CHAPTER 13 RATES OF REACTION Vanessa N PrasadPermaul





![Rate Expression and Rate Constant Rate expression / rate law: rate = k[A] where Rate Expression and Rate Constant Rate expression / rate law: rate = k[A] where](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-11.jpg)


![Example 1 CH 3 CHO(g) CH 4(g) + CO(g) [CH 3 CHO] . 10 Example 1 CH 3 CHO(g) CH 4(g) + CO(g) [CH 3 CHO] . 10](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-16.jpg)
![Example 1 Rate 2 = [A 2]m Rate 1 [A 1]m 4 = 2 Example 1 Rate 2 = [A 2]m Rate 1 [A 1]m 4 = 2](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-17.jpg)
![Example 1 rate = k[CH 3 CHO]2 rate =. 085 mol/L s conc =. Example 1 rate = k[CH 3 CHO]2 rate =. 085 mol/L s conc =.](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-18.jpg)
![Example 1 rate = k[CH 3 CHO]2 rate = 8. 5 L/mol s [. Example 1 rate = k[CH 3 CHO]2 rate = 8. 5 L/mol s [.](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-19.jpg)


![Reactant concentration and time Rate expression rate = k[A] Shows how the rate of Reactant concentration and time Rate expression rate = k[A] Shows how the rate of](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-23.jpg)

![Zero Order Zero order: A Products rate = k CONC-TIME RELATION [A]0 – [A] Zero Order Zero order: A Products rate = k CONC-TIME RELATION [A]0 – [A]](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-25.jpg)
![First Order: A Products rate = k[A] ln [A]o/[A] = kt CONC-TIME RELATION HALF First Order: A Products rate = k[A] ln [A]o/[A] = kt CONC-TIME RELATION HALF](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-26.jpg)
![Second Order Second order: A Products rate = k[A]2 CONC-TIME RELATION HALF LIFE 1 Second Order Second order: A Products rate = k[A]2 CONC-TIME RELATION HALF LIFE 1](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-27.jpg)
![DETERMINING THE RATE LAW FROM INITIAL REACTIONS Rate = k[H 2 O 2]m [I-]n DETERMINING THE RATE LAW FROM INITIAL REACTIONS Rate = k[H 2 O 2]m [I-]n](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-29.jpg)
![DETERMINING THE RATE LAW FROM INITIAL REACTIONS Rate = k[H 2 O 2] [I-] DETERMINING THE RATE LAW FROM INITIAL REACTIONS Rate = k[H 2 O 2] [I-]](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-30.jpg)
![[HI] vs. time not linear so it is not zero order [A] vs. TIME [HI] vs. time not linear so it is not zero order [A] vs. TIME](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-33.jpg)
![ln [HI] vs. time not linear so it is not first order ln[A] vs. ln [HI] vs. time not linear so it is not first order ln[A] vs.](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-34.jpg)
![1/[HI] vs. time is linear so it is second order 1/ln[A] vs. TIME FOR 1/[HI] vs. time is linear so it is second order 1/ln[A] vs. TIME FOR](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-35.jpg)
![Order Rate Expression Conc-Time Relation Half-life 0 Rate = k [A]0 – [A] = Order Rate Expression Conc-Time Relation Half-life 0 Rate = k [A]0 – [A] =](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-36.jpg)

























- Slides: 87

CHAPTER 13: RATES OF REACTION Vanessa N. Prasad-Permaul CHM 1046 Valencia Community College

Introduction 1. Chemical kinetics is the study of reaction rates 2. For a chemical reaction to be useful it must occur at a reasonable rate 3. It is important to be able to control the rate of reaction 4. Factors that influence rate a) Concentration of reactants (molarity) b) Nature of reaction, process by which the reaction takes place c) Temperature d) Reaction mechanism (rate determining step) e) Catalyst

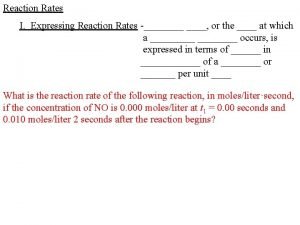
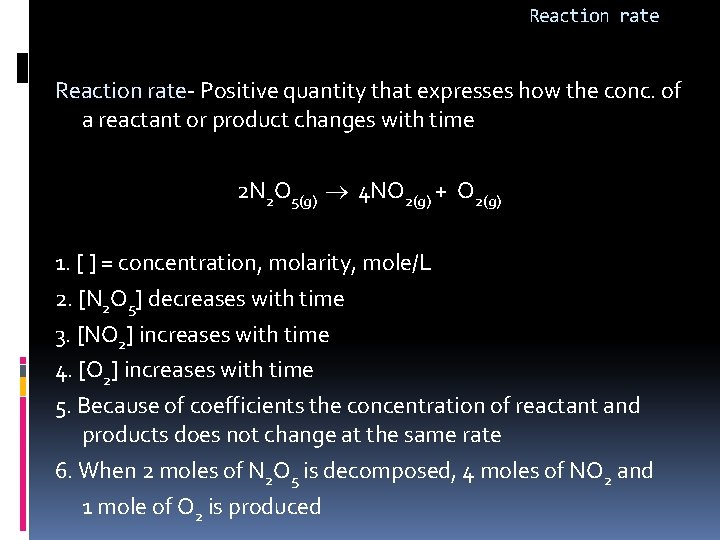
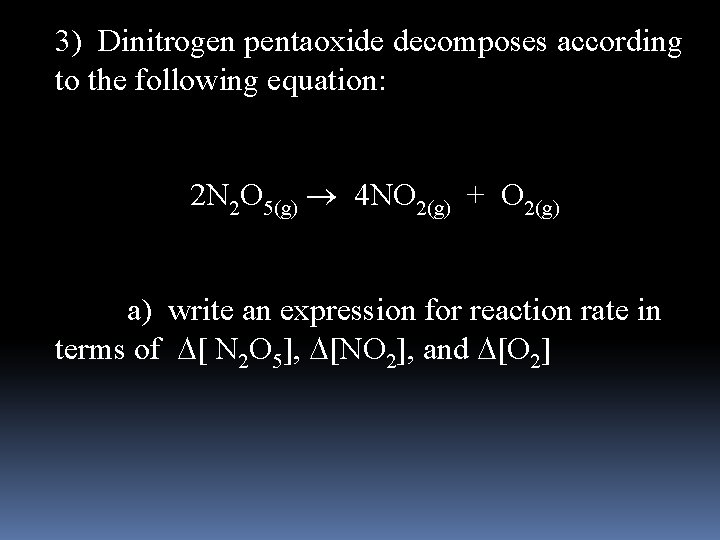
Reaction rate- Positive quantity that expresses how the conc. of a reactant or product changes with time 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) 1. [ ] = concentration, molarity, mole/L 2. [N 2 O 5] decreases with time 3. [NO 2] increases with time 4. [O 2] increases with time 5. Because of coefficients the concentration of reactant and products does not change at the same rate 6. When 2 moles of N 2 O 5 is decomposed, 4 moles of NO 2 and 1 mole of O 2 is produced

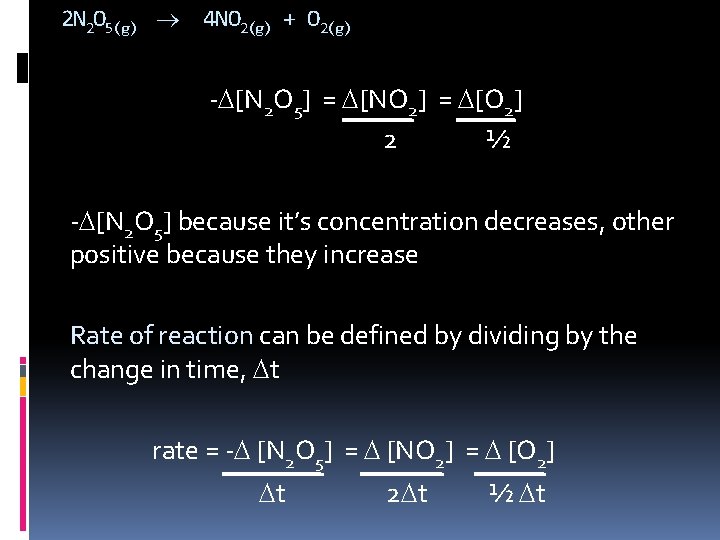
2 N 2 O 5(g) 4 NO 2(g) + O 2(g) - [N 2 O 5] = [NO 2] = [O 2] 2 ½ - [N 2 O 5] because it’s concentration decreases, other positive because they increase Rate of reaction can be defined by dividing by the change in time, t rate = - [N 2 O 5] = [NO 2] = [O 2] t 2 t ½ t

Reaction Rates Generic Formula: a. A + b. B c. C + d. D rate = - [A] = - [B] = [D] = [C] a t b t d t c t

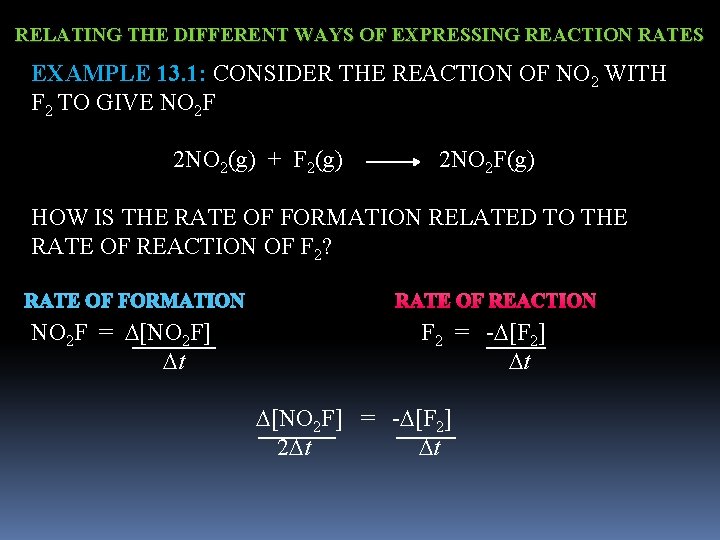
RELATING THE DIFFERENT WAYS OF EXPRESSING REACTION RATES EXAMPLE 13. 1: CONSIDER THE REACTION OF NO 2 WITH F 2 TO GIVE NO 2 F 2 NO 2(g) + F 2(g) 2 NO 2 F(g) HOW IS THE RATE OF FORMATION RELATED TO THE RATE OF REACTION OF F 2? RATE OF FORMATION RATE OF REACTION NO 2 F = [NO 2 F] F 2 = - [F 2] t [NO 2 F] = - [F 2] 2 t t

RELATING THE DIFFERENT WAYS OF EXPRESSING REACTION RATES EXERCISE 13. 1: CONSIDER THE REACTION OF NO 2 WITH F 2 TO GIVE NO 2 F 2 NO 2(g) + F 2(g) 2 NO 2 F(g) HOW IS THE RATE OF FORMATION RELATED TO THE RATE OF REACTION OF NO 2?

