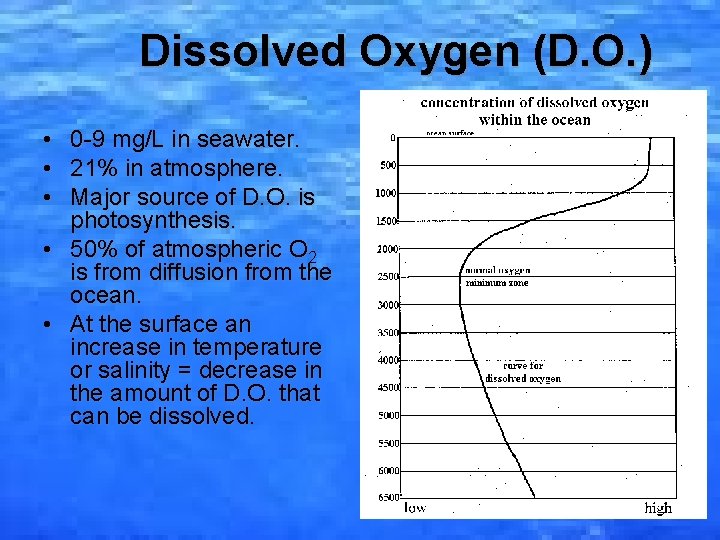
Dissolved Oxygen (D. O. ) • • • 0 -9 mg/L in seawater. 21% in atmosphere. Major source of D. O. is photosynthesis. • 50% of atmospheric O 2 is from diffusion from the ocean. • At the surface an increase in temperature or salinity = decrease in the amount of D. O. that can be dissolved

Transparency • Seawater is relatively transparent so sunlight can penetrate fairly deep into the ocean (which helps plants to grow). • Transparency depends on what is suspended and dissolved in the water. • Different colors of light penetrate to different depths of the ocean. Blue light penetrates the deepest, red light the least.