CHAPTER 5 Water and Seawater 2011 Pearson Education






- Slides: 24

CHAPTER 5 Water and Seawater © 2011 Pearson Education, Inc.

Earth’s Water • • • 97. 2% in the world ocean 2. 15% frozen in glaciers and ice caps 0. 62% in groundwater and soil moisture 0. 02% in streams and lakes 0. 001% as water vapor in the atmosphere © 2011 Pearson Education, Inc.

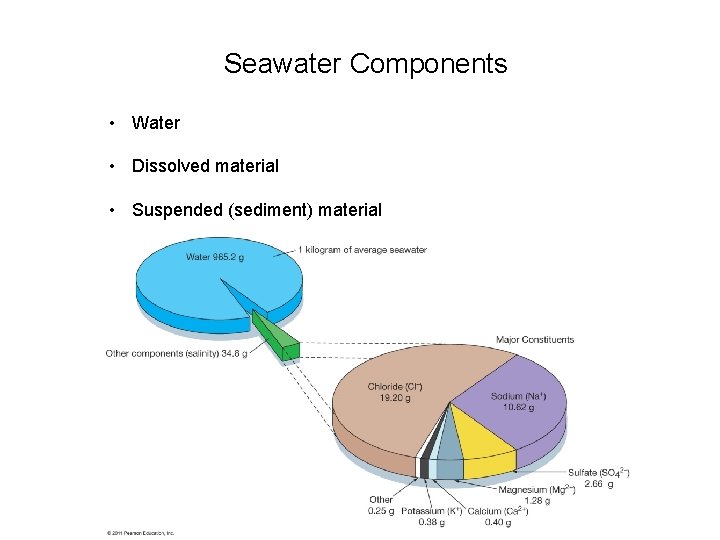
Seawater Components • Water • Dissolved material • Suspended (sediment) material © 2011 Pearson Education, Inc.

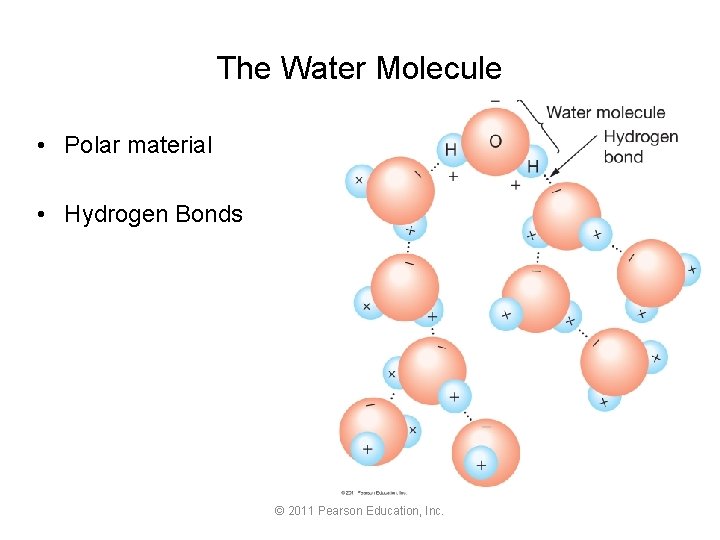
The Water Molecule • Polar material • Hydrogen Bonds © 2011 Pearson Education, Inc.

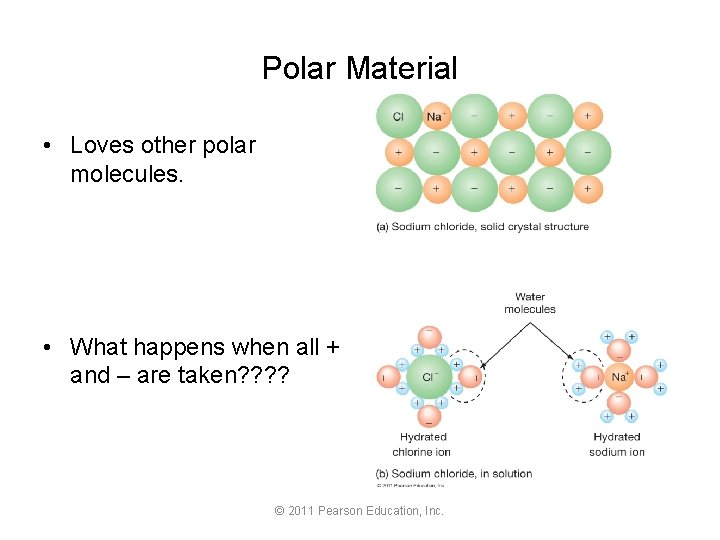
Polar Material • Loves other polar molecules. • What happens when all + and – are taken? ? © 2011 Pearson Education, Inc.

Hydrogen Bonding • Hydrogen bonds (between water) and polarity result in unique properties • See Coursenotes…… © 2011 Pearson Education, Inc.

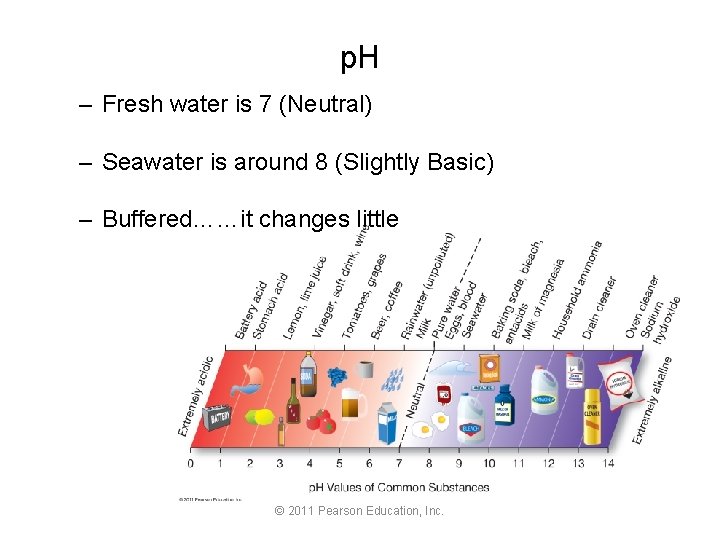
p. H – Fresh water is 7 (Neutral) – Seawater is around 8 (Slightly Basic) – Buffered……it changes little © 2011 Pearson Education, Inc.

Freezing and Boiling Points • Freezing point = melting point: 32°F • Boiling point = condensation point: 212°F © 2011 Pearson Education, Inc.

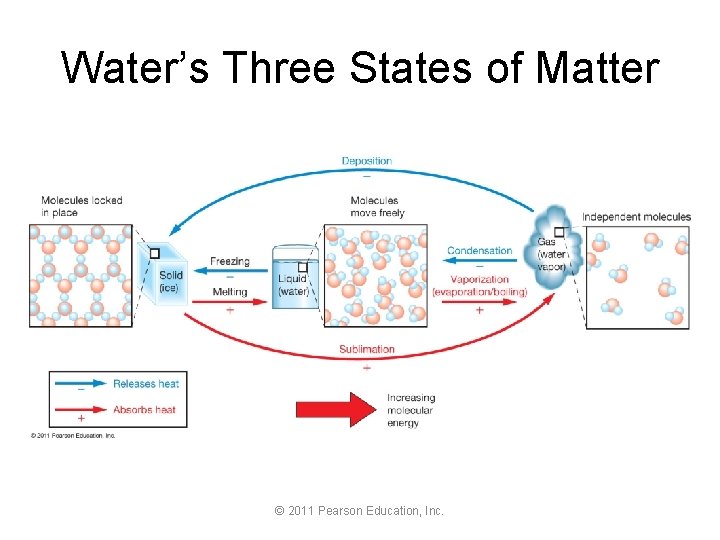
Water’s Three States of Matter © 2011 Pearson Education, Inc.

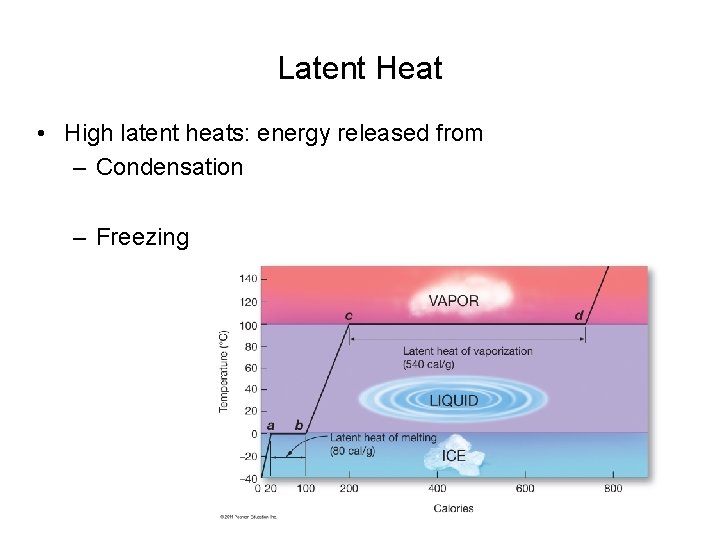
Latent Heat • High latent heats: energy released from – Condensation – Freezing © 2011 Pearson Education, Inc.

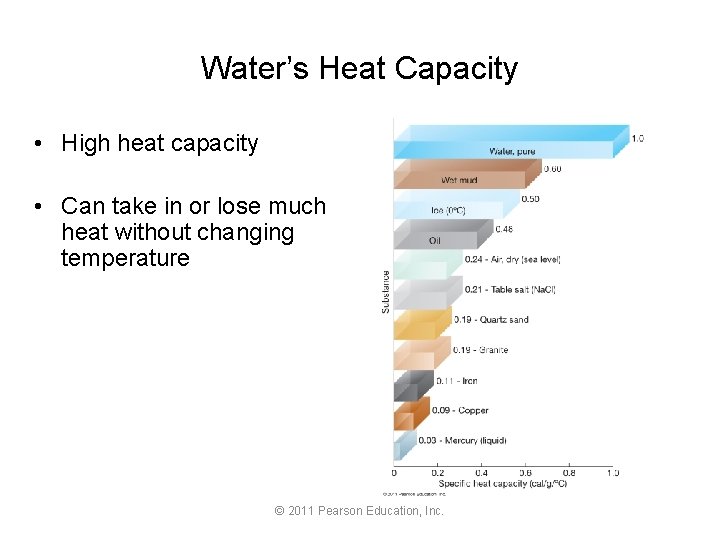
Water’s Heat Capacity • High heat capacity • Can take in or lose much heat without changing temperature © 2011 Pearson Education, Inc.

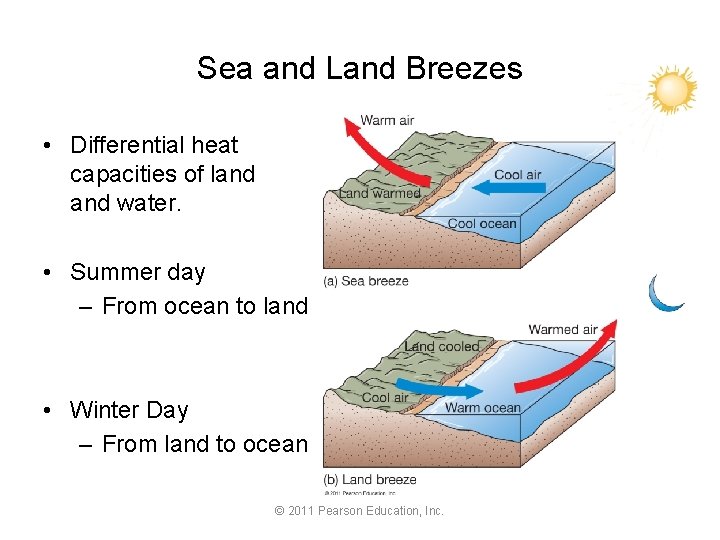
Sea and Land Breezes • Differential heat capacities of land water. • Summer day – From ocean to land • Winter Day – From land to ocean © 2011 Pearson Education, Inc.

Water In The Ocean • Moderates temperatures on Earth • Moderates temperatures in the Ocean © 2011 Pearson Education, Inc.

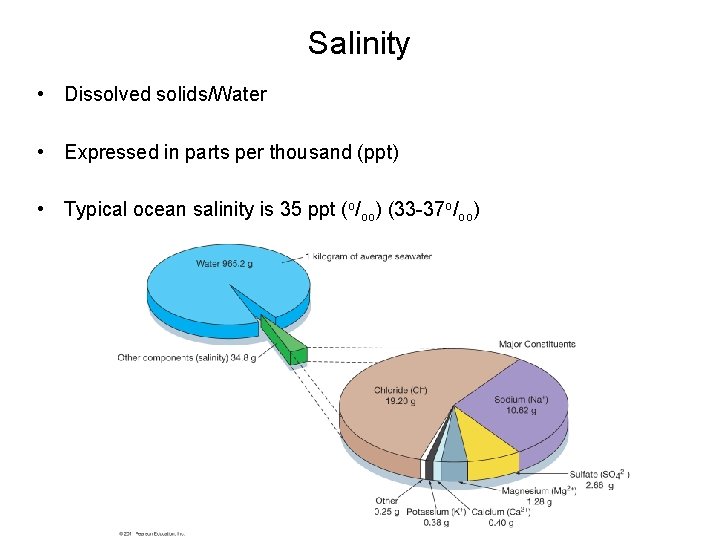
Salinity • Dissolved solids/Water • Expressed in parts per thousand (ppt) • Typical ocean salinity is 35 ppt (o/oo) (33 -37 o/oo) © 2011 Pearson Education, Inc.

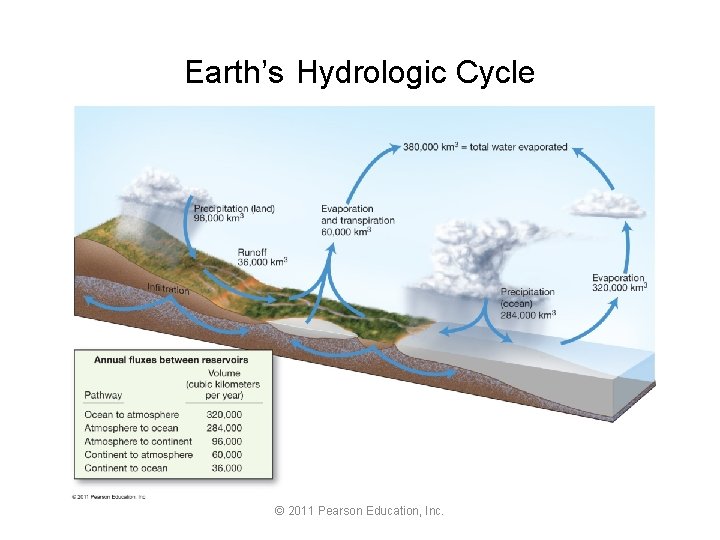
Earth’s Hydrologic Cycle © 2011 Pearson Education, Inc.

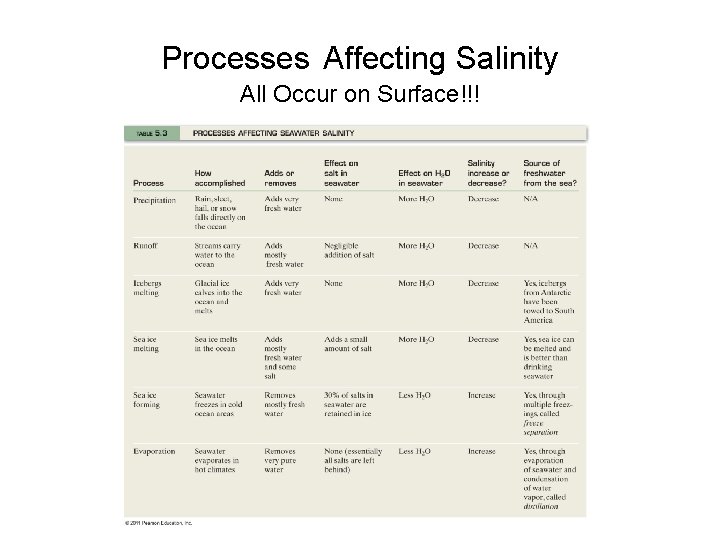
Processes Affecting Salinity All Occur on Surface!!! © 2011 Pearson Education, Inc.

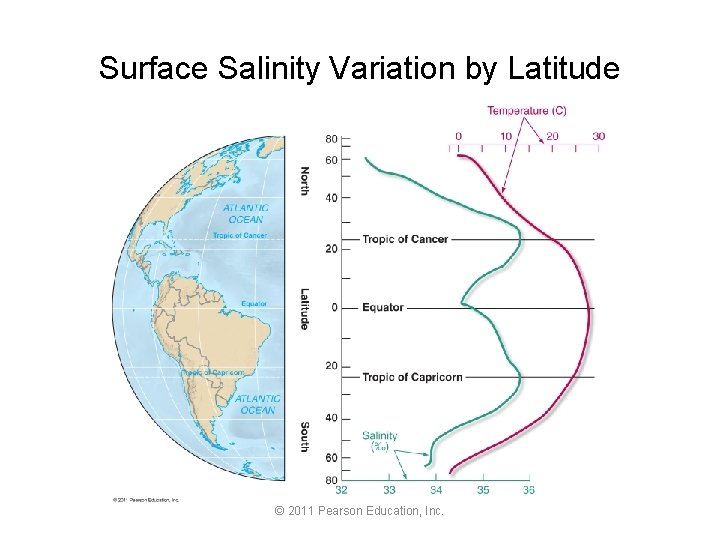
Surface Salinity Variation by Latitude © 2011 Pearson Education, Inc.

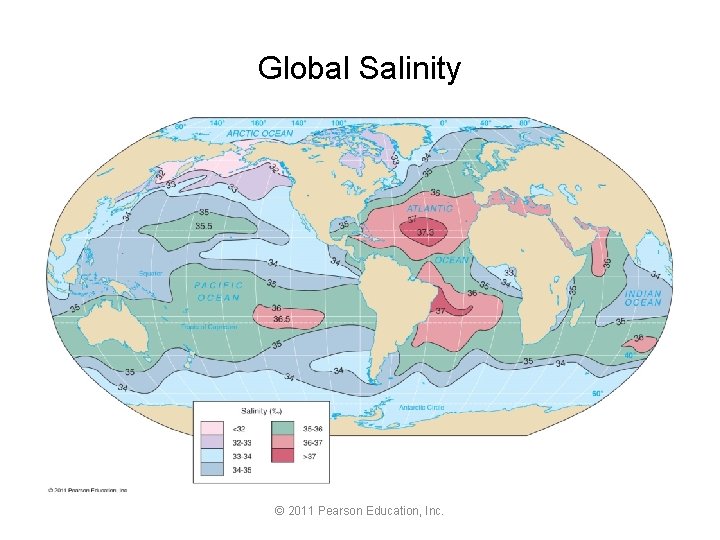
Global Salinity © 2011 Pearson Education, Inc.

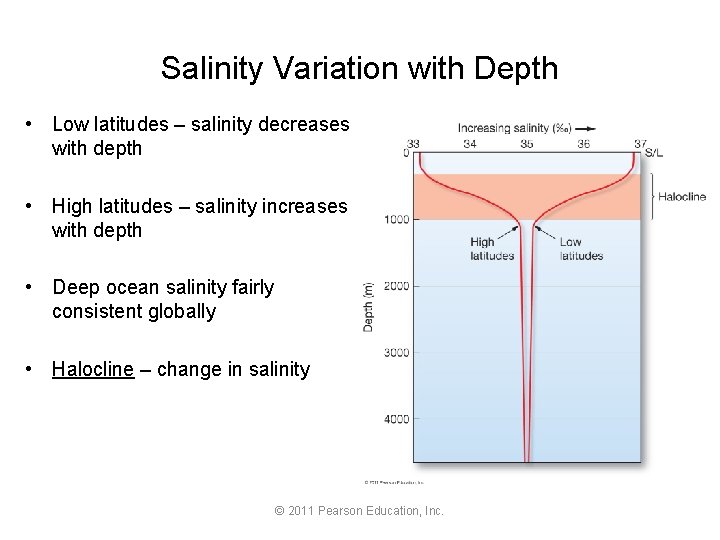
Salinity Variation with Depth • Low latitudes – salinity decreases with depth • High latitudes – salinity increases with depth • Deep ocean salinity fairly consistent globally • Halocline – change in salinity © 2011 Pearson Education, Inc.

Seawater Density • Freshwater density = 1. 000 g/cm 3 • Ocean surface water =1. 022 to 1. 030 g/cm 3 • Ocean layered according to density © 2011 Pearson Education, Inc.

Seawater Density • Density increases with decreasing temperature • Density increases with increasing salinity © 2011 Pearson Education, Inc.

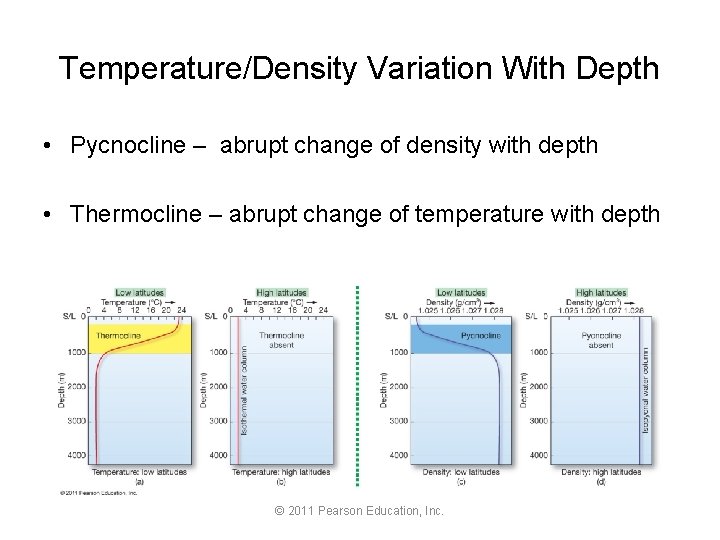
Temperature/Density Variation With Depth • Pycnocline – abrupt change of density with depth • Thermocline – abrupt change of temperature with depth © 2011 Pearson Education, Inc.

Layered Ocean Three distinct water masses based on density: • Mixed surface layer – above thermocline • Upper water – thermocline and pycnocline • Deep water – below thermocline to ocean floor • High latitude oceans – thermocline and pycnocline rarely develop © 2011 Pearson Education, Inc.

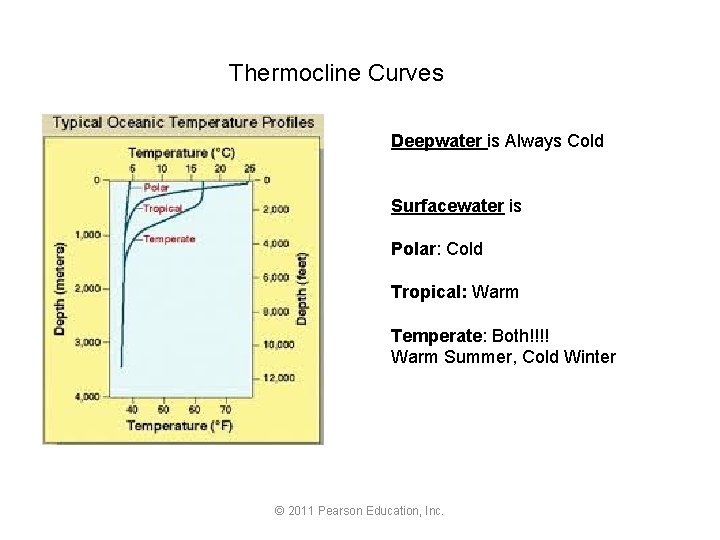
Thermocline Curves Deepwater is Always Cold Surfacewater is Polar: Cold Tropical: Warm Temperate: Both!!!! Warm Summer, Cold Winter © 2011 Pearson Education, Inc.