Chapter 2 Matter Matter Anything that has mass















- Slides: 33

Chapter 2 Matter

• Matter: Anything that has mass and takes up space • Atom: is the basic unit of matter • Mass: is the amount of material an object has (do not confuse with weight) • Chemistry: is the study of matter and how it changes • Element: is a substance that cannot be separated into other kinds of substances (lead into gold)





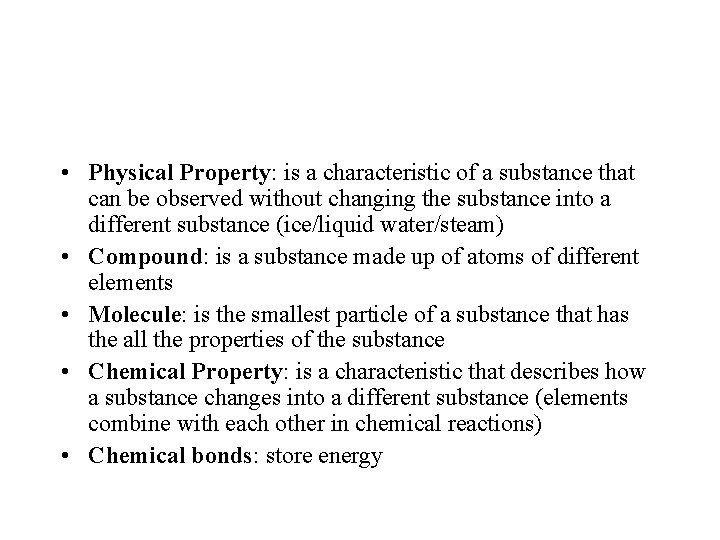
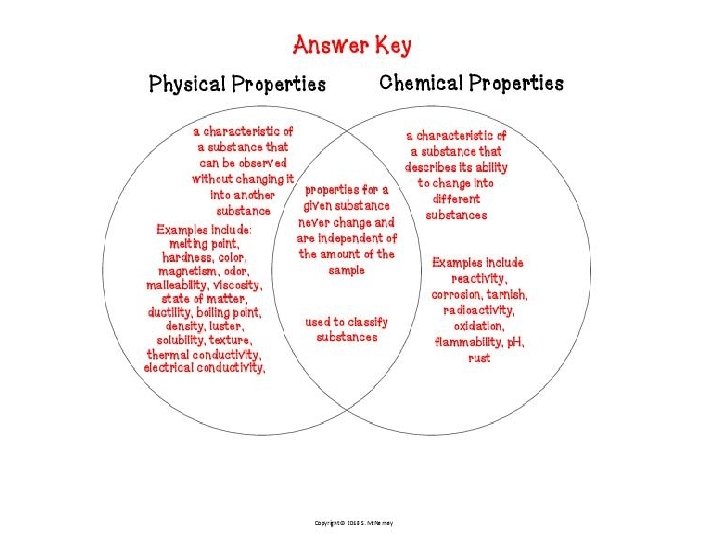
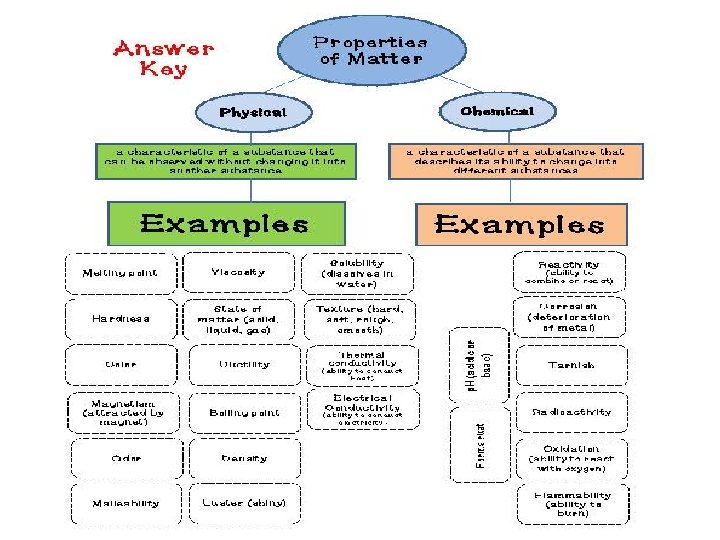
• Physical Property: is a characteristic of a substance that can be observed without changing the substance into a different substance (ice/liquid water/steam) • Compound: is a substance made up of atoms of different elements • Molecule: is the smallest particle of a substance that has the all the properties of the substance • Chemical Property: is a characteristic that describes how a substance changes into a different substance (elements combine with each other in chemical reactions) • Chemical bonds: store energy

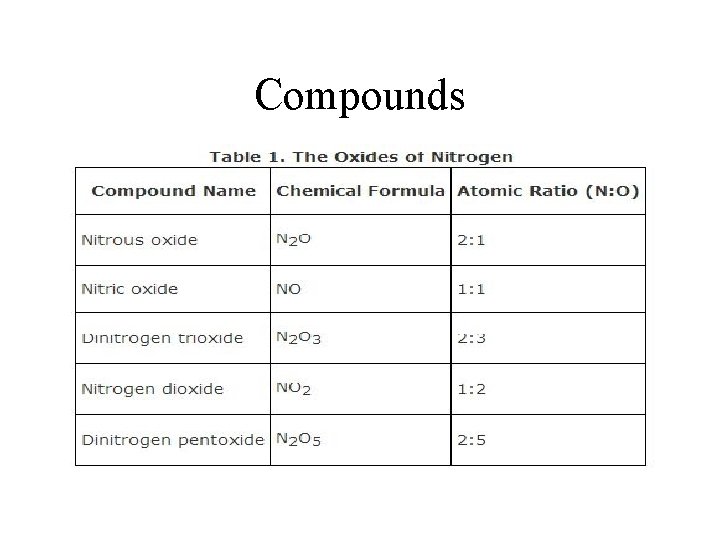
Compounds

Chemical Formula • Represents compounds • Example: – Molecule of indigo Dye for blue jeans • C 16 H 10 N 2 O 2 – Three molecules of table sugar • 3 C 12 H 22 O 11

Pure Substance • A sample of matter, either a single element (O) or a single compound (H 2 O), that has definite chemical and physical properties – Elements: Na or Cl – Compound: Na. Cl • Elements and compounds are pure substances, but mixtures are not.

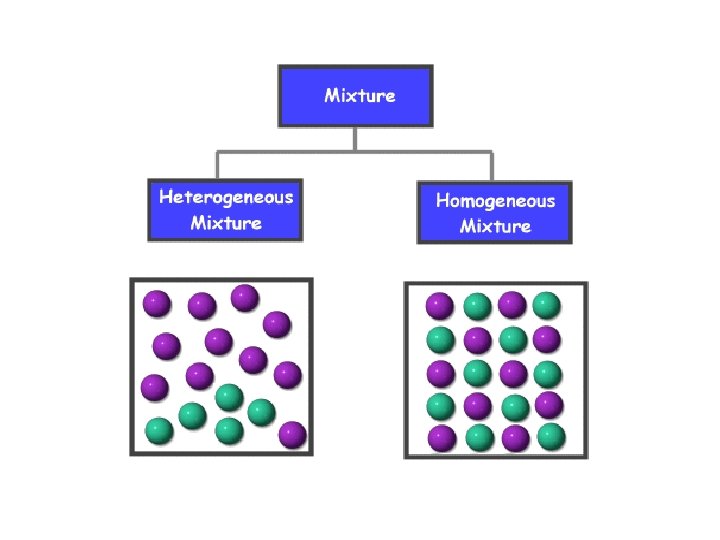
Mixtures • Classified by how well the substances mix – Heterogeneous: mixture that is NOT evenly distributed • Salad – Homogeneous: mixture that is evenly distributed • Vinegar and water • Gasoline (hundreds of liquids that are miscible) • Carbonated beverage


Mixture

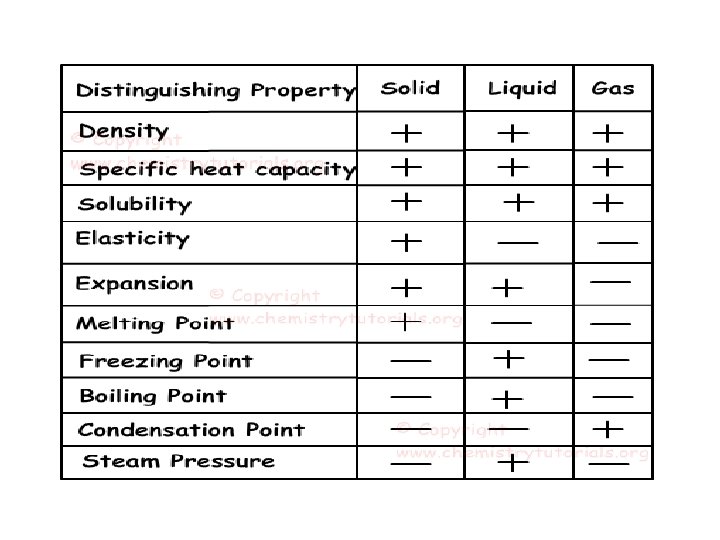
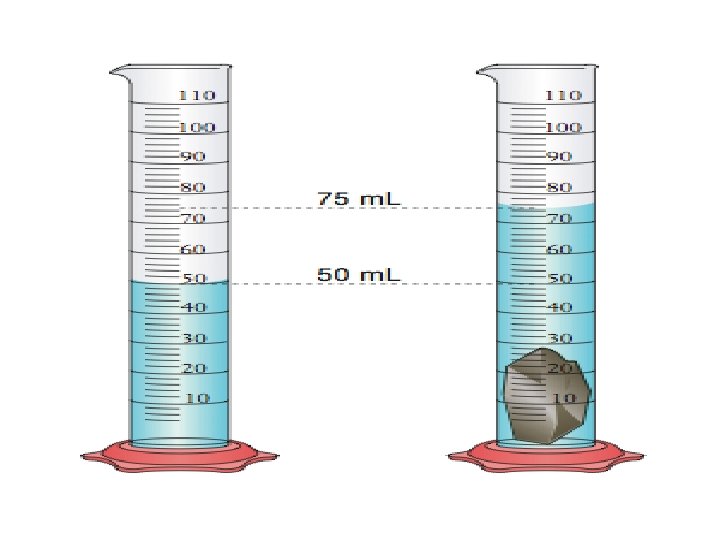
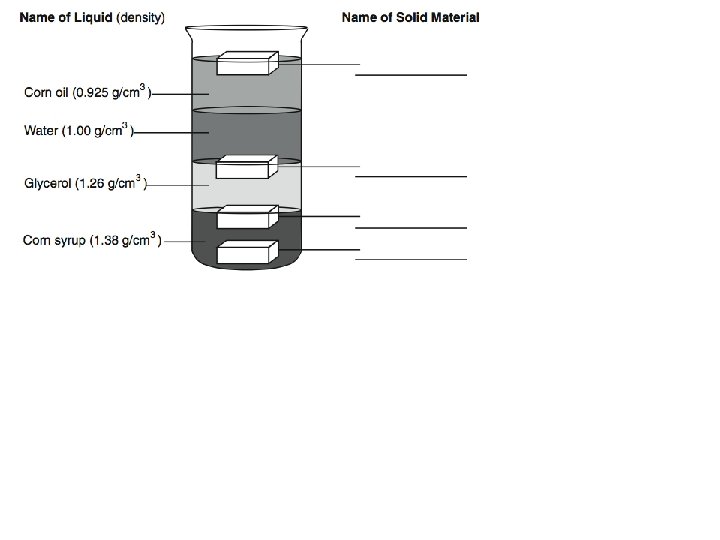
Properties of Matter • Physical: characteristics that can be observed without changing the identity of the substance – Density – Melting point (solid to a liquid) – Boiling point (liquid to a gas) – Reactivity (chemical property) • Can be separated by physical means



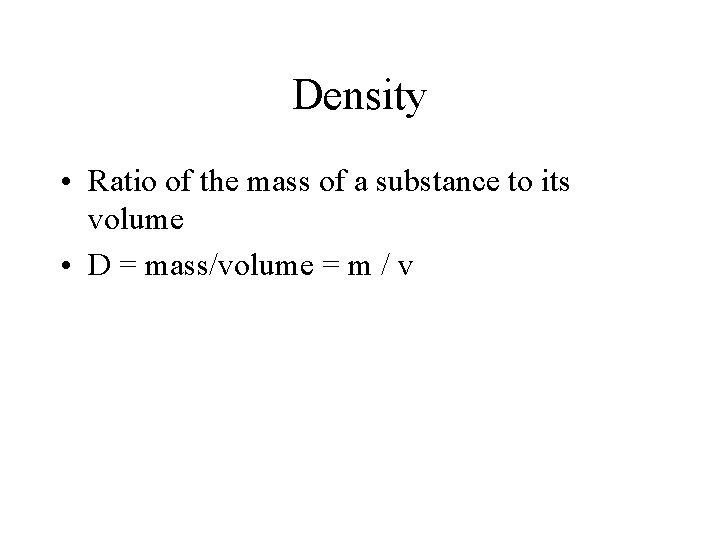
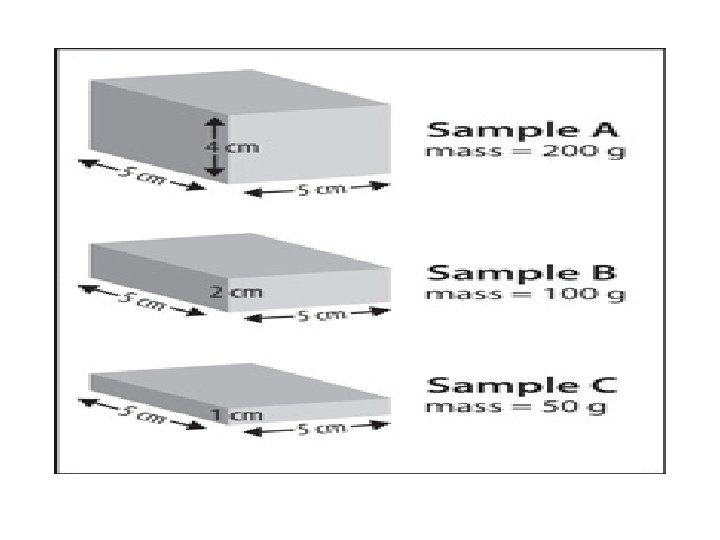
Density • Ratio of the mass of a substance to its volume • D = mass/volume = m / v





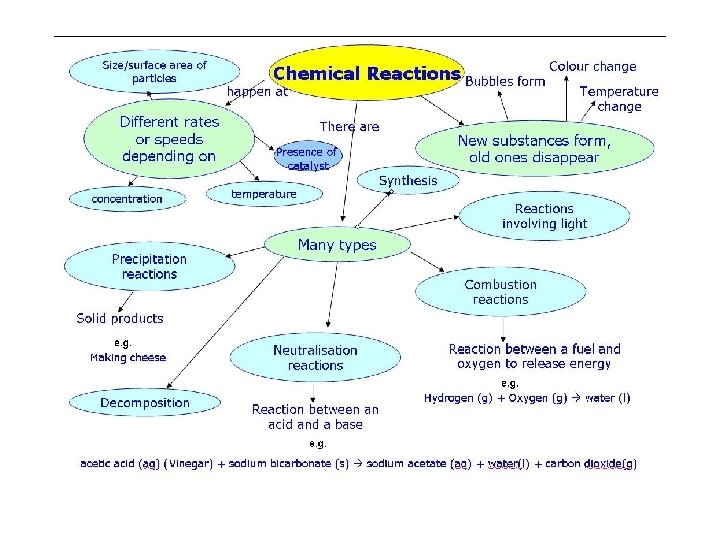
Chemical Property • Reactivity: the capacity of a substance to combine chemically with another substance • Describes how a substance changes into a new substance, either by combining with other elements or by breaking apart into new substances – Flammability (ability to burn) – Iron and oxygen yielding rust – Na and Cl yielding salt (Na. Cl) • Some are reversible – Example of carbonic acid in soda



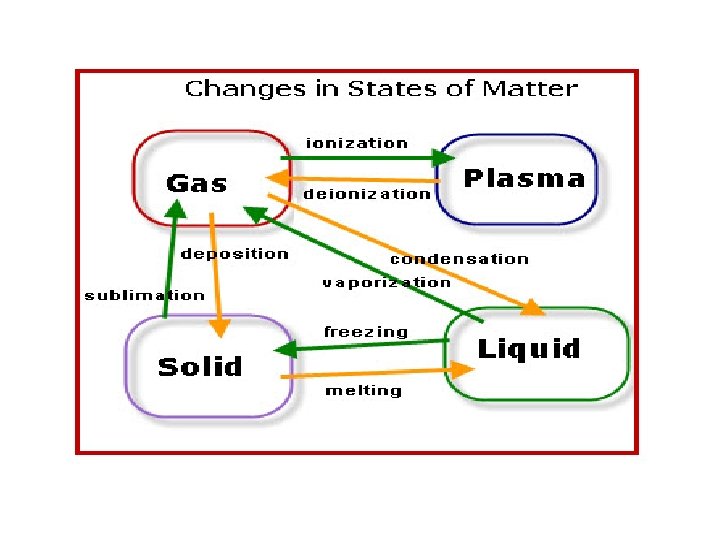
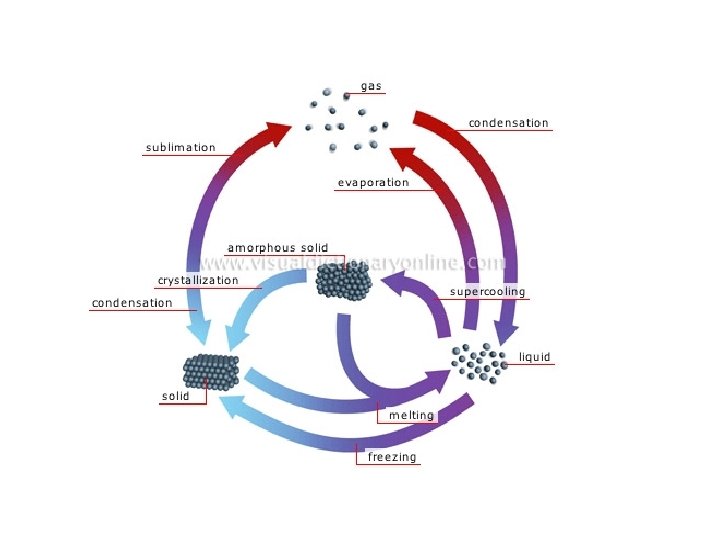
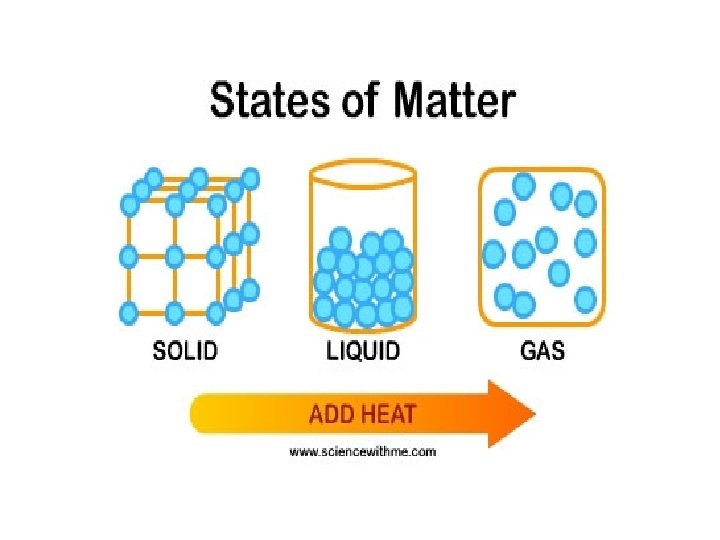
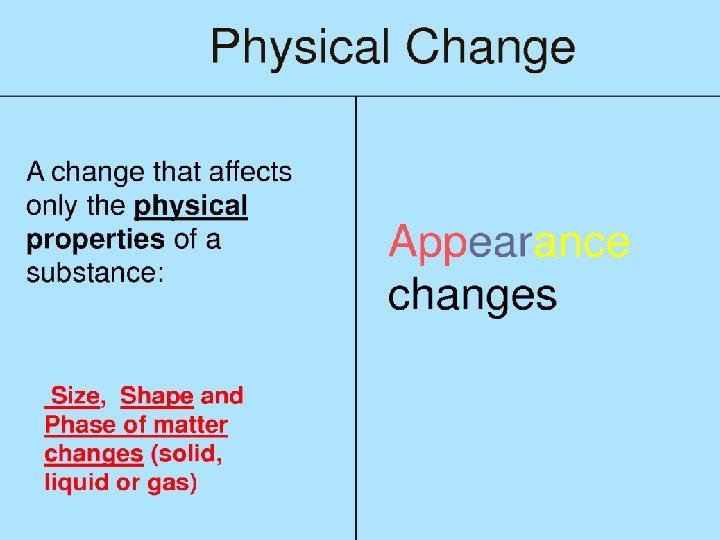
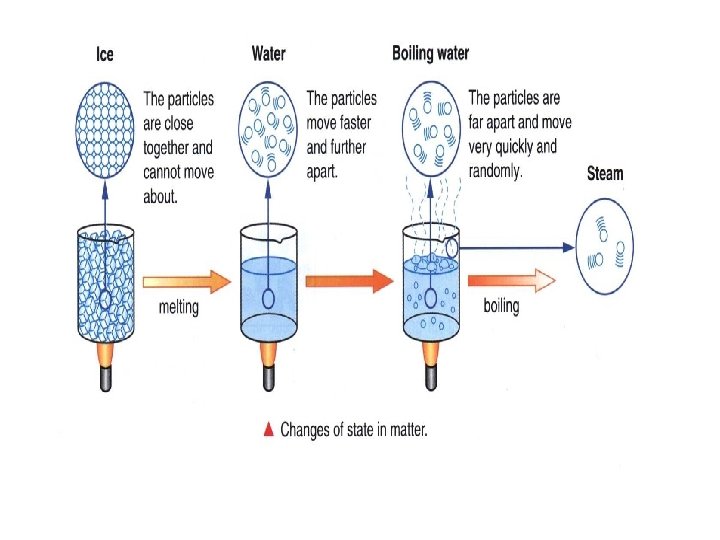
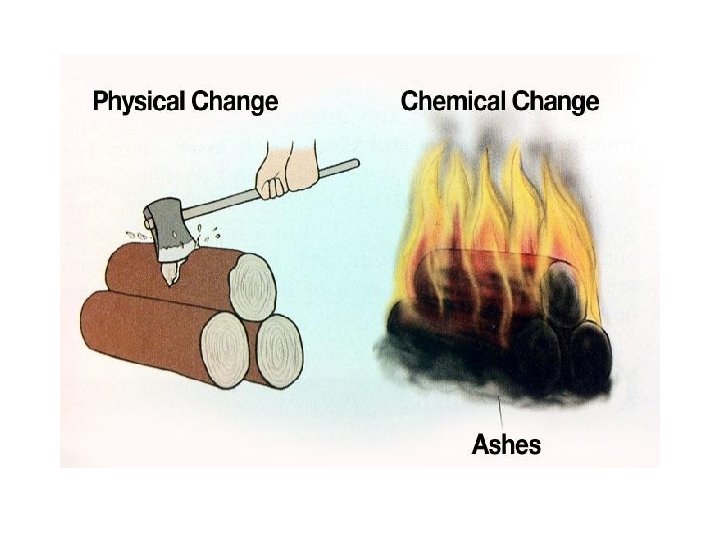
Changes of Matter • Physical change affects one or more physical properties of a substance without changing the identity of the substance – Melting of ice (solid to liquid) – Crushing of a can – Dissolving of sugar in water – Dissolving of CO 2 in water – Dissolving of air in ice cream (soft ice cream)





Changes of Matter • Chemical change happens when one or more substances are changed into entirely new substances that have different properties (new substance has formed). – Digesting of food • Burn with oxygen – Na and Cl to form Na. Cl – Iron rusting – Copper turning green