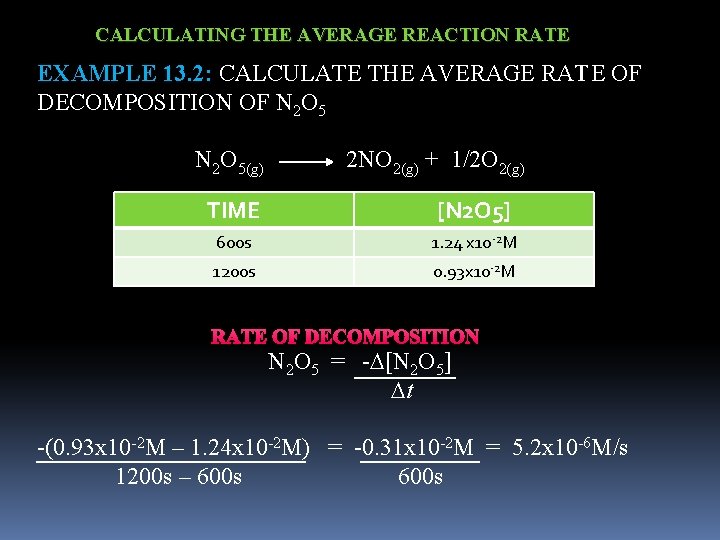
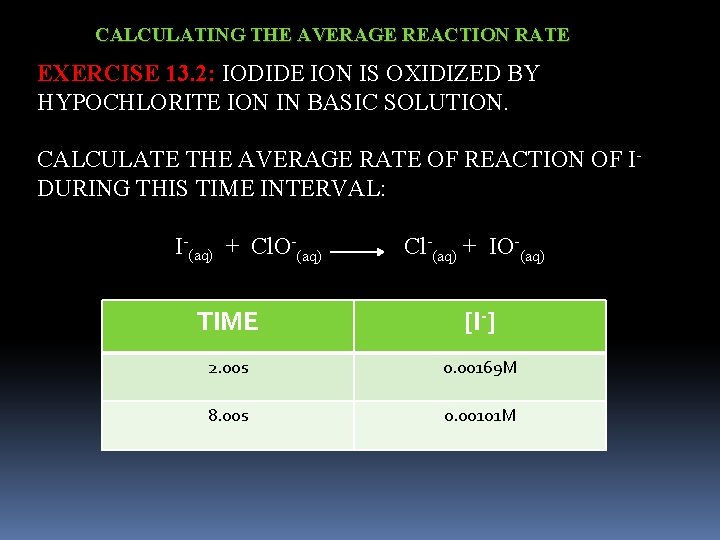
CALCULATING THE AVERAGE REACTION RATE EXAMPLE 13. 2: CALCULATE THE AVERAGE RATE OF DECOMPOSITION OF N 2 O 5(g) 2 NO 2(g) + 1/2 O 2(g) TIME [N 2 O 5] 600 s 1. 24 x 10 -2 M 1200 s 0. 93 x 10 -2 M RATE OF DECOMPOSITION N 2 O 5 = - [N 2 O 5] t -(0. 93 x 10 -2 M – 1. 24 x 10 -2 M) = -0. 31 x 10 -2 M = 5. 2 x 10 -6 M/s 1200 s – 600 s 600 s

CALCULATING THE AVERAGE REACTION RATE EXERCISE 13. 2: IODIDE ION IS OXIDIZED BY HYPOCHLORITE ION IN BASIC SOLUTION. CALCULATE THE AVERAGE RATE OF REACTION OF I- DURING THIS TIME INTERVAL: I-(aq) + Cl. O-(aq) Cl-(aq) + IO-(aq) TIME [I-] 2. 00 s 0. 00169 M 8. 00 s 0. 00101 M

Reaction Rate and Concentration The higher the conc. of starting reactant the more rapidly a reaction takes place 1. Reactions occur as the result of collisions between reactant molecules 2. The higher the concentration of molecules, the greater the # of collisions in unit time and a faster reaction 3. As the reactants are consumed the concentration decreases, collisions decrease, reaction rate decreases 4. Reaction rate decreases with time and eventually = 0, all reactants consumed 5. Instantaneous rate, rate at a particular time 6. Initial rate at t = 0
![Rate Expression and Rate Constant Rate expression rate law rate kA where Rate Expression and Rate Constant Rate expression / rate law: rate = k[A] where](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-11.jpg)
Rate Expression and Rate Constant Rate expression / rate law: rate = k[A] where k = rate constant, varies w/ nature and temp. [A] = concentration of A Rate law: an equation that related the rate of a reaction to the concentration of reactants (and catalyst) raised to various powers

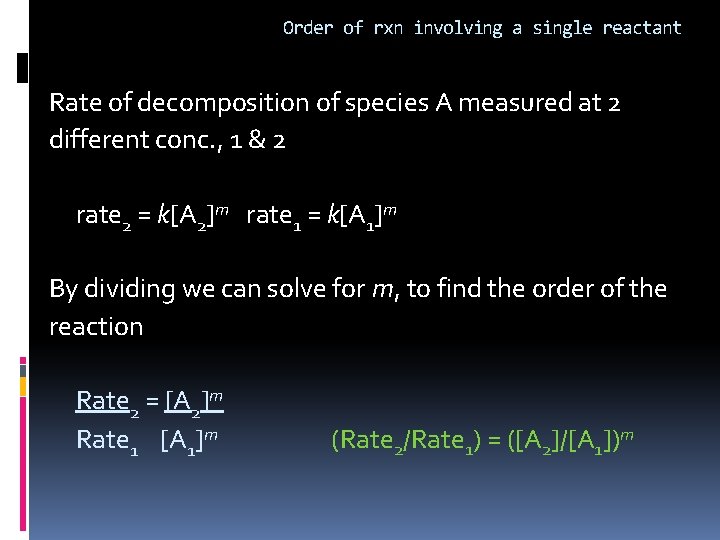
Order of rxn involving a single reactant General equation A products rate expression rate = k[A]m where m=order of reaction m=0 zero order m=1 first order m=2 second order m, can’t be deduced from the coefficient of the balanced equation. Must be determined experimentally!

Order of rxn involving a single reactant Rate of decomposition of species A measured at 2 different conc. , 1 & 2 rate 2 = k[A 2]m rate 1 = k[A 1]m By dividing we can solve for m, to find the order of the reaction Rate 2 = [A 2]m Rate 1 [A 1]m (Rate 2/Rate 1) = ([A 2]/[A 1])m

DETERMINING THE ORDER OF REACTION FROM RATE LAW EXAMPLE 13. 3: BROMIDE ION IS OXIDIZED BY BROMATE ION IN ACIDIC SOLUTION 5 Br -(aq) + Br. O 3 -(aq) + 6 H+(aq) 3 Br 2(aq) + 3 H 2 O(l) Rate = k[Br -][Br. O 3 -][H+]2 WHAT IS THE ORDER OF REACTION WITH RESPECT TO EACH REACTANT SPECIES? WHAT IS THE OVERALL ORDER OF REACTION? Br - IS FIRST ORDER Br. O 3 - IS FIRST ORDER H+ IS SECOND ORDER THE REACTION IS FOURTH ORDER OVERALL (1+1+2)=4

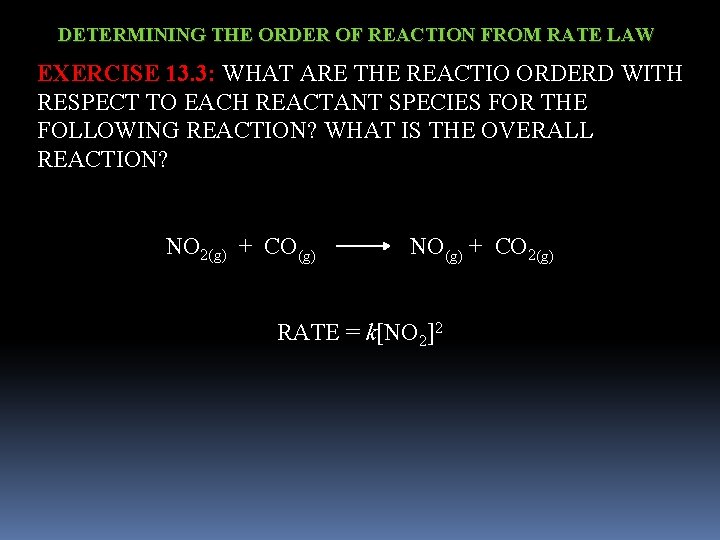
DETERMINING THE ORDER OF REACTION FROM RATE LAW EXERCISE 13. 3: WHAT ARE THE REACTIO ORDERD WITH RESPECT TO EACH REACTANT SPECIES FOR THE FOLLOWING REACTION? WHAT IS THE OVERALL REACTION? NO 2(g) + CO(g) NO(g) + CO 2(g) RATE = k[NO 2]2
![Example 1 CH 3 CHOg CH 4g COg CH 3 CHO 10 Example 1 CH 3 CHO(g) CH 4(g) + CO(g) [CH 3 CHO] . 10](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-16.jpg)
Example 1 CH 3 CHO(g) CH 4(g) + CO(g) [CH 3 CHO] . 10 M. 20 M Rate (mol/L s). 085 . 34 . 30 M . 40 M . 76 1. 4 Using the given data determine the reaction order
![Example 1 Rate 2 A 2m Rate 1 A 1m 4 2 Example 1 Rate 2 = [A 2]m Rate 1 [A 1]m 4 = 2](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-17.jpg)
Example 1 Rate 2 = [A 2]m Rate 1 [A 1]m 4 = 2 m log 4 log 2 second order m = 2 rate = k[CH 3 CHO]2 once the order of the rxn is known, rate constant can be calculated, let’s calculate the rate constant, k
![Example 1 rate kCH 3 CHO2 rate 085 molL s conc Example 1 rate = k[CH 3 CHO]2 rate =. 085 mol/L s conc =.](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-18.jpg)
Example 1 rate = k[CH 3 CHO]2 rate =. 085 mol/L s conc =. 10 mol/L k = rate = 0. 085 mol/L s = 8. 5 L/mol s [CH 3 CHO]2 (0. 10 mol/L)2 now we can calc. the rate at any concentration, let’s try . 55 M
![Example 1 rate kCH 3 CHO2 rate 8 5 Lmol s Example 1 rate = k[CH 3 CHO]2 rate = 8. 5 L/mol s [.](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-19.jpg)
Example 1 rate = k[CH 3 CHO]2 rate = 8. 5 L/mol s [. 55]2 = 2. 6 mol/ L s Rate when [CH 3 CHO] =. 55 M is 2. 6 mol/L s

Order of rxn with more than 1 reactant a. A + b. B prod rate exp: rate = k [A]m [B]n m = order of rxn with respect to A n = order of rxn with respect to B Overall order of the rxn is the sum, m + n

Key When more than 1 reactant is involved the order can be determined by holding the concentration of 1 reactant constant while varying the other reactant. From the measured rates you can calculate the order of the rxn with respect to the varying reactant

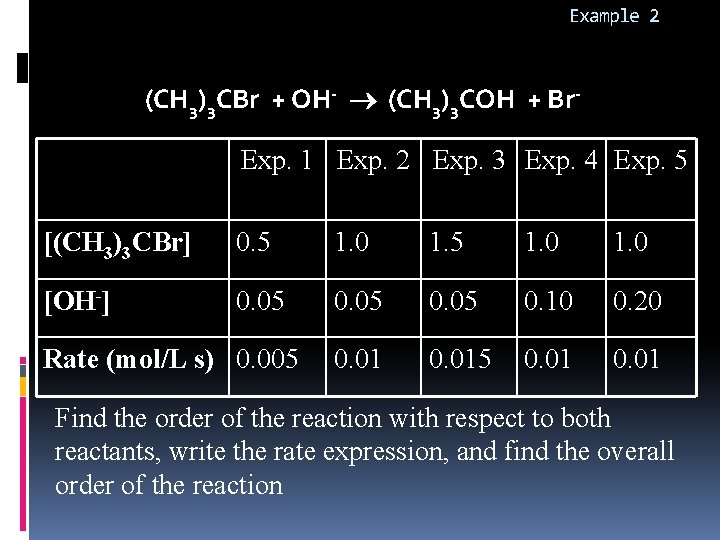
Example 2 (CH 3)3 CBr + OH- (CH 3)3 COH + Br. Exp. 1 Exp. 2 Exp. 3 Exp. 4 Exp. 5 [(CH 3)3 CBr] 0. 5 1. 0 [OH-] 0. 05 0. 10 0. 20 0. 015 0. 01 Rate (mol/L s) 0. 005 Find the order of the reaction with respect to both reactants, write the rate expression, and find the overall order of the reaction
![Reactant concentration and time Rate expression rate kA Shows how the rate of Reactant concentration and time Rate expression rate = k[A] Shows how the rate of](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-23.jpg)
Reactant concentration and time Rate expression rate = k[A] Shows how the rate of decomposition of A changes with concentration More important to know the relation between concentration and time Using calculus: Integrated rate equations relating react conc. to time

For the following rate law, what is the overall order of the reaction? Rate = k [A]2 [B] 1. 1 2. 2 3. 3 4. 4
![Zero Order Zero order A Products rate k CONCTIME RELATION A0 A Zero Order Zero order: A Products rate = k CONC-TIME RELATION [A]0 – [A]](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-25.jpg)
Zero Order Zero order: A Products rate = k CONC-TIME RELATION [A]0 – [A] = kt HALF LIFE t½ = [A]0 2 k m = 0: zero order - rate is independent of the concentration of reactant. Doubling the concentration has no effect on rate.
![First Order A Products rate kA ln AoA kt CONCTIME RELATION HALF First Order: A Products rate = k[A] ln [A]o/[A] = kt CONC-TIME RELATION HALF](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-26.jpg)
First Order: A Products rate = k[A] ln [A]o/[A] = kt CONC-TIME RELATION HALF LIFE t½ =. 693/k [A]o = original conc. of A [A] = Conc. of A at time, t k = first order rate constant ln = natural logarithm m = 1: first order - rate is directly proportional to the concentration of the reactant. Doubling the concentration increases the rate by a factor of 2.
![Second Order Second order A Products rate kA2 CONCTIME RELATION HALF LIFE 1 Second Order Second order: A Products rate = k[A]2 CONC-TIME RELATION HALF LIFE 1](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-27.jpg)
Second Order Second order: A Products rate = k[A]2 CONC-TIME RELATION HALF LIFE 1 – 1 = kt t½ = 1 [A]0 k[A]0 m = 2: second order - the rate is to the square of the concentration of the reactant. Doubling the concentration increases the rate by a factor of 4.

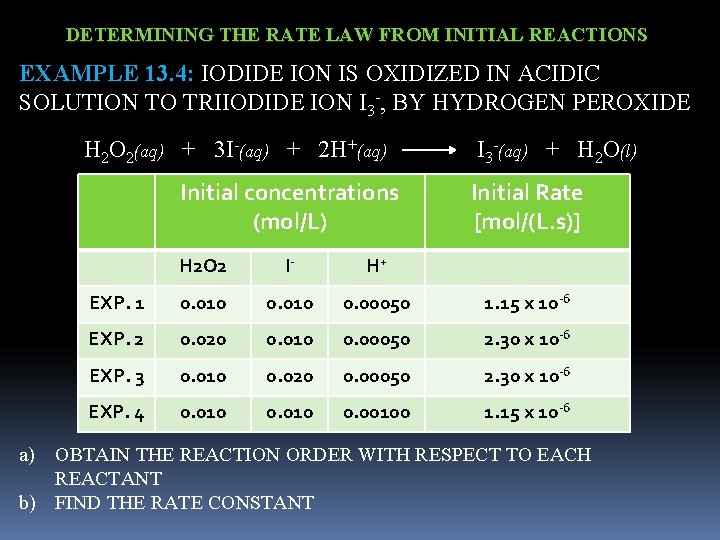
DETERMINING THE RATE LAW FROM INITIAL REACTIONS EXAMPLE 13. 4: IODIDE ION IS OXIDIZED IN ACIDIC SOLUTION TO TRIIODIDE ION I 3 -, BY HYDROGEN PEROXIDE H 2 O 2(aq) + 3 I-(aq) + 2 H+(aq) I 3 -(aq) + H 2 O(l) Initial concentrations (mol/L) Initial Rate [mol/(L. s)] H 2 O 2 I- H+ EXP. 1 0. 010 0. 00050 1. 15 x 10 -6 EXP. 2 0. 020 0. 010 0. 00050 2. 30 x 10 -6 EXP. 3 0. 010 0. 020 0. 00050 2. 30 x 10 -6 EXP. 4 0. 010 0. 00100 1. 15 x 10 -6 a) OBTAIN THE REACTION ORDER WITH RESPECT TO EACH REACTANT b) FIND THE RATE CONSTANT
![DETERMINING THE RATE LAW FROM INITIAL REACTIONS Rate kH 2 O 2m In DETERMINING THE RATE LAW FROM INITIAL REACTIONS Rate = k[H 2 O 2]m [I-]n](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-29.jpg)
DETERMINING THE RATE LAW FROM INITIAL REACTIONS Rate = k[H 2 O 2]m [I-]n [H+]p (Rate) 2 = k[H 2 O 2]m [I-]n [H+]p (Rate) 1 = k[H 2 O 2]m [I-]n [H+]p Rate constant cancels 2. 30 x 10 -6 = [0. 020]m [0. 010]n [0. 00050]p 1. 15 x 10 -6 = [0. 010]m [0. 010]n [0. 00050]p 2 = 2 m m = 1 doubling the H 2 O 2 concentration doubles the rate Rate = k[H 2 O 2] [I-] Reaction order = 1, 1, 0
![DETERMINING THE RATE LAW FROM INITIAL REACTIONS Rate kH 2 O 2 I DETERMINING THE RATE LAW FROM INITIAL REACTIONS Rate = k[H 2 O 2] [I-]](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-30.jpg)
DETERMINING THE RATE LAW FROM INITIAL REACTIONS Rate = k[H 2 O 2] [I-] 1. 15 x 10 -6 mol = k x 0. 010 mol L. s L k = 1. 15 x 10 -6 mol L. s 0. 010 mol x 0. 010 mol L k = 1. 2 x 10 -2 L/(mol. s)

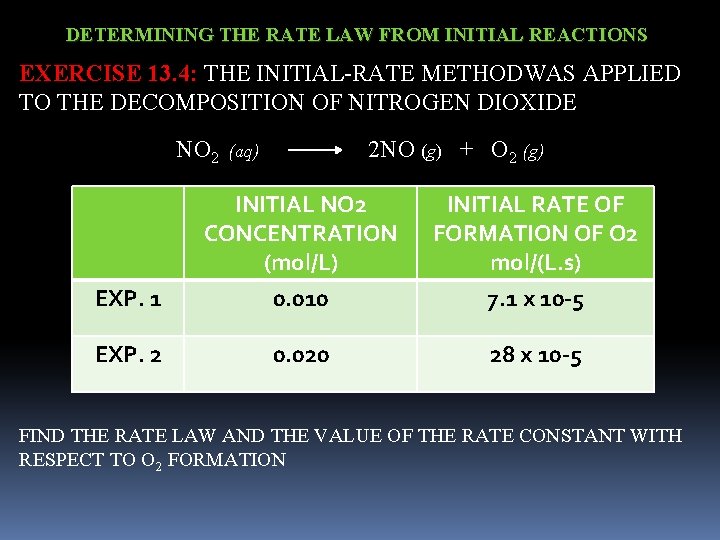
DETERMINING THE RATE LAW FROM INITIAL REACTIONS EXERCISE 13. 4: THE INITIAL-RATE METHODWAS APPLIED TO THE DECOMPOSITION OF NITROGEN DIOXIDE NO 2 (aq) 2 NO (g) + O 2 (g) INITIAL NO 2 CONCENTRATION (mol/L) INITIAL RATE OF FORMATION OF O 2 mol/(L. s) EXP. 1 0. 010 7. 1 x 10 -5 EXP. 2 0. 020 28 x 10 -5 FIND THE RATE LAW AND THE VALUE OF THE RATE CONSTANT WITH RESPECT TO O 2 FORMATION

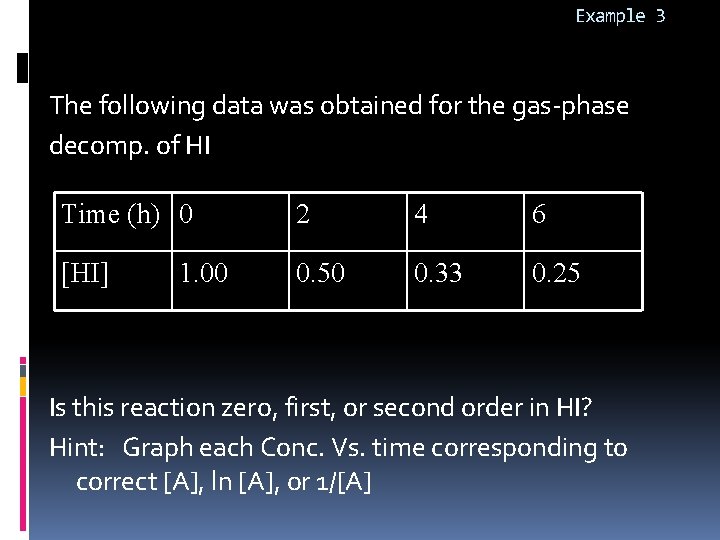
Example 3 The following data was obtained for the gas-phase decomp. of HI Time (h) 0 2 4 6 [HI] 0. 50 0. 33 0. 25 1. 00 Is this reaction zero, first, or second order in HI? Hint: Graph each Conc. Vs. time corresponding to correct [A], ln [A], or 1/[A]
![HI vs time not linear so it is not zero order A vs TIME [HI] vs. time not linear so it is not zero order [A] vs. TIME](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-33.jpg)
[HI] vs. time not linear so it is not zero order [A] vs. TIME FOR ZERO ORDER REACTION
![ln HI vs time not linear so it is not first order lnA vs ln [HI] vs. time not linear so it is not first order ln[A] vs.](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-34.jpg)
ln [HI] vs. time not linear so it is not first order ln[A] vs. TIME FOR 1 ST ORDER REACTION
![1HI vs time is linear so it is second order 1lnA vs TIME FOR 1/[HI] vs. time is linear so it is second order 1/ln[A] vs. TIME FOR](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-35.jpg)
1/[HI] vs. time is linear so it is second order 1/ln[A] vs. TIME FOR 2 ND ORDER REACTION
![Order Rate Expression ConcTime Relation Halflife 0 Rate k A0 A Order Rate Expression Conc-Time Relation Half-life 0 Rate = k [A]0 – [A] =](https://slidetodoc.com/presentation_image_h/76544061db160aa33f63a6a40508b809/image-36.jpg)
Order Rate Expression Conc-Time Relation Half-life 0 Rate = k [A]0 – [A] = kt [A]0 2 k [A] vs. t 1 Rate = k[A] ln [A]0 = kt [A] ln [A] vs. t 2 Rate = k[A]2 1 – 1 = kt [A]0 0. 693 k 1 k[A]0 Linear Plot 1 vs. t [A]

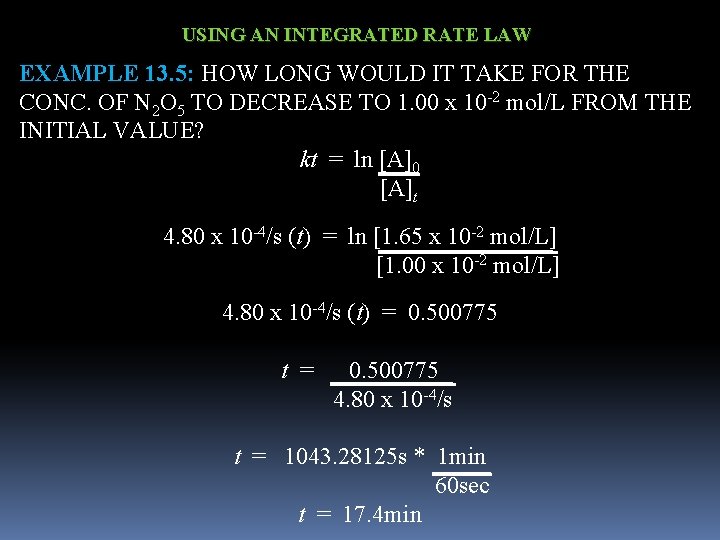
USING AN INTEGRATED RATE LAW EXAMPLE 13. 5: HOW LONG WOULD IT TAKE FOR THE CONC. OF N 2 O 5 TO DECREASE TO 1. 00 x 10 -2 mol/L FROM THE INITIAL VALUE? kt = ln [A]0 [A]t 4. 80 x 10 -4/s (t) = ln [1. 65 x 10 -2 mol/L] [1. 00 x 10 -2 mol/L] 4. 80 x 10 -4/s (t) = 0. 500775 t = 0. 500775 4. 80 x 10 -4/s t = 1043. 28125 s * 1 min 60 sec t = 17. 4 min

USING AN INTEGRATED RATE LAW EXERCISE 13. 5: A) WHAT WOULD THE CONCENTRATION BE OF DINITROGEN PENTOXIDE IN THE EXPERIMENT DESCRIBED IN EXAMPLE 13. 5 AFTER 6. 00 x 102 s? B) HOW LONG WOULD IT TAKE FOR THE CONCENTRATION OF N 2 O 5 TO DECREASE TO 10% OF ITS INITIAL VALUE?

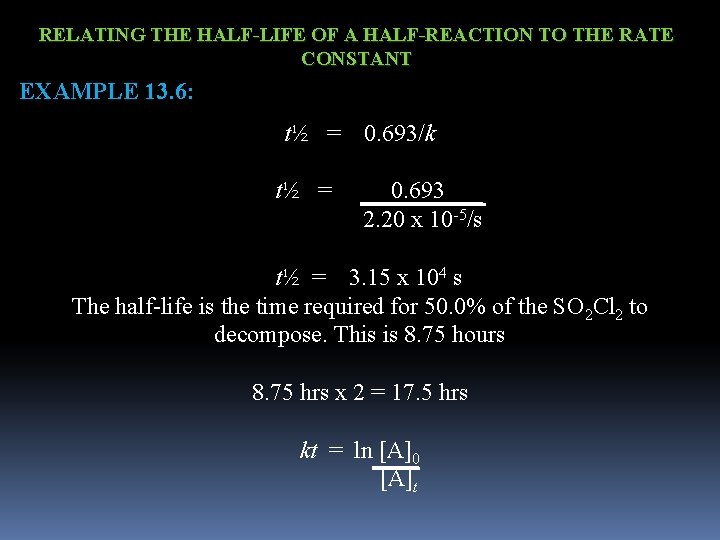
RELATING THE HALF-LIFE OF A HALF-REACTION TO THE RATE CONSTANT EXAMPLE 13. 6: SULFURYL CHLORIDE, SOC 2 Cl 2, IS A COLORLESS CORROSIVE LIQUID WHOSE VAPOR DECOMPOSES IN A FIRST-ORDER REACTION TO SULFUR DIOXIDE AND CHLORINE. SO 2 Cl 2 (g) SO 2 (g) + Cl 2 (g) AT 320 o. C, THE RATE CONSTANT IS 2. 20 x 10 -5/s. WHAT IS THE HALF-LIFE OF SO 2 Cl 2 VAPOR AT THIS TEMPERATURE? A) HOW LONG (IN HOURS) WOULD IT TAKE FOR 50. 0% OF THE SO 2 Cl 2 TO DECOMPOSE? B) HOW LONG WOULD IT FOR 75. 0% OF THE SO 2 Cl 2?

RELATING THE HALF-LIFE OF A HALF-REACTION TO THE RATE CONSTANT EXAMPLE 13. 6: t½ = 0. 693/k t½ = 0. 693 2. 20 x 10 -5/s t½ = 3. 15 x 104 s The half-life is the time required for 50. 0% of the SO 2 Cl 2 to decompose. This is 8. 75 hours 8. 75 hrs x 2 = 17. 5 hrs kt = ln [A]0 [A]t

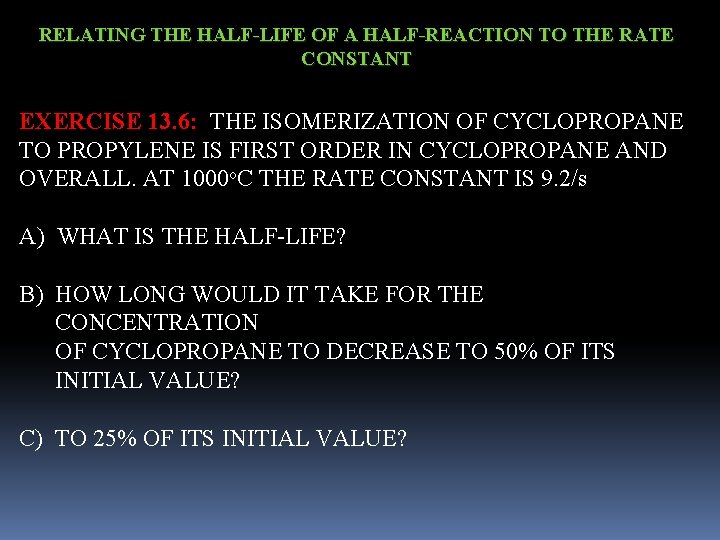
RELATING THE HALF-LIFE OF A HALF-REACTION TO THE RATE CONSTANT EXERCISE 13. 6: THE ISOMERIZATION OF CYCLOPROPANE TO PROPYLENE IS FIRST ORDER IN CYCLOPROPANE AND OVERALL. AT 1000 o. C THE RATE CONSTANT IS 9. 2/s A) WHAT IS THE HALF-LIFE? B) HOW LONG WOULD IT TAKE FOR THE CONCENTRATION OF CYCLOPROPANE TO DECREASE TO 50% OF ITS INITIAL VALUE? C) TO 25% OF ITS INITIAL VALUE?

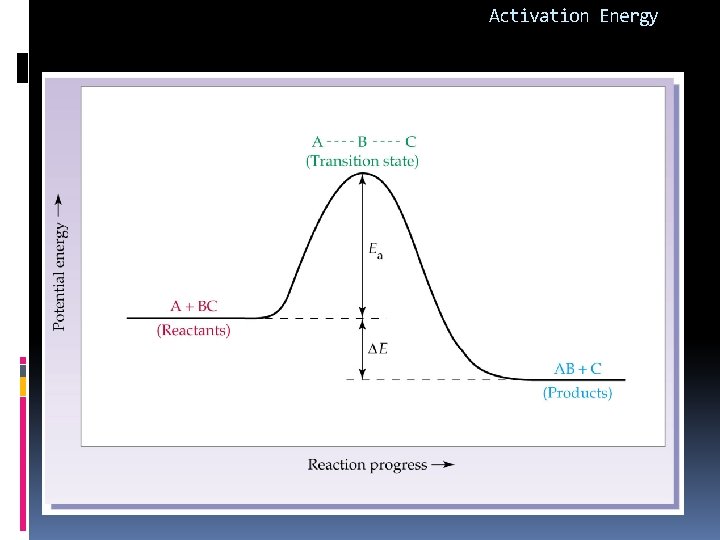
Activation Energy: Ea (k. J) For every reaction there is a certain minimum energy that molecules must possess for collisions to be effective. 1. Positive quantity (Ea>0) 2. Depends only upon the nature of reaction 3. Fast reaction = small Ea 4. Is independent of temp and concentration

For the following reaction: A + B C If the concentration of A is doubled, and B is constant, the rate doubles. What is the order of the reaction with respect to A? 1. 0 2. 1 3. 2

Activation Energy

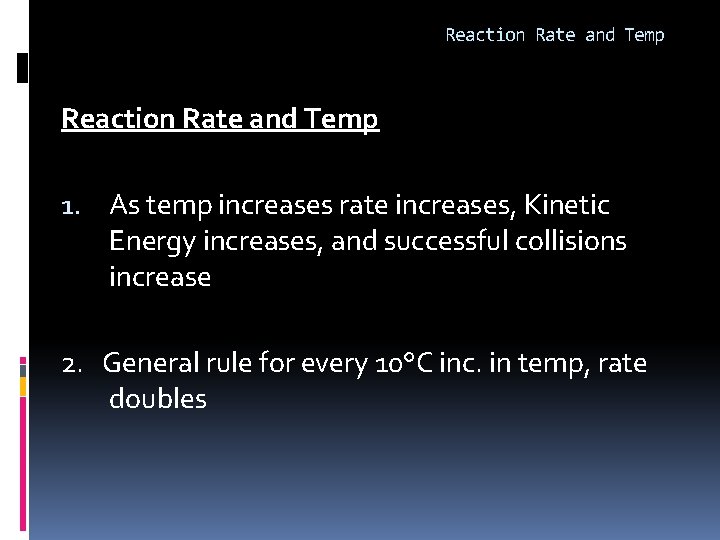
Reaction Rate and Temp 1. As temp increases rate increases, Kinetic Energy increases, and successful collisions increase 2. General rule for every 10°C inc. in temp, rate doubles

If I increase the temperature of a reaction from 110 K to 120 K, what happens to the rate of the reaction? 1. Stay the same 2. Doubles 3. Triples

The Arrhenius Equation f = e-Ea/RT f = fraction of molecules having an En. equal to or greater than E a R = gas constant A = constant T = temp in K ln k = ln A –Ea/RT plot of ln k Vs. 1/T linear slope = -Ea/R Two-point equation relating k & T ln k 2 = Ea [1/T 1 – 1/T 2] k 1 R

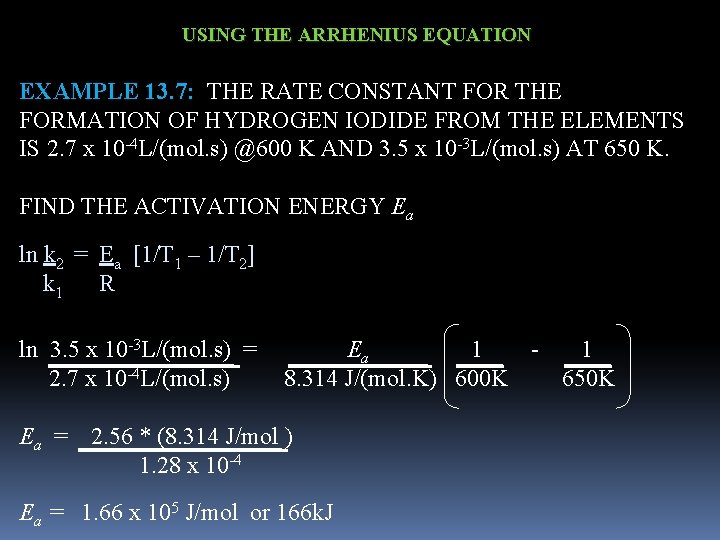
USING THE ARRHENIUS EQUATION EXAMPLE 13. 7: THE RATE CONSTANT FOR THE FORMATION OF HYDROGEN IODIDE FROM THE ELEMENTS IS 2. 7 x 10 -4 L/(mol. s) @600 K AND 3. 5 x 10 -3 L/(mol. s) AT 650 K. FIND THE ACTIVATION ENERGY Ea ln k 2 = Ea [1/T 1 – 1/T 2] k 1 R ln 3. 5 x 10 -3 L/(mol. s) = Ea 1 - 1 2. 7 x 10 -4 L/(mol. s) 8. 314 J/(mol. K) 600 K 650 K Ea = 2. 56 * (8. 314 J/mol ) 1. 28 x 10 -4 Ea = 1. 66 x 105 J/mol or 166 k. J

USING THE ARRHENIUS EQUATION EXERCISE 13. 7: ACETALDEHYDE DECOMPOSES WHEN HEATED. THE RATE OF DECOMPOSITION IS 1. 05 x 10 -3 L/mol. s AT 759 K AND 2. 14 x 10 -2 L/mol. s AT 836 K. CH 3 CHO(g) CH 4(g) + CO(g) WHAT IS ACTIVATION ENERGY FOR THIS DECOMPOSITION? WHAT IS THE RATE CONSTANT AT 865 K?

Example 4 For a certain rxn the rate constant doubles when the temp increases from 15 to 25°C. a) Calc. The activation energy, Ea b) Calc. the rate constant at 100°C, taking k at 25°C to be 1. 2 x 10 -2 L/mol s

Reaction Mechanism Description of a path, or a sequence of steps, by which a reaction occurs at the molecular level. Simplest case- only a single step, collision between two reactant molecules

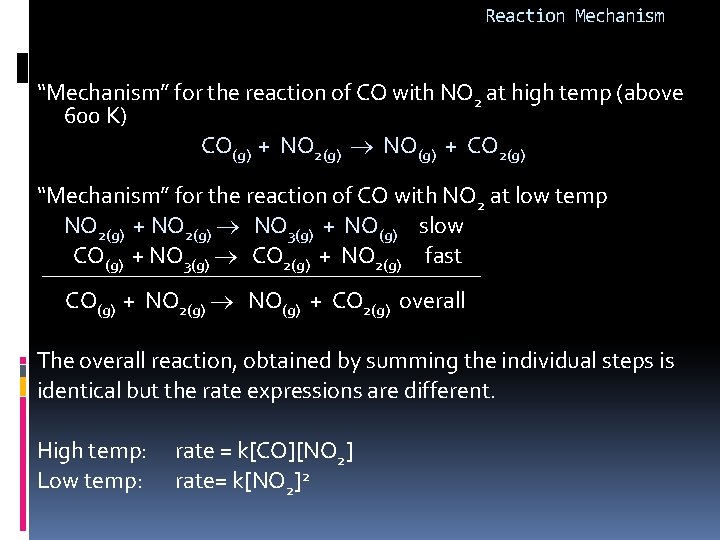
Reaction Mechanism “Mechanism” for the reaction of CO with NO 2 at high temp (above 600 K) CO(g) + NO 2(g) NO(g) + CO 2(g) “Mechanism” for the reaction of CO with NO 2 at low temp NO 2(g) + NO 2(g) NO 3(g) + NO(g) slow CO(g) + NO 3(g) CO 2(g) + NO 2(g) fast CO(g) + NO 2(g) NO(g) + CO 2(g) overall The overall reaction, obtained by summing the individual steps is identical but the rate expressions are different. High temp: rate = k[CO][NO 2] Low temp: rate= k[NO 2]2

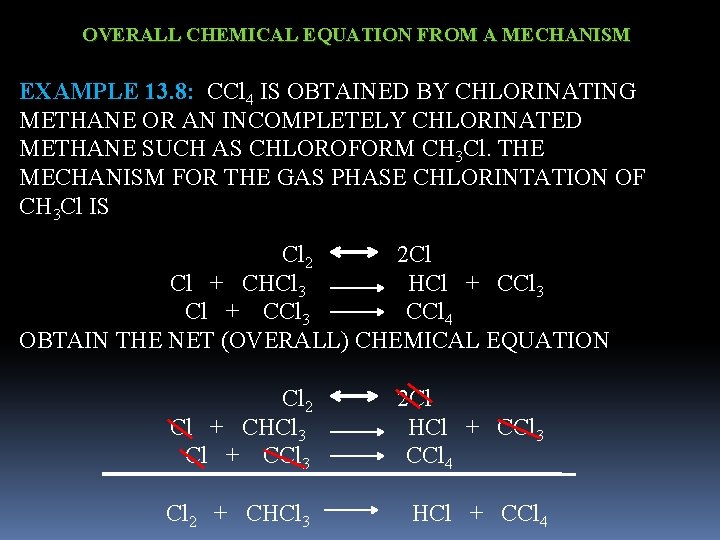
OVERALL CHEMICAL EQUATION FROM A MECHANISM EXAMPLE 13. 8: CCl 4 IS OBTAINED BY CHLORINATING METHANE OR AN INCOMPLETELY CHLORINATED METHANE SUCH AS CHLOROFORM CH 3 Cl. THE MECHANISM FOR THE GAS PHASE CHLORINTATION OF CH 3 Cl IS Cl 2 2 Cl Cl + CHCl 3 HCl + CCl 3 CCl 4 OBTAIN THE NET (OVERALL) CHEMICAL EQUATION Cl 2 2 Cl Cl + CHCl 3 HCl + CCl 3 CCl 4 Cl 2 + CHCl 3 HCl + CCl 4

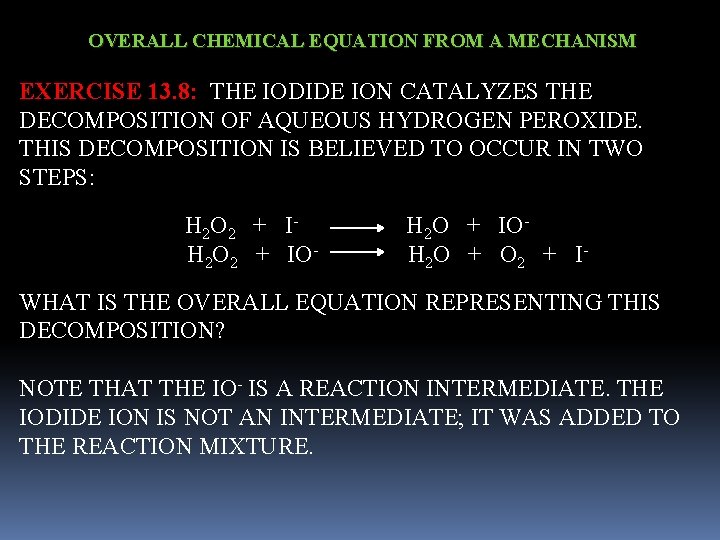
OVERALL CHEMICAL EQUATION FROM A MECHANISM EXERCISE 13. 8: THE IODIDE ION CATALYZES THE DECOMPOSITION OF AQUEOUS HYDROGEN PEROXIDE. THIS DECOMPOSITION IS BELIEVED TO OCCUR IN TWO STEPS: H 2 O 2 + I- H 2 O + IO H 2 O 2 + IO- H 2 O + O 2 + IWHAT IS THE OVERALL EQUATION REPRESENTING THIS DECOMPOSITION? NOTE THAT THE IO- IS A REACTION INTERMEDIATE. THE IODIDE ION IS NOT AN INTERMEDIATE; IT WAS ADDED TO THE REACTION MIXTURE.

Reaction Mechanism Elementary Steps: Individual steps that constitute a reaction mechanism Unimolecular A B + C rate = k[A] Bimolecular A + A B + C rate = k[A][A] = [A]2 Termolecular A + B + C D + E rate = k[A][B][C] The rate of an elementary step is equal to a rate constant, k, multiplied by the concentration of each reactant molecule. You can treat all reactants as if they were first order. If a reactant is second order it will appear twice in the general equation.

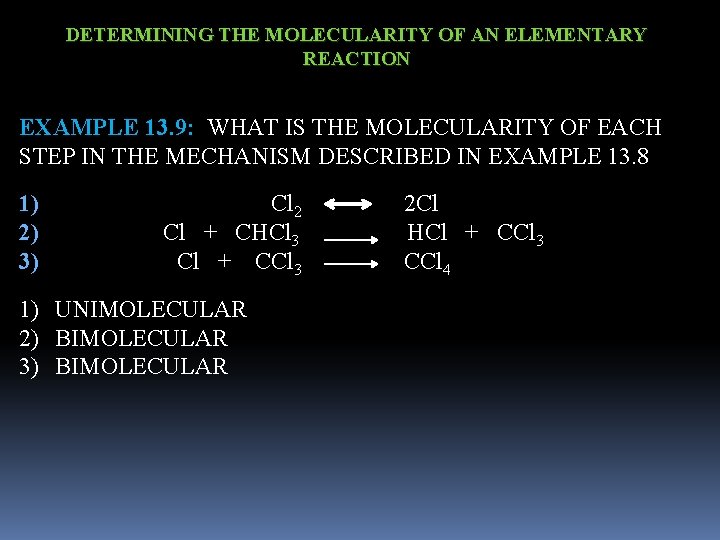
DETERMINING THE MOLECULARITY OF AN ELEMENTARY REACTION EXAMPLE 13. 9: WHAT IS THE MOLECULARITY OF EACH STEP IN THE MECHANISM DESCRIBED IN EXAMPLE 13. 8 1) 2) 3) Cl 2 2 Cl Cl + CHCl 3 HCl + CCl 3 CCl 4 1) UNIMOLECULAR 2) BIMOLECULAR 3) BIMOLECULAR

DETERMINING THE MOLECULARITY OF AN ELEMENTARY REACTION EXAMPLE 13. 9: THE FOLLOWING IS AN ELEMENTARY REACTION THAT OCCURS IN THE DECOMPOSITION OF OZONE IN THE STRATOSPHERE BY NITROGEN MONOXIDE NO + O 3 O 2 + NO 2 WHAT IS THE MOLECULARITY OF THIS REACTION?

Reaction Mechanism Slow Steps- A step that is much slower than any other in a reaction mechanism. Rate-determining step - The rate of the overall reaction can be taken to be that of the slow step Step 1: A B fast Step 2: B C slow Step 3: C D fast A D The rate A D (overall reaction) is approx. equal to the rate of B C the slow step

Deducing a Rate Expression from a Proposed Mechanism 1. Find the slowest step and equate the rate of the overall reaction to the rate of that step. 2. Find the rate expression for the slowest step. NO 2(g) + NO 2(g) NO 3(g) + NO(g) CO(g) + NO 2(g) CO 2(g) + NO 2(g) slow fast CO(g) + NO 2(g) CO 2(g) + NO(g) Rate of overall reaction = rate of 1 st step = k[NO 2]2

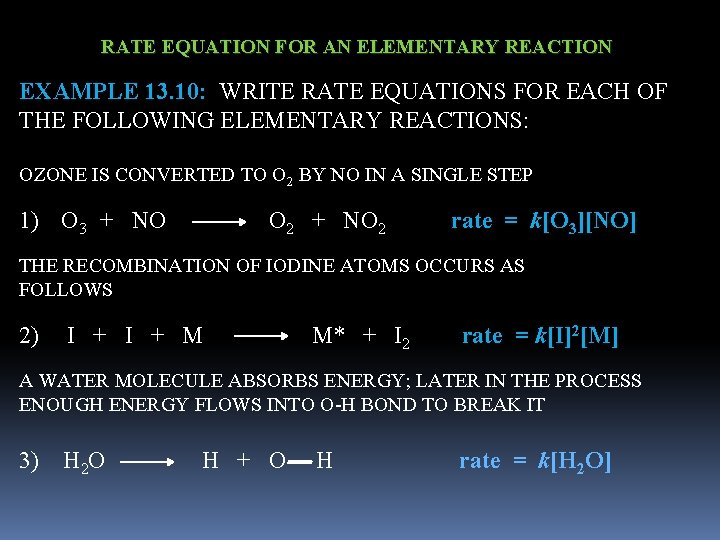
RATE EQUATION FOR AN ELEMENTARY REACTION EXAMPLE 13. 10: WRITE RATE EQUATIONS FOR EACH OF THE FOLLOWING ELEMENTARY REACTIONS: OZONE IS CONVERTED TO O 2 BY NO IN A SINGLE STEP 1) O 3 + NO O 2 + NO 2 rate = k[O 3][NO] THE RECOMBINATION OF IODINE ATOMS OCCURS AS FOLLOWS 2) I + M M* + I 2 rate = k[I]2[M] A WATER MOLECULE ABSORBS ENERGY; LATER IN THE PROCESS ENOUGH ENERGY FLOWS INTO O-H BOND TO BREAK IT 3) H 2 O H + O H rate = k[H 2 O]

RATE EQUATION FOR AN ELEMENTARY REACTION EXAMPLE 13. 10: WRITE RATE EQUATIONS FOR THE FOLLOWING ELEMENTARY REACTION: NO 2 + NO 2 + N 2 O 4

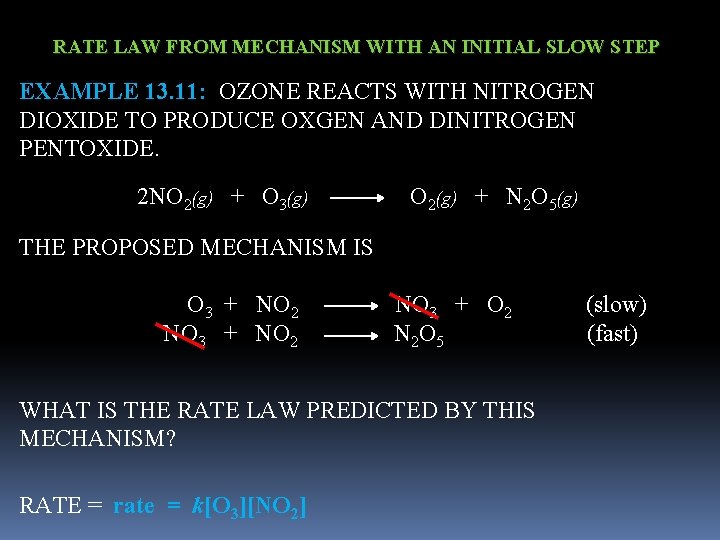
RATE LAW FROM MECHANISM WITH AN INITIAL SLOW STEP EXAMPLE 13. 11: OZONE REACTS WITH NITROGEN DIOXIDE TO PRODUCE OXGEN AND DINITROGEN PENTOXIDE. 2 NO 2(g) + O 3(g) O 2(g) + N 2 O 5(g) THE PROPOSED MECHANISM IS O 3 + NO 2 NO 3 + O 2 (slow) NO 3 + NO 2 N 2 O 5 (fast) WHAT IS THE RATE LAW PREDICTED BY THIS MECHANISM? RATE = rate = k[O 3][NO 2]

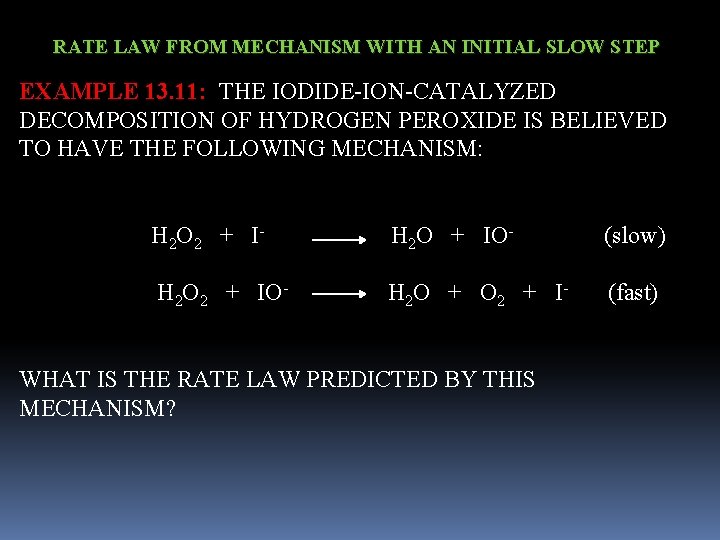
RATE LAW FROM MECHANISM WITH AN INITIAL SLOW STEP EXAMPLE 13. 11: THE IODIDE-ION-CATALYZED DECOMPOSITION OF HYDROGEN PEROXIDE IS BELIEVED TO HAVE THE FOLLOWING MECHANISM: H 2 O 2 + I- H 2 O + IO- (slow) H 2 O 2 + IO- H 2 O + O 2 + I- (fast) WHAT IS THE RATE LAW PREDICTED BY THIS MECHANISM?

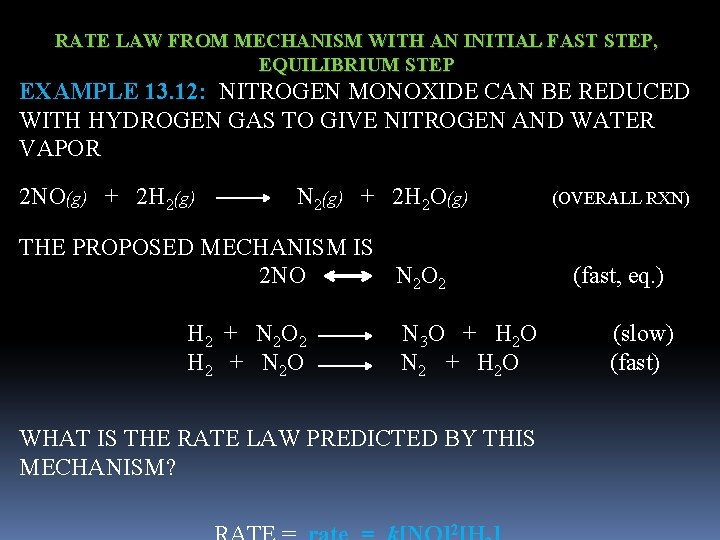
RATE LAW FROM MECHANISM WITH AN INITIAL FAST STEP, EQUILIBRIUM STEP EXAMPLE 13. 12: NITROGEN MONOXIDE CAN BE REDUCED WITH HYDROGEN GAS TO GIVE NITROGEN AND WATER VAPOR 2 NO(g) + 2 H 2(g) N 2(g) + 2 H 2 O(g) (OVERALL RXN) THE PROPOSED MECHANISM IS 2 NO N 2 O 2 (fast, eq. ) H 2 + N 2 O 2 N 3 O + H 2 O (slow) H 2 + N 2 O N 2 + H 2 O (fast) WHAT IS THE RATE LAW PREDICTED BY THIS MECHANISM? 2

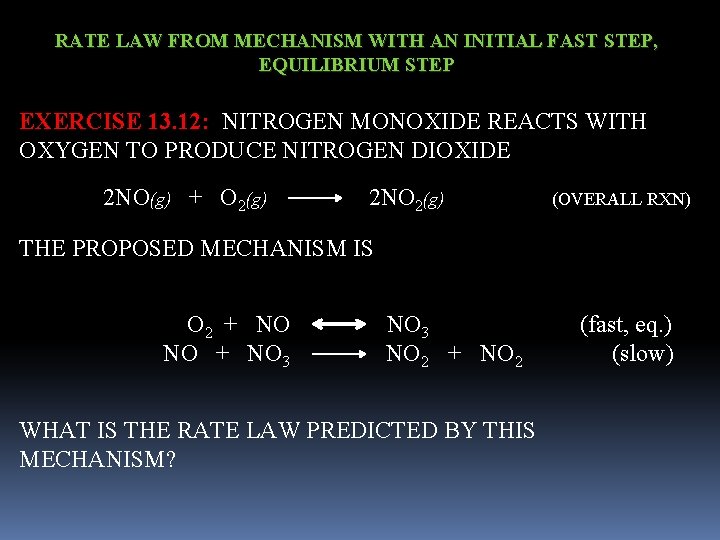
RATE LAW FROM MECHANISM WITH AN INITIAL FAST STEP, EQUILIBRIUM STEP EXERCISE 13. 12: NITROGEN MONOXIDE REACTS WITH OXYGEN TO PRODUCE NITROGEN DIOXIDE 2 NO(g) + O 2(g) 2 NO 2(g) (OVERALL RXN) THE PROPOSED MECHANISM IS O 2 + NO 3 (fast, eq. ) NO + NO 3 NO 2 + NO 2 (slow) WHAT IS THE RATE LAW PREDICTED BY THIS MECHANISM?

Elimination of Intermediates Intermediate 1. A species produced in one step of the mechanism and consumed in a later step. 2. Concentration too small to determine experimentally 3. Must be eliminated from rate expression 4. The final rate expression must include only those species that appear in the balanced equation for the overall reaction

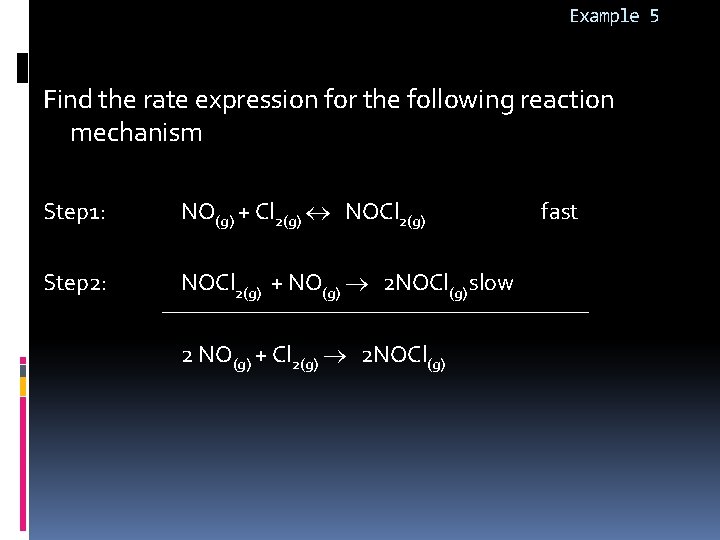
Example 5 Find the rate expression for the following reaction mechanism Step 1: Step 2: NO(g) + Cl 2(g) NOCl 2(g) + NO(g) 2 NOCl(g) slow 2 NO(g) + Cl 2(g) 2 NOCl(g) fast

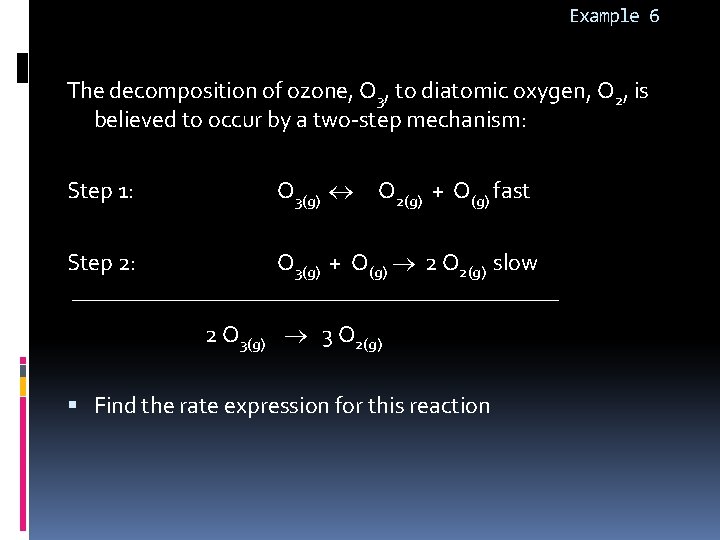
Example 6 The decomposition of ozone, O 3, to diatomic oxygen, O 2, is believed to occur by a two-step mechanism: Step 1: O 3(g) O 2(g) + O(g) fast Step 2: O 3(g) + O(g) 2 O 2(g) slow 2 O 3(g) 3 O 2(g) Find the rate expression for this reaction

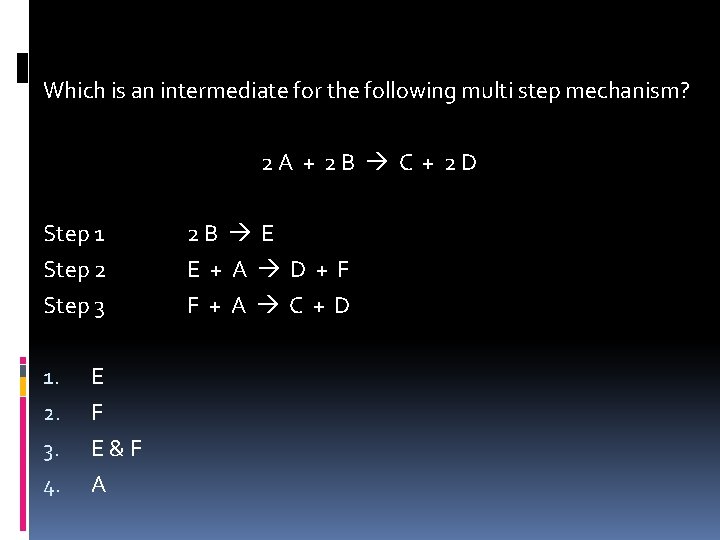
Which is an intermediate for the following multi step mechanism? 2 A + 2 B C + 2 D Step 1 Step 2 Step 3 1. 2. 3. 4. E F E & F A 2 B E E + A D + F F + A C + D

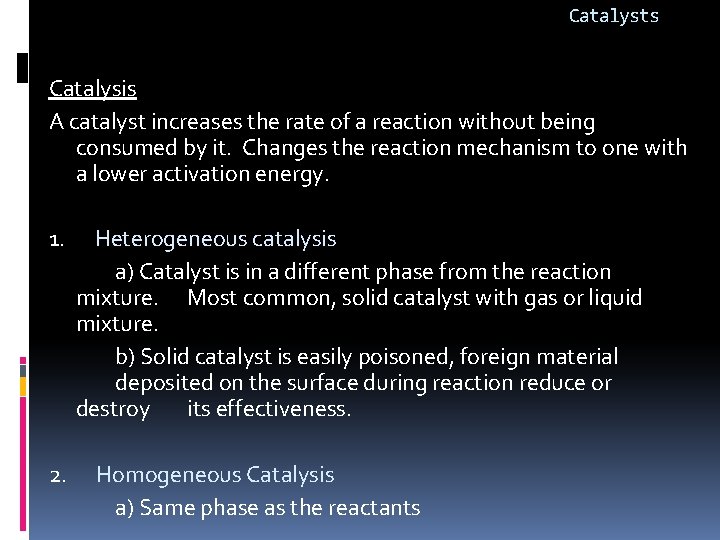
Catalysts Catalysis A catalyst increases the rate of a reaction without being consumed by it. Changes the reaction mechanism to one with a lower activation energy. 1. Heterogeneous catalysis a) Catalyst is in a different phase from the reaction mixture. Most common, solid catalyst with gas or liquid mixture. b) Solid catalyst is easily poisoned, foreign material deposited on the surface during reaction reduce or destroy its effectiveness. 2. Homogeneous Catalysis a) Same phase as the reactants

Which is the catalyst for the following multi step mechanism? 2 A + 2 B C + 2 D Step 1 2 B + G E Step 2 E + A D + F Step 3 F + A C + D + G 1. 2. 3. 4. E G E & F A

SUMMARY PROBLEM AND HOMEWORK PROBLEMS Chapter 12: Kinetics

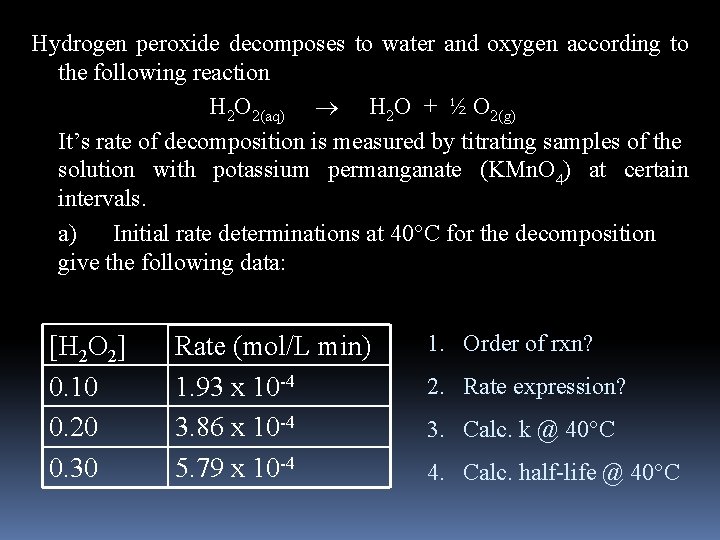
Hydrogen peroxide decomposes to water and oxygen according to the following reaction H 2 O 2(aq) H 2 O + ½ O 2(g) It’s rate of decomposition is measured by titrating samples of the solution with potassium permanganate (KMn. O 4) at certain intervals. a) Initial rate determinations at 40 C for the decomposition give the following data: [H 2 O 2] 0. 10 0. 20 0. 30 Rate (mol/L min) 1. 93 x 10 -4 3. 86 x 10 -4 5. 79 x 10 -4 1. Order of rxn? 2. Rate expression? 3. Calc. k @ 40°C 4. Calc. half-life @ 40°C

b) Hydrogen peroxide is sold commercially as a 30. 0% solution. If the solution is kept at 40 C, how long will it take for the solution to become 10. 0% H 2 O 2?

c) It has been determined that at 50 C, the rate constant for the reaction is 4. 32 x 10 -3/min. Calculate the activation energy for the decomposition of H 2 O 2

d) Manufacturers recommend that solutions of hydrogen peroxide be kept in a refrigerator at 4 C. How long will it take for a 30. 0% solution to decompose to 10. 0% if the solution is kept at 4 C?

e) The rate constant for the uncatalyzed reaction at 25 C is 5. 21 x 10 -4/min. The rate constant for the catalyzed reaction at 25 C is 2. 95 x 108/min. 1) What is the half-life of the uncatalyzed reaction at 25 C? 2) What is the half-life of the catalyzed reaction?

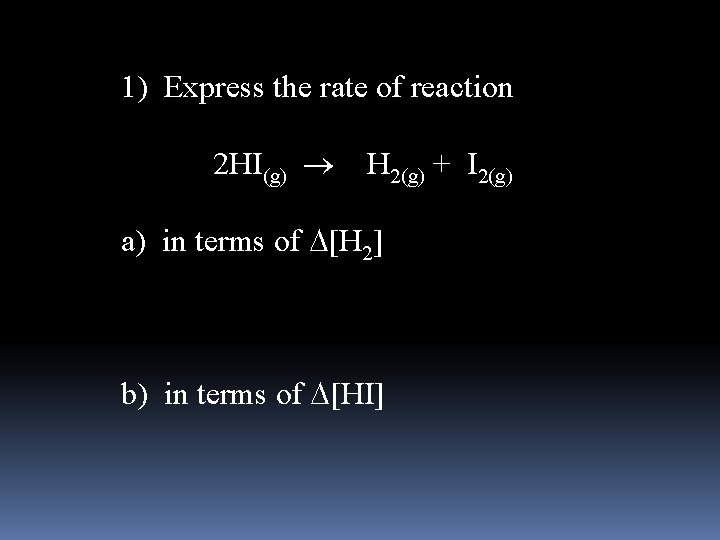
1) Express the rate of reaction 2 HI(g) H 2(g) + I 2(g) a) in terms of [H 2] b) in terms of [HI]

3) Dinitrogen pentaoxide decomposes according to the following equation: 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) a) write an expression for reaction rate in terms of [ N 2 O 5], [NO 2], and [O 2]

4) What is the order with respect to each reactant and the overall order of the reactions described by the following rate expressions? a) rate = k 1[A]3 b) rate = k 2[A][B] c) rate = k 3[A][B]2 d) rate = k 4[B] e) rate = k

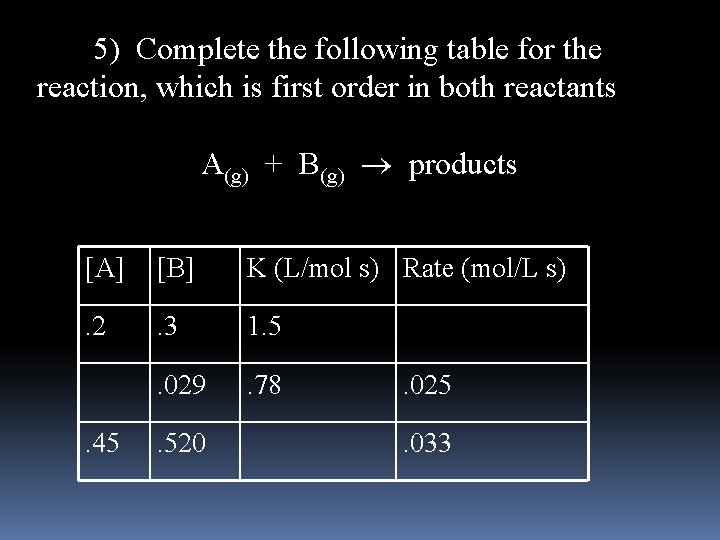
5) Complete the following table for the reaction, which is first order in both reactants A(g) + B(g) products [A] [B] K (L/mol s) Rate (mol/L s) . 2 . 3 1. 5 . 029 . 78 . 45 . 520 . 025. 033

7) The decomposition of ammonia on tungsten at 1100 C is zero-order, with a rate constant of 2. 5 x 10 -4 mol/L min a) write the rate expression b) calculate the rate when the concentration of ammonia is 0. 080 M c) At what concentration of ammonia is the rate equal to the rate constant?

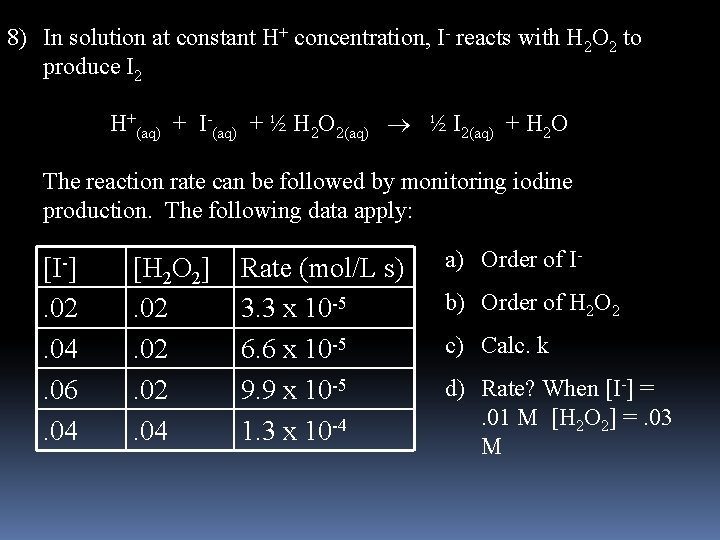
8) In solution at constant H+ concentration, I- reacts with H 2 O 2 to produce I 2 H+(aq) + I-(aq) + ½ H 2 O 2(aq) ½ I 2(aq) + H 2 O The reaction rate can be followed by monitoring iodine production. The following data apply: [I-]. 02. 04. 06. 04 [H 2 O 2]. 02. 02. 04 Rate (mol/L s) 3. 3 x 10 -5 6. 6 x 10 -5 9. 9 x 10 -5 1. 3 x 10 -4 a) Order of Ib) Order of H 2 O 2 c) Calc. k d) Rate? When [I-] = . 01 M [H 2 O 2] =. 03 M

9) In the first-order decomposition of acetone at 500 C it is found that the concentration is 0. 0300 M after 200 min and 0. 0200 M after 400 min. H 3 C-CO-CH 3(g) products Calculate a) The rate constant b) The half-life c) The initial concentration

10) The decomposition of hydrogen iodide is second-order. Its half-life is 85 seconds when the initial conc. is 0. 15 M HI(g) ½ H 2(g) + ½ I 2(g) a) What is k for the reaction? b) How long will it take to go from 0. 300 M to 0. 100 M?

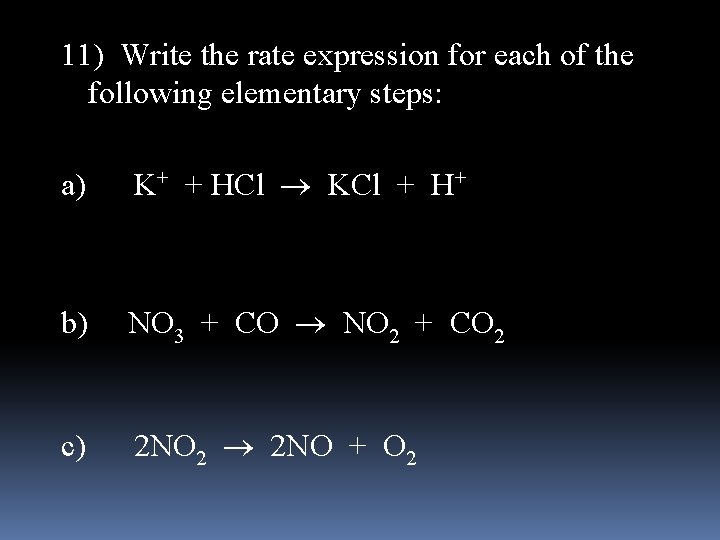
11) Write the rate expression for each of the following elementary steps: a) K+ + HCl KCl + H+ b) NO 3 + CO NO 2 + CO 2 c) 2 NO 2 2 NO + O 2

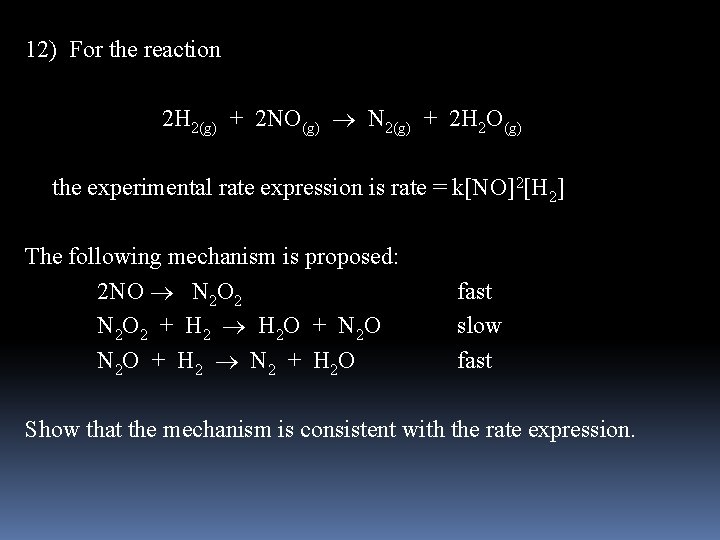
12) For the reaction 2 H 2(g) + 2 NO(g) N 2(g) + 2 H 2 O(g) the experimental rate expression is rate = k[NO]2[H 2] The following mechanism is proposed: 2 NO N 2 O 2 fast N 2 O 2 + H 2 O + N 2 O slow N 2 O + H 2 N 2 + H 2 O fast Show that the mechanism is consistent with the rate expression.
 Is a ratio a rate
Is a ratio a rate Equivalent ratios guided notes
Equivalent ratios guided notes Ratios rates and unit rates
Ratios rates and unit rates Ratios rates and unit rates
Ratios rates and unit rates Reaction rates and equilibrium worksheet answers chapter 19
Reaction rates and equilibrium worksheet answers chapter 19 Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Chapter 18 reaction rates and equilibrium
Chapter 18 reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Mini unit reaction rates and equilibrium
Mini unit reaction rates and equilibrium Section 4 reaction rates and equilibrium
Section 4 reaction rates and equilibrium Expressing reaction rates
Expressing reaction rates Rates of reaction can be positive or negative. true false
Rates of reaction can be positive or negative. true false Expressing reaction rates
Expressing reaction rates Did a chemical reaction occur
Did a chemical reaction occur Rate of reaction
Rate of reaction E1cb elimination reaction
E1cb elimination reaction Leukoerythroblastic reaction vs leukemoid reaction
Leukoerythroblastic reaction vs leukemoid reaction Half life formula
Half life formula Chapter 7 interest rates and bond valuation
Chapter 7 interest rates and bond valuation Chapter 8 risk and rates of return problem solutions
Chapter 8 risk and rates of return problem solutions Your uncle would like to restrict his interest rate risk
Your uncle would like to restrict his interest rate risk Chapter 6 interest rates and bond valuation
Chapter 6 interest rates and bond valuation Chapter 7 interest rates and bond valuation
Chapter 7 interest rates and bond valuation Chapter 6 interest rates and bond valuation
Chapter 6 interest rates and bond valuation Vanessa borilot
Vanessa borilot Vanessa jason www.biology-roots.com
Vanessa jason www.biology-roots.com Vanessa bouchet
Vanessa bouchet Vagga
Vagga Vanessa charest
Vanessa charest Vanessa graber
Vanessa graber Vanessa manni
Vanessa manni Mononucleres
Mononucleres Vanessa loi
Vanessa loi Cyrille las chicas de alambre
Cyrille las chicas de alambre Vanessa manni
Vanessa manni Vanessa prasad
Vanessa prasad Vanessa tipton
Vanessa tipