CHAPTER 5 WATER AND SEAWATER Basic chemistry Atomic

CHAPTER 5 WATER AND SEAWATER
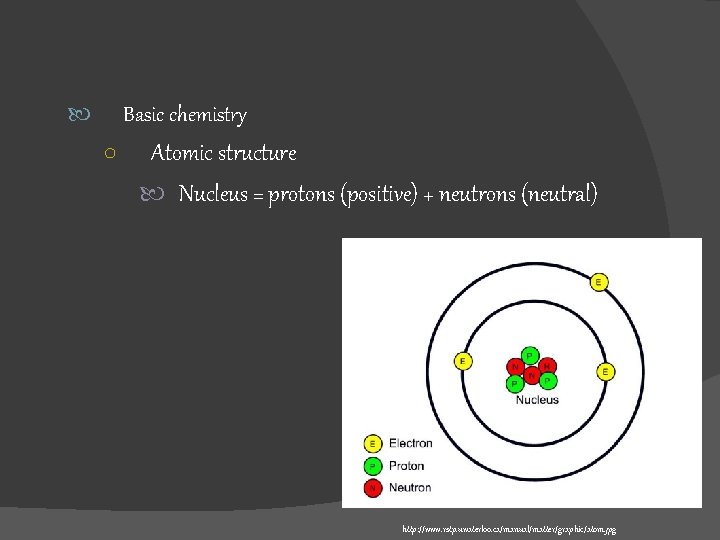
Basic chemistry ○ Atomic structure Nucleus = protons (positive) + neutrons (neutral) http: //www. rstp. uwaterloo. ca/manual/matter/graphic/atom. jpg
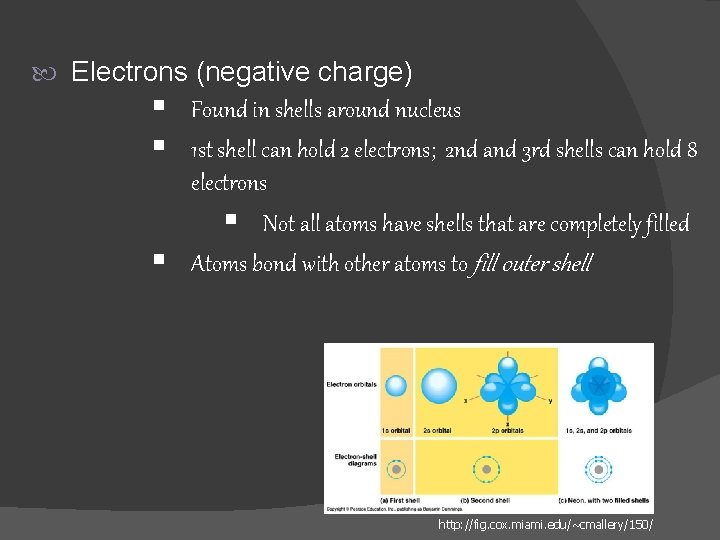
Electrons (negative charge) § Found in shells around nucleus § 1 st shell can hold 2 electrons; 2 nd and 3 rd shells can hold 8 § electrons § Not all atoms have shells that are completely filled Atoms bond with other atoms to fill outer shell http: //fig. cox. miami. edu/~cmallery/150/
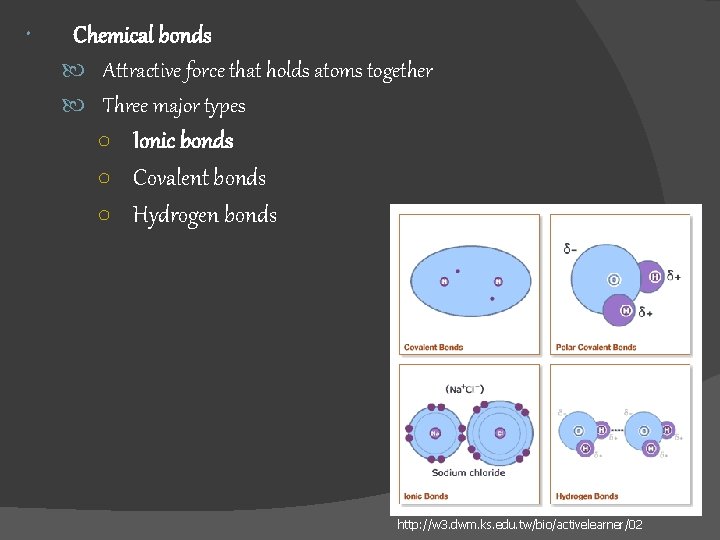
Chemical bonds Attractive force that holds atoms together Three major types ○ Ionic bonds ○ Covalent bonds ○ Hydrogen bonds http: //w 3. dwm. ks. edu. tw/bio/activelearner/02
http: //serc. carleton. edu/images/usingdata/nasaimages
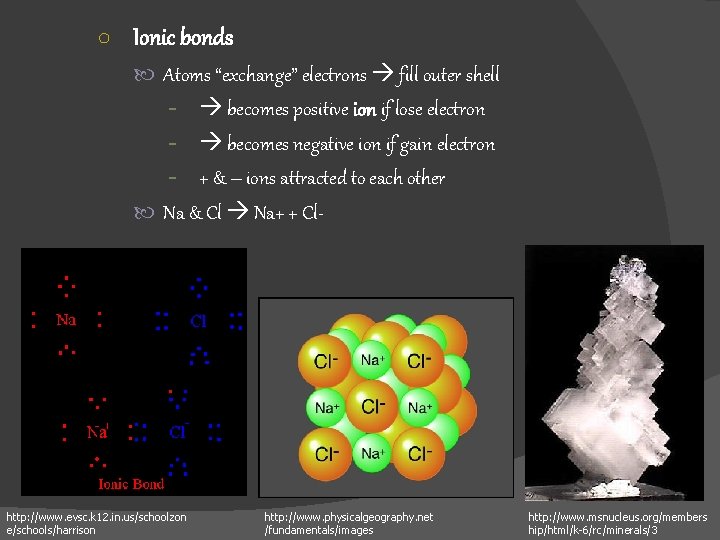
○ Ionic bonds Atoms “exchange” electrons fill outer shell - becomes positive ion if lose electron - becomes negative ion if gain electron - + & – ions attracted to each other Na & Cl Na+ + Cl- http: //www. evsc. k 12. in. us/schoolzon e/schools/harrison http: //www. physicalgeography. net /fundamentals/images http: //www. msnucleus. org/members hip/html/k-6/rc/minerals/3
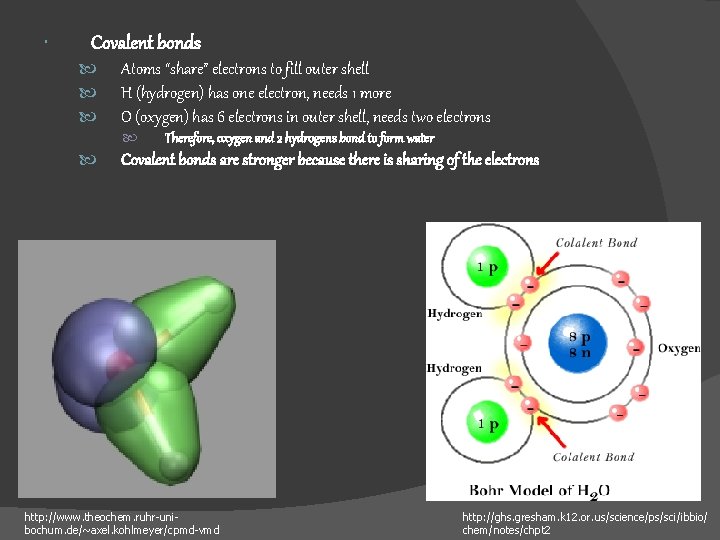
Covalent bonds Atoms “share” electrons to fill outer shell H (hydrogen) has one electron, needs 1 more O (oxygen) has 6 electrons in outer shell, needs two electrons Therefore, oxygen and 2 hydrogens bond to form water Covalent bonds are stronger because there is sharing of the electrons http: //www. theochem. ruhr-unibochum. de/~axel. kohlmeyer/cpmd-vmd http: //ghs. gresham. k 12. or. us/science/ps/sci/ibbio/ chem/notes/chpt 2
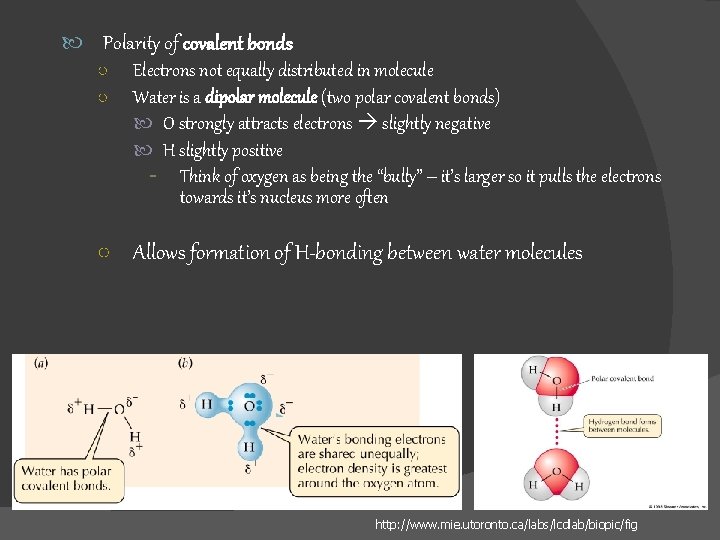
Polarity of covalent bonds ○ Electrons not equally distributed in molecule ○ Water is a dipolar molecule (two polar covalent bonds) O strongly attracts electrons slightly negative H slightly positive - Think of oxygen as being the “bully” – it’s larger so it pulls the electrons towards it’s nucleus more often ○ Allows formation of H-bonding between water molecules http: //www. mie. utoronto. ca/labs/lcdlab/biopic/fig

