Properties of Matter Matter anything that has mass













- Slides: 23

Properties of Matter

Matter – anything that has mass and volume Pretty much everything!

How Can We Describe Matter? Properties – the characteristics of a substance What are the properties of matter? l. Mass, volume l. Color, size, shape, etc.

What’s the Matter? l Matter is grouped into three phases l Solids l Liquids l Gases

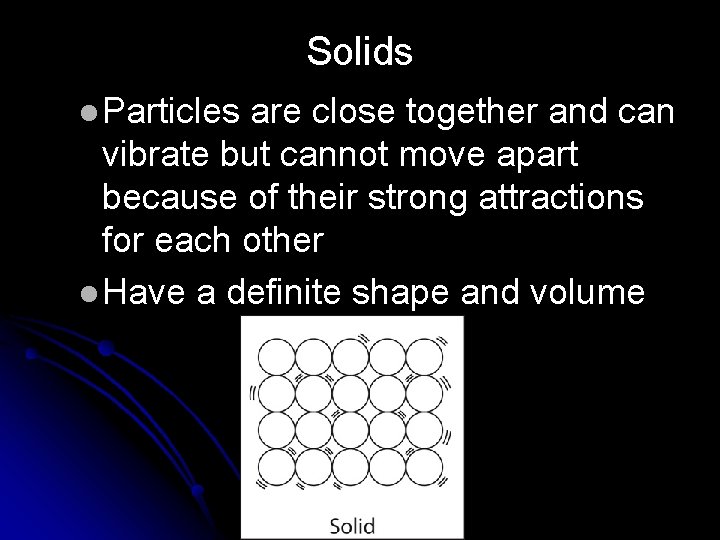
Solids l Particles are close together and can vibrate but cannot move apart because of their strong attractions for each other l Have a definite shape and volume

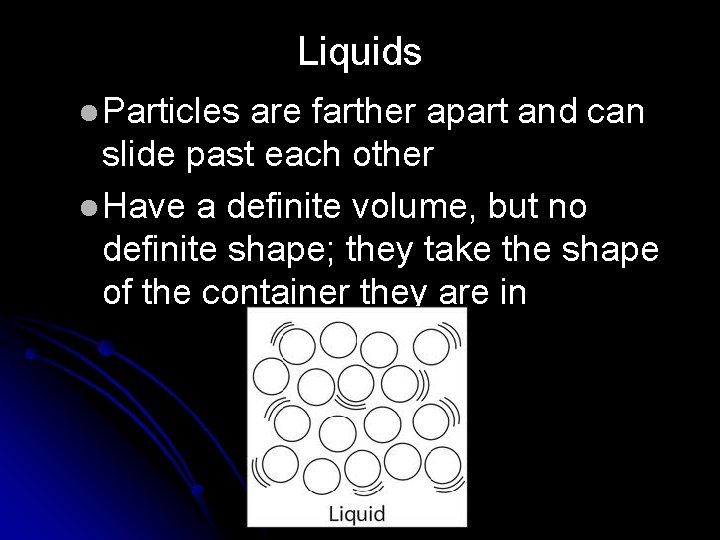
Liquids l Particles are farther apart and can slide past each other l Have a definite volume, but no definite shape; they take the shape of the container they are in

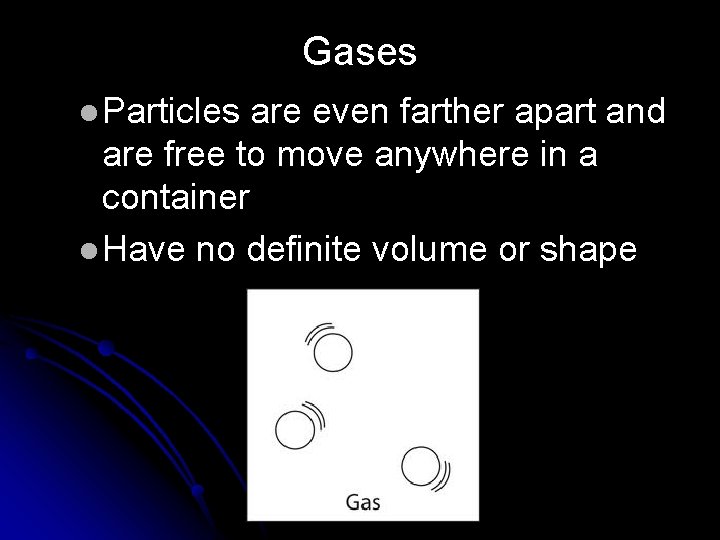
Gases l Particles are even farther apart and are free to move anywhere in a container l Have no definite volume or shape

Let’s Compare Particles in Matter

Your Task l Make a tri-fold brochure comparing solids, liquids and gases. It must include: l. Definitions l. Drawings of the particle arrangements l. Examples – at least two!

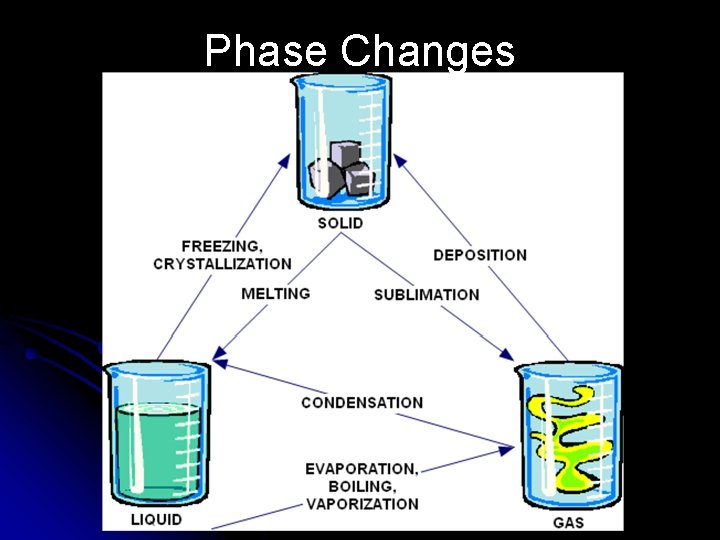
Phase Changes

How Phases Change l Solid to Liquid l Melting – the change of a substance from a solid to a liquid when heat is added l Liquid to Solid l Freezing – the change of a substance from a liquid to a solid when heat is removed

How Phases Change l Liquid to Gas l Vaporization – the change of a substance from a liquid to a gas when heat is added l Gas to Liquid l Condensation – the change of a substance from a gas to a liquid when heat is removed

3 Minute Chat l Discuss the following, explaining your answers: l What are pure substances? l What are mixtures? l How are they alike? l How are they different?

Elements l. Element – pure substance that cannot be separated by physical or chemical means - atoms l. Any one element on the periodic table! l. Ex.

Compounds l Compound – substance made of two or more elements that are joined by chemical bonds - molecules l Can only be separated by chemical means, not physical l H 2 O l Na. Cl l Two or more elements

Mixtures l. Mixture – a combination of two or more pure substances that are not chemically combined l. Can physically separate

Stuff Lab Is the “stuff” an element, a compound, or a mixture? l If stuff is a mixture, how can you separate the components? l Physical methods l

How to Fold Filter Paper

Homogeneous Mixtures l Two or more substances spread out evenly without settling - the same throughout l Appears clear l Also called solutions l Iced tea l Salt water

Heterogeneous Mixtures l. A mixture in which different parts are easily distinguished l Sandwich pencil (lead, wood, eraser)

Heterogeneous Mixtures l Colloid – heterogeneous mixture that never settles l Milk l Can tell by Tyndall Effect l Scattering of light by the particles in the mixture

Heterogeneous Mixtures l Suspensions – contain a liquid with visible particles that settle out when it stands – also have Tyndall Effect l Orange juice l Different from a colloid or solution because particles will settle out

Whiteboard Check What type of mixture (homogeneous or heterogeneous) is each of the following: l Chocolate chip cookie l Coffee l Apple juice l Lucky Charms® cereal l Trail mix l
 Is anything that has mass and occupies space
Is anything that has mass and occupies space Anything that has mass and volume
Anything that has mass and volume Anything that has mass and takes up space is
Anything that has mass and takes up space is Matter vs mass
Matter vs mass Anything that occupies space
Anything that occupies space It is anything that has mass and occupies space
It is anything that has mass and occupies space It is anything that has mass and occupies space
It is anything that has mass and occupies space Anything that has mass and take up space
Anything that has mass and take up space Everything that has mass and volume
Everything that has mass and volume Is anything that has mass and takes up space.
Is anything that has mass and takes up space. Anything that takes up space and has mass is
Anything that takes up space and has mass is Defintion of matter
Defintion of matter Anything that takes up space and has mass
Anything that takes up space and has mass Anything defintion
Anything defintion Something that takes up space
Something that takes up space Matter is anything that occupies space and has -----------
Matter is anything that occupies space and has ----------- Phân độ lown
Phân độ lown Block av độ 2
Block av độ 2 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Con hãy đưa tay khi thấy người vấp ngã
Con hãy đưa tay khi thấy người vấp ngã Tôn thất thuyết là ai
Tôn thất thuyết là ai








