H 2 O molecule One hydrogen H and two oxygen O atoms bonded by sharing electrons Both H atoms on same side of O atom Dipolar covalent bond
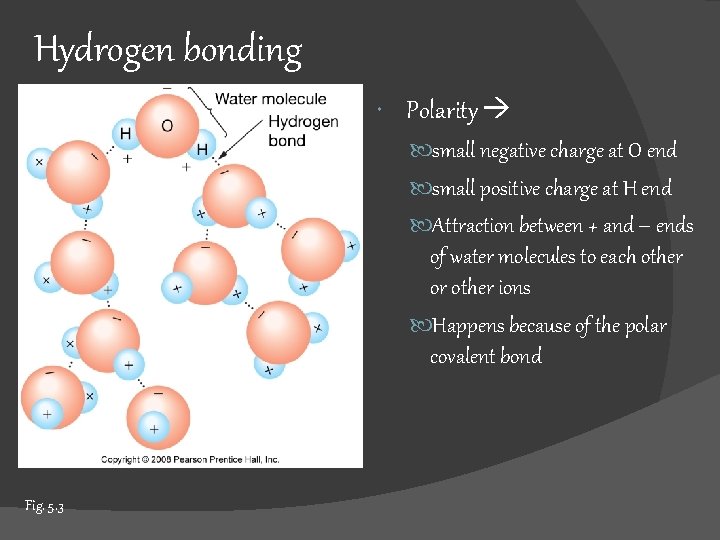
Hydrogen bonding Polarity small negative charge at O end small positive charge at H end Attraction between + and – ends of water molecules to each other or other ions Happens because of the polar covalent bond Fig. 5. 3
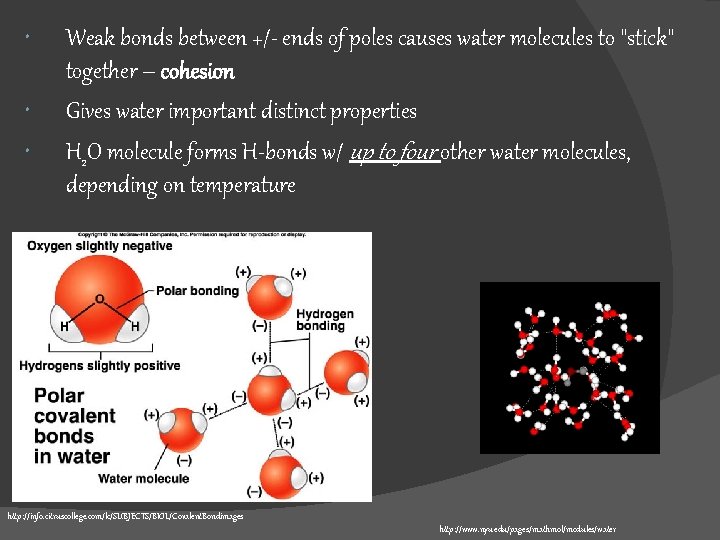
Weak bonds between +/- ends of poles causes water molecules to "stick" together – cohesion Gives water important distinct properties H 2 O molecule forms H-bonds w/ up to four other water molecules, depending on temperature http: //info. citruscollege. com/lc/SUBJECTS/BIOL/Covalent. Bondimages http: //www. nyu. edu/pages/mathmol/modules/water

Hydrogen bonding and water Hydrogen bonds are weaker than covalent bonds but still strong enough to result in unique properties of water Cohesion = sticks to other water molecules Adhesion = sticks to other types of molecules High surface tension http: //faculty. uca. edu/~benw/biol 1400 http: //ucsu. colorado. edu/~meiercl/photography

Hydrogen bonding and water H-bonds absorb red light, reflect blue light blue color High solubility of chemical compounds in water Solid, liquid, gas at Earth’s surface Unusual thermal properties Unusual density http: //www. pacific-promotion. com. fr/Phototek
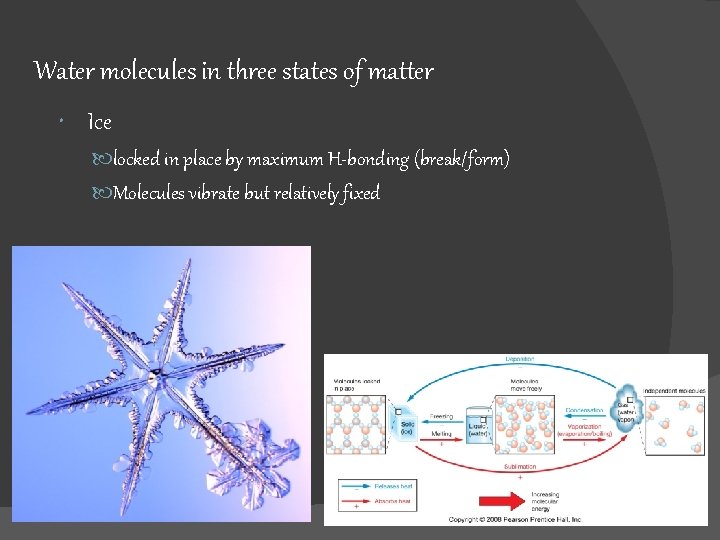
Water molecules in three states of matter Ice locked in place by maximum H-bonding (break/form) Molecules vibrate but relatively fixed Fig. 5. 5

Changes of state due to adding or subtracting heat Heat is energy of moving molecules calorie is amount of heat needed to raise the temperature of 1 gram of water by 1 o C Temperature is measurement of average kinetic energy http: //www. magnet. fsu. edu/education/tutorials/magnetacademy/superconductivity 101/images/superco nductivity-temperature. jpg
Unusual thermal properties of H 2 O has high boiling point H 2 O has high freezing point Most H 2 O is in liquid form of water on Earth’s surface VERY important for life http: //www. magnet. fsu. edu/education/tutorials/magnetacademy/superconductivity 101/images/superco nductivity-temperature. jpg
Unusual thermal properties of H 2 O High latent (hidden) heats of Vaporization/condensation Melting/freezing Evaporation – cools ocean surface H-bonds holding water together require extra energy (heat) to break bonds change states without change in temperature (a to b, c to d in figure)
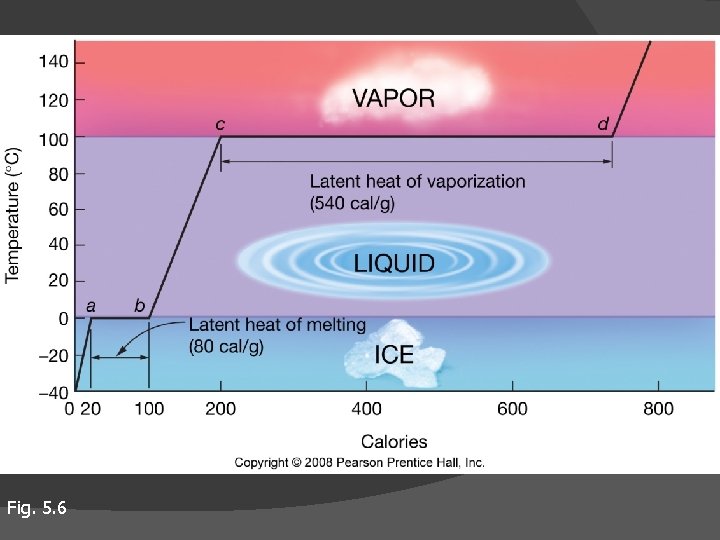
Fig. 5. 6

Water Phase Changes
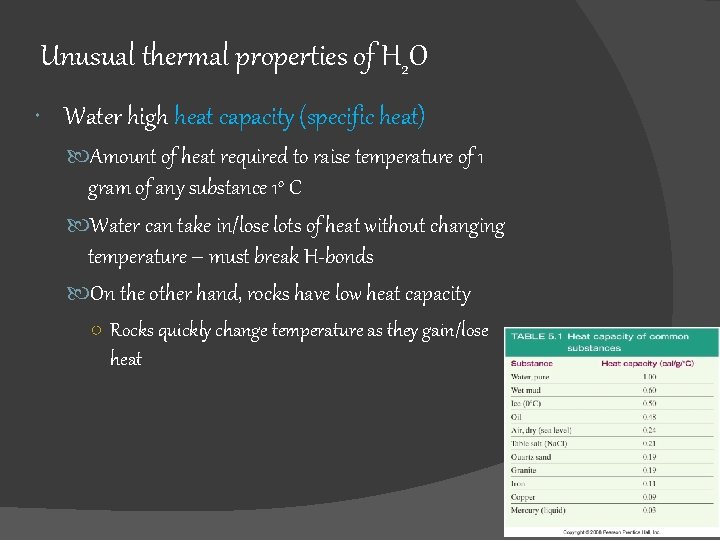
Unusual thermal properties of H 2 O Water high heat capacity (specific heat) Amount of heat required to raise temperature of 1 gram of any substance 1 o C Water can take in/lose lots of heat without changing temperature – must break H-bonds On the other hand, rocks have low heat capacity ○ Rocks quickly change temperature as they gain/lose heat
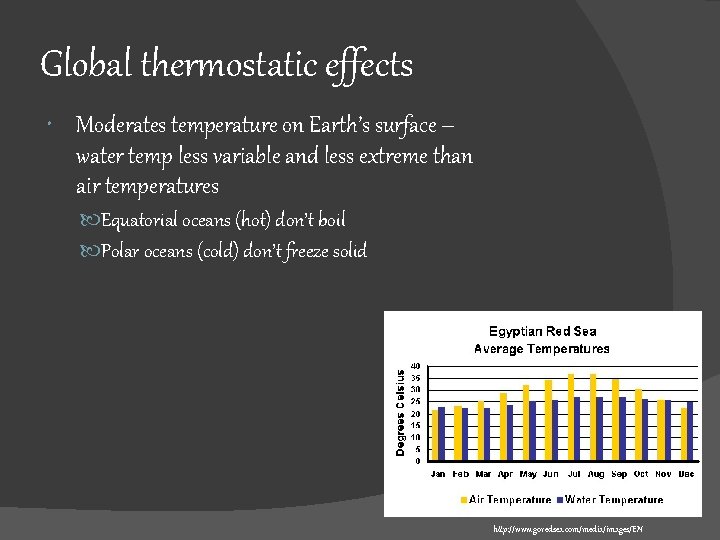
Global thermostatic effects Moderates temperature on Earth’s surface – water temp less variable and less extreme than air temperatures Equatorial oceans (hot) don’t boil Polar oceans (cold) don’t freeze solid http: //www. goredsea. com/media/images/EN
Global thermostatic effects Marine effect Oceans moderate temperature changes day/night; different seasons Continental effect Land areas have greater range of temperatures day/night and during different seasons Look at the differences between coastal Florida compared to Orlando

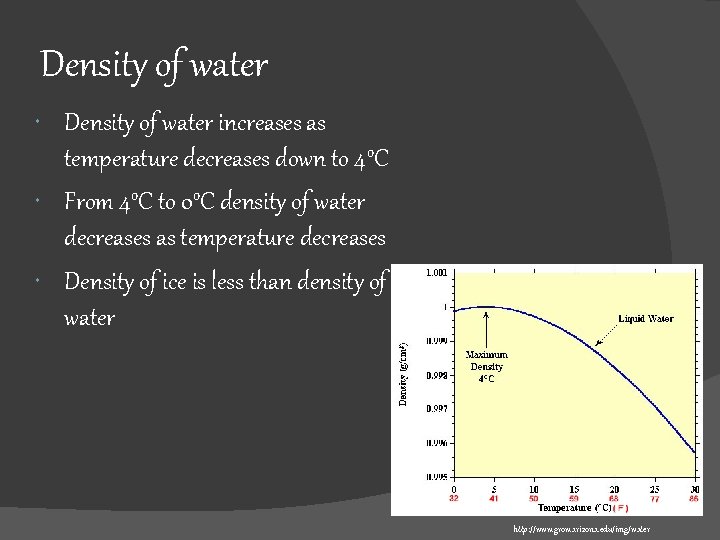
Density of water increases as temperature decreases down to 4 o. C From 4 o. C to 0 o. C density of water decreases as temperature decreases Density of ice is less than density of water http: //www. grow. arizona. edu/img/water
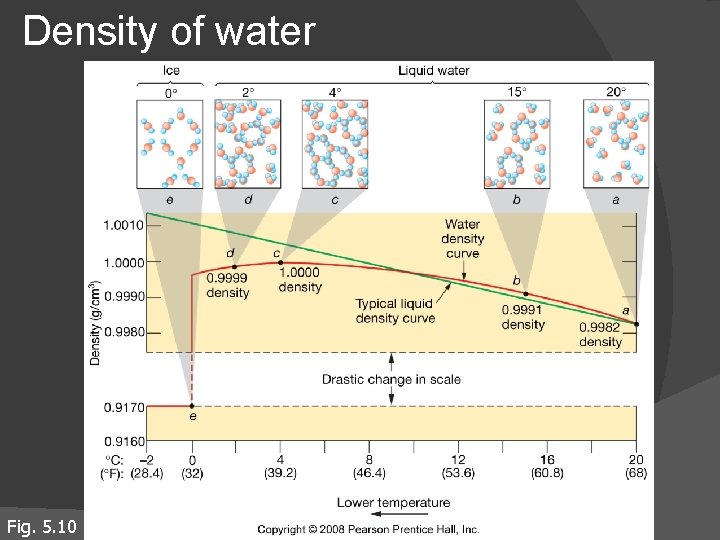
Density of water Fig. 5. 10

Density of water Dissolved solids reduce freezing point of water As water freezes, the crystalline structure “pushes out” much of the dissolved solids Creates icy “slush” and surrounding waters become saltier Putting salt on icy roads melts ice ○ Salt lowers freezing point of water on roads allowing it to remain liquid at colder temps http: //www. ibarron. net/users/robert/pics/2003/Norway/Oslo. Fjord 11. jpg

Table 5. 2

Water = Life • Summary: • Unique properties of water that make life possible • High heat capacity and specific heat • Moderates climates • Keeps equatorial regions from boiling and pole regions from freezing solid • High latent heat – when undergoing change of state, large amount of heat is absorbed or released • Sweat evaporating from your skin draws heat from your body, keep you cool • Ice is less dense than liquid water • Cohesion • Water moving up xylem in plants • Surface tension – allows plankton to stay near surface of water
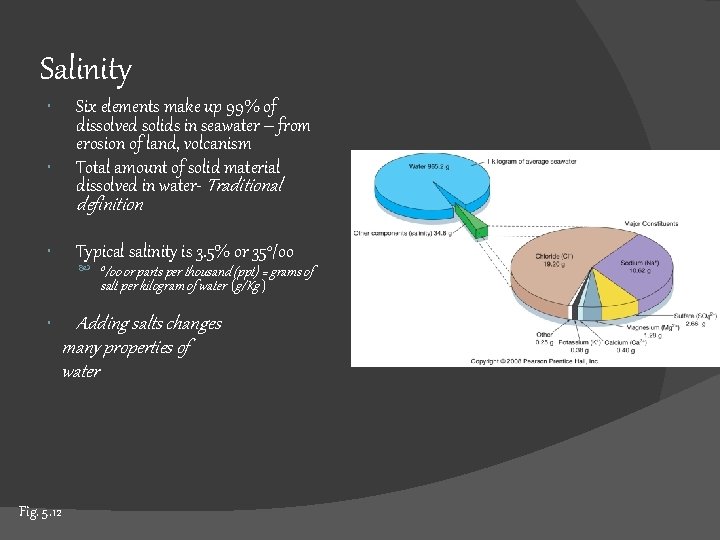
Salinity Six elements make up 99% of dissolved solids in seawater – from erosion of land, volcanism Total amount of solid material dissolved in water- Traditional definition Typical salinity is 3. 5% or 35 o/oo or parts per thousand (ppt) = grams of salt per kilogram of water (g/Kg ) Adding salts changes many properties of water Fig. 5. 12

http: //static. howstuffworks. com/gif/beer-hydrometer. jpg Measuring salinity Evaporation Chemical analysis - titration Principle of constant proportions Major dissolved constituents in same proportion regardless of total salinity Measure amount of halogens (Cl, Br, I, F) (chlorinity) Salinity = 1. 80655 * Chlorinity (ppt) Specific gravity (1. 028 g/ml) Hydrometer Electrical conductivity Salinometer http: //iodeweb 5. vliz. be/oceanteacher/resources/other/Anderson. Book/images/salmeter. jpg
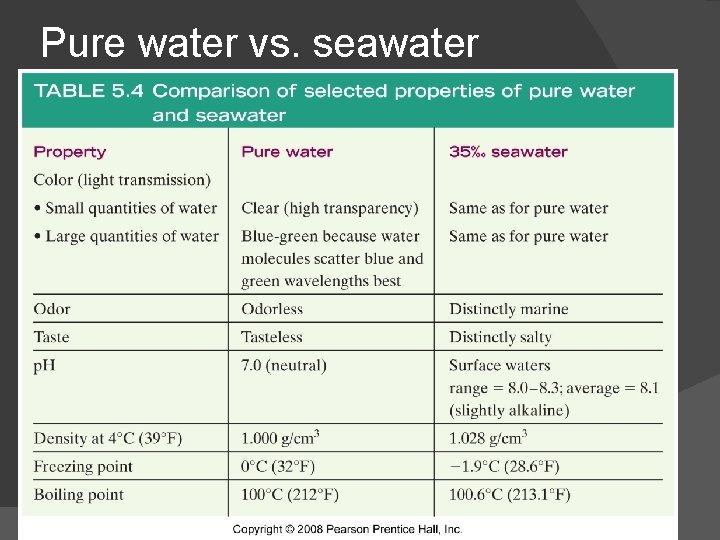
Pure water vs. seawater

http: //farm 1. static. flickr. com/58/186020843_205 a 03 e 35 e. jpg? v=0 Salinity variations Open ocean salinity 33 to 38 o/oo However, coastal areas salinity varies more widely Influx of freshwater lowers salinity or creates brackish conditions Greater rate of evaporation raises salinity or creates hypersaline conditions Salinity may vary with seasons (dry/rain) Salt flats in Puerto Rico http: //www. caborojopr. com/images/cabo-rojo-salt-flats-las-salinas-puerto-rico-55. jpg
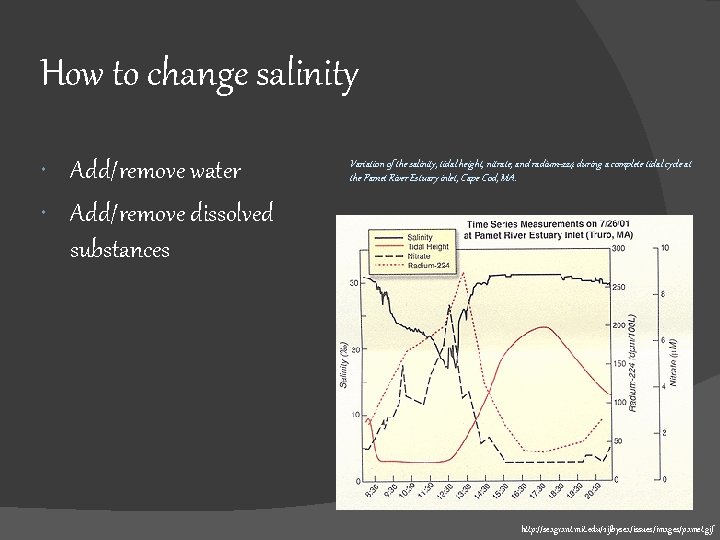
How to change salinity Add/remove water Add/remove dissolved substances Variation of the salinity, tidal height, nitrate, and radium-224 during a complete tidal cycle at the Pamet River Estuary inlet, Cape Cod, MA. http: //seagrant. mit. edu/2 ifbysea/issues/images/pamet. gif

Processes that add/subtract water from oceans Salinity decreases through: Salinity increases through: Precipitation (rain or snow) Runoff (river flow) Melting icebergs Melting sea ice Floating in the Dead Sea Evaporation Formation of sea ice
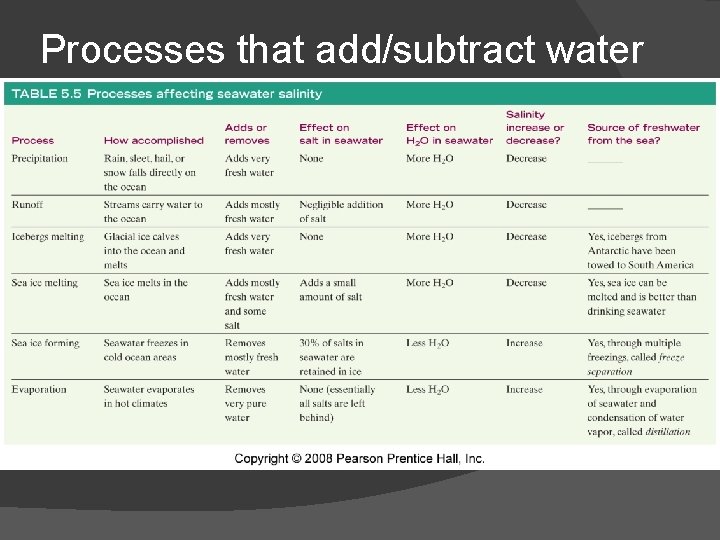
Processes that add/subtract water
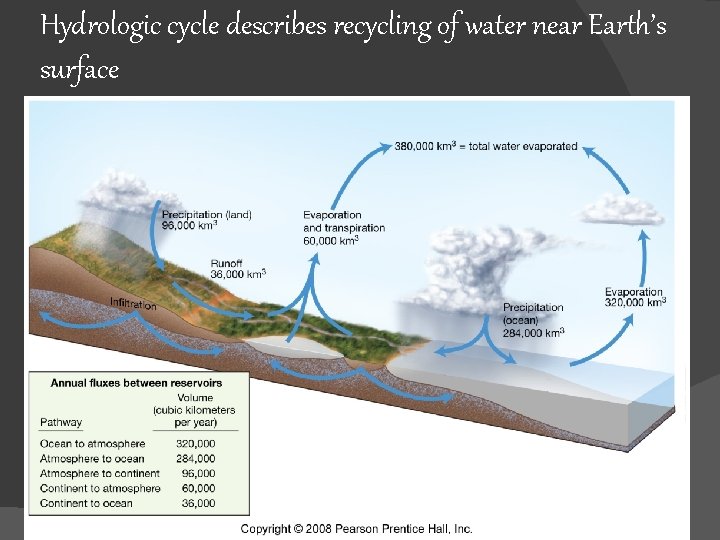
Hydrologic cycle describes recycling of water near Earth’s surface Fig. 5. 15

Processes that add/subtract dissolved substances Salinity increases through: Salinity decreases through: River flow Volcanic eruptions Atmosphere Biologic interactions Salt spray Chemical reactions at seawater-sea floor interface Biologic interactions Evaporite formation Adsorption Physical attachment to sinking clay or biological particles

Residence time Average length of time a substance remains dissolved in seawater Ions with long residence time are in high concentration in seawater (Na +, Cl-) Ions with short residence time are in low concentration in seawater percipitate out (K+, Ca 2+ ) Steady state condition
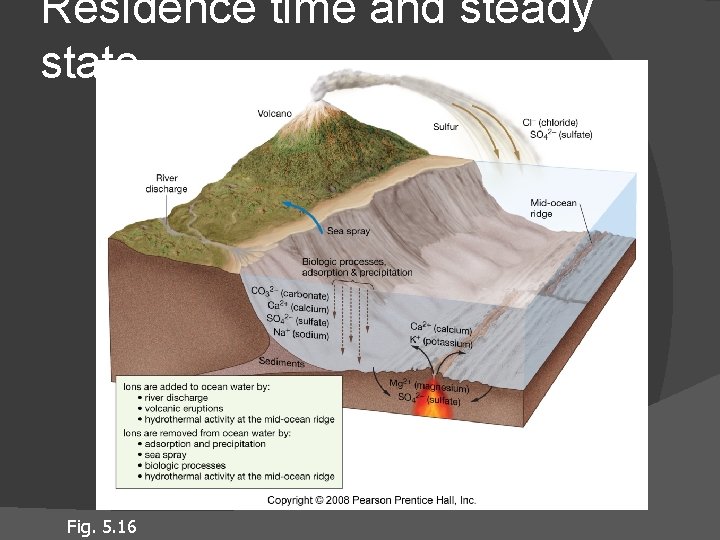
Residence time and steady state Fig. 5. 16
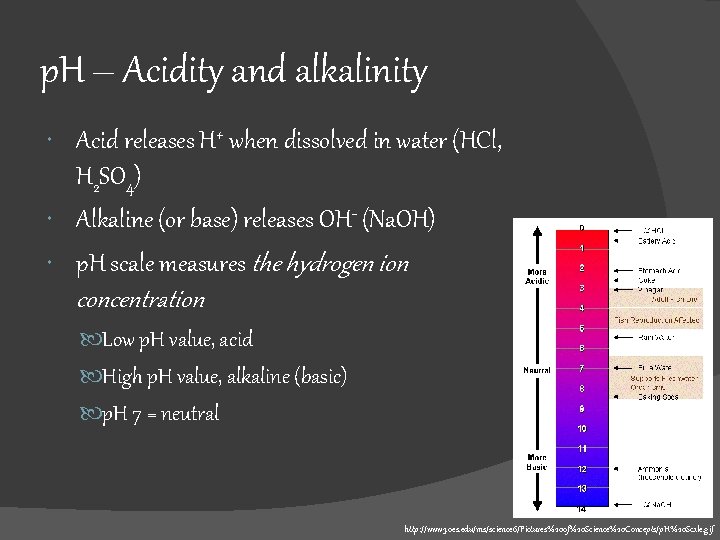
p. H – Acidity and alkalinity Acid releases H+ when dissolved in water (HCl, H 2 SO 4) Alkaline (or base) releases OH- (Na. OH) p. H scale measures the hydrogen ion concentration Low p. H value, acid High p. H value, alkaline (basic) p. H 7 = neutral http: //www 3. oes. edu/ms/science 6/Pictures%20 of%20 Science%20 Concepts/p. H%20 Scale. gif
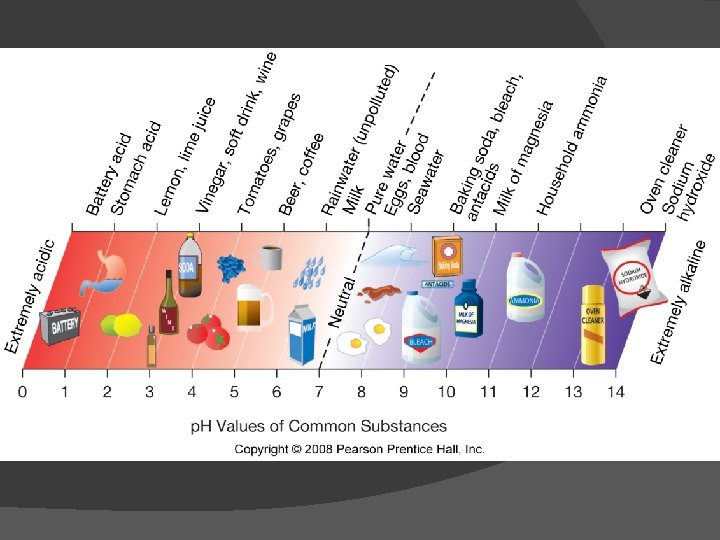
Figure 5. 17
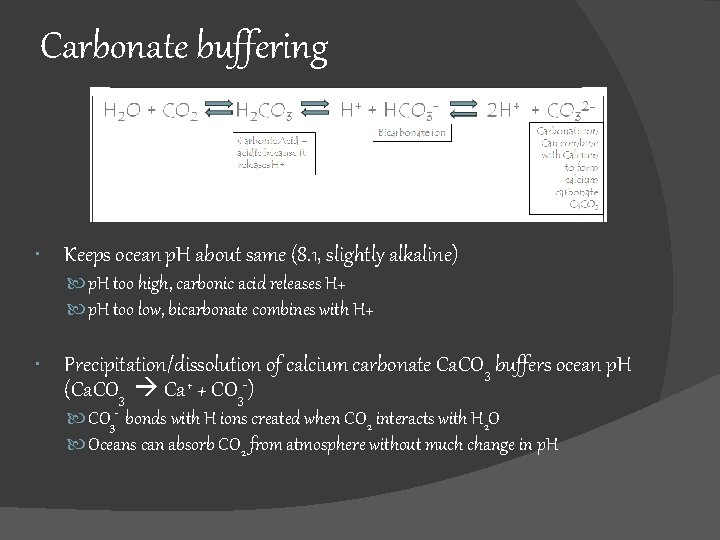
Carbonate buffering Keeps ocean p. H about same (8. 1, slightly alkaline) p. H too high, carbonic acid releases H+ p. H too low, bicarbonate combines with H+ Precipitation/dissolution of calcium carbonate Ca. CO 3 buffers ocean p. H (Ca. CO 3 Ca+ + CO 3 -) CO 3 - bonds with H ions created when CO 2 interacts with H 2 O Oceans can absorb CO 2 from atmosphere without much change in p. H

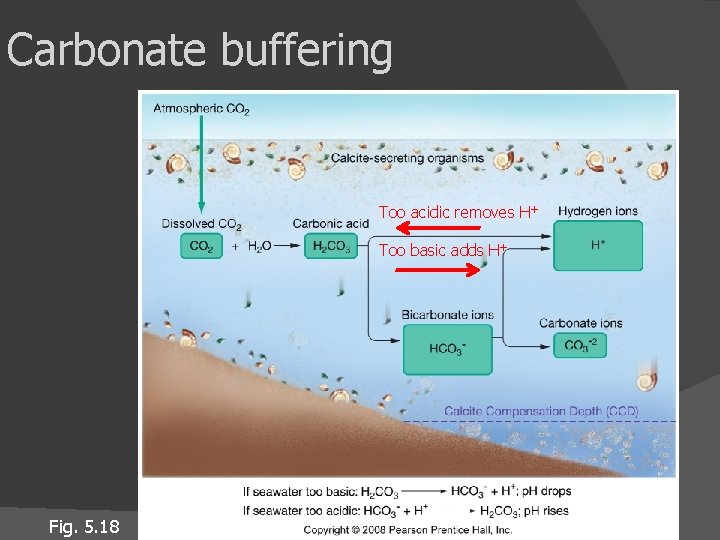
Carbonate buffering Too acidic removes H+ Too basic adds H+ Fig. 5. 18
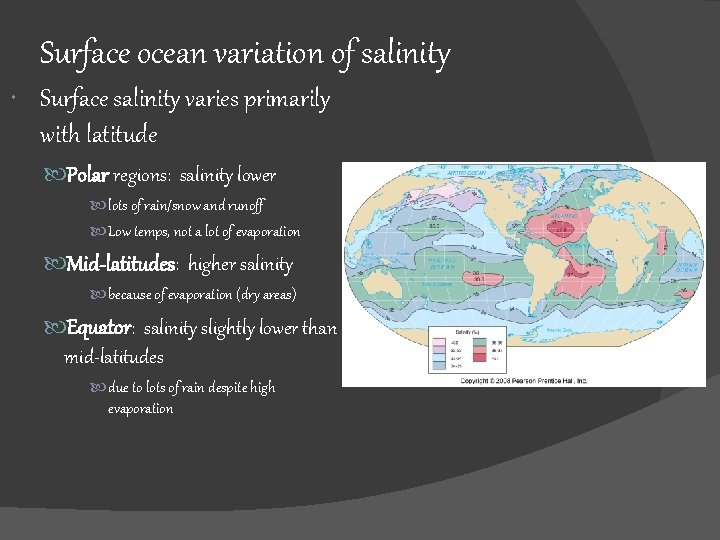
Surface ocean variation of salinity Surface salinity varies primarily with latitude Polar regions: salinity lower lots of rain/snow and runoff Low temps, not a lot of evaporation Mid-latitudes: higher salinity because of evaporation (dry areas) Equator: salinity slightly lower than mid-latitudes due to lots of rain despite high evaporation
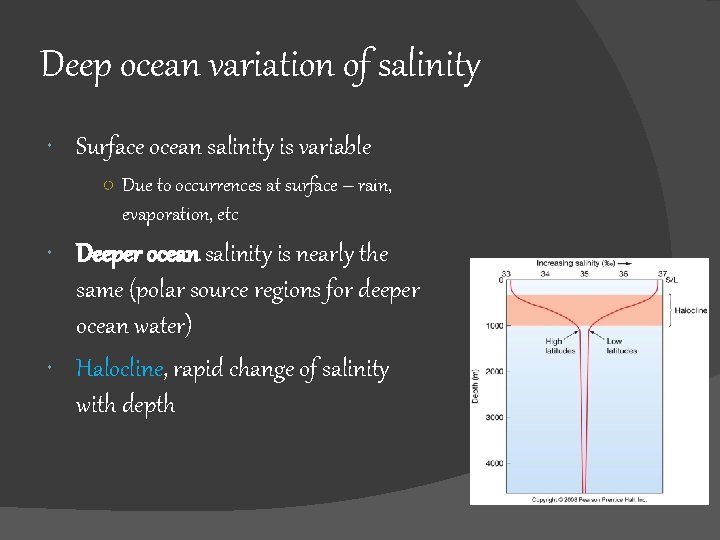
Deep ocean variation of salinity Surface ocean salinity is variable ○ Due to occurrences at surface – rain, evaporation, etc Deeper ocean salinity is nearly the same (polar source regions for deeper ocean water) Halocline, Halocline rapid change of salinity with depth
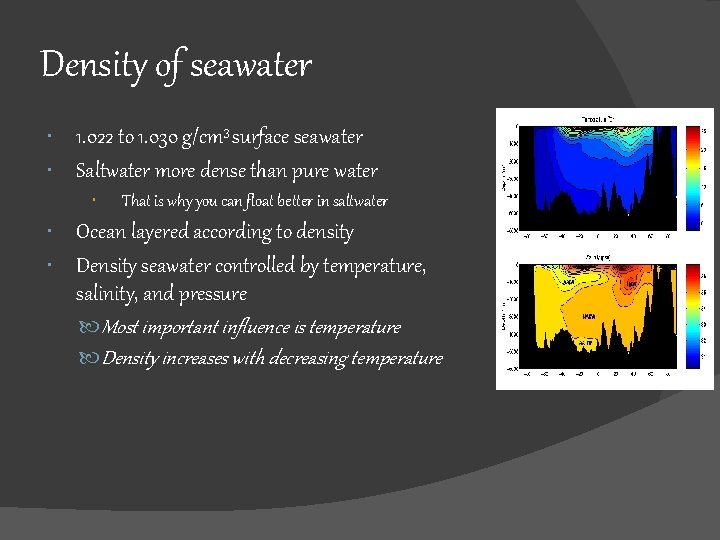
Density of seawater 1. 022 to 1. 030 g/cm 3 surface seawater Saltwater more dense than pure water That is why you can float better in saltwater Ocean layered according to density Density seawater controlled by temperature, salinity, and pressure Most important influence is temperature Density increases with decreasing temperature


Density of seawater Overall, temp has greatest effect on density However, salinity greatest influence on density in polar oceans polar ocean is isothermal (same temperature as depth increases) Currents from lower latitudes bring higher salinity water into polar areas But polar waters are overall isothermal AND isopycnal http: //www. waterencyclopedia. com/images/wsci_03_img 0394. jpg

Density versus depth Pycnocline, Pycnocline abrupt change of density with depth Thermocline, Thermocline abrupt change of temperature with depth Density differences cause a layered ocean Mixed surface water Pycnocline and thermocline Deep water

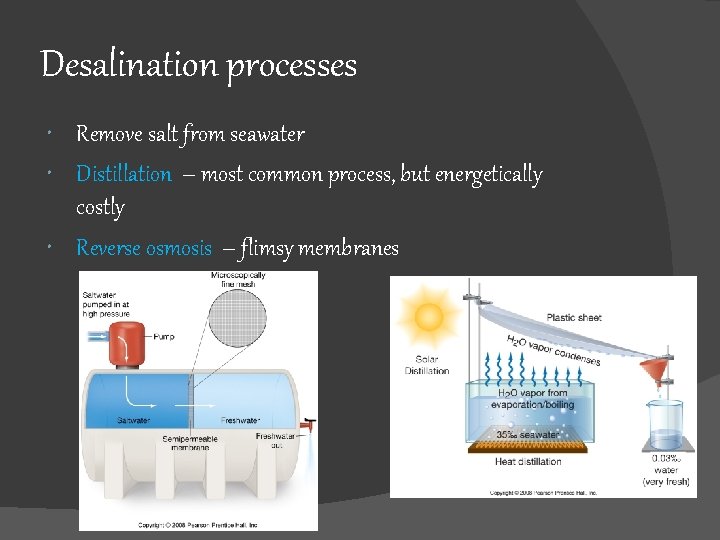
Desalination processes Remove salt from seawater Distillation – most common process, but energetically costly Reverse osmosis – flimsy membranes

Misconceptions Increases in global temperatures in the atmosphere and the consequent warming of the oceans will only create a problem for people living along the coast. Water exists in the ground in actual rivers or lakes that are constantly renewed. People drink bottle water because it is better for our health; the safety of tap water is below consumption standards.

Ocean Literacy Principles 1 e - Most of Earth’s water (97%) is in the ocean. Seawater has unique properties: it is saline, its freezing point is slightly lower than fresh water, its density is slightly higher, its electrical conductivity is much higher, and it is slightly basic. The salt in seawater comes from eroding land, volcanic emissions, reactions at the seafloor, and atmospheric deposition. 1 g - The ocean is connected to major lakes, watersheds and waterways because all major watersheds on Earth drain to the ocean. Rivers and streams transport nutrients, salts, sediments and pollutants from watersheds to estuaries and to the ocean. 3 a - The ocean controls weather and climate by dominating the Earth’s energy, water and carbon systems.

Sunshine State Standards SC. 6. E. 7. 1 Differentiate among radiation, conduction, and convection, the three mechanisms by which heat is transferred through Earth's system. SC. 6. E. 7. 6 Differentiate between weather and climate. SC. 8. P. 8. 1 Explore the scientific theory of atoms (also known as atomic theory) by using models to explain the motion of particles in solids, liquids, and gases. SC. 8. P. 8. 4 Classify and compare substances on the basis of characteristic physical properties that can be demonstrated or measured; for example, density, thermal or electrical conductivity, solubility, magnetic properties, melting and boiling points, and know that these properties are independent of the amount of the sample. SC. 8. P. 8. 6 Recognize that elements are grouped in the periodic table according to similarities of their properties. SC. 8. P. 8. 8 Identify basic examples of and compare and classify the properties of compounds, including acids, bases, and salts. SC. 912. E. 7. 9 Cite evidence that the ocean has had a significant influence on climate change by absorbing, storing, and moving heat, carbon, and water. SC. 912. P. 8. 4 Explore the scientific theory of atoms (also known as atomic theory) by describing the structure of atoms in terms of protons, neutrons and electrons, and differentiate among these particles in terms of their mass, electrical charges and locations within the atom SC. 912. P. 8. 5 Relate properties of atoms and their position in the periodic table to the arrangement of their electrons.
- Slides: 56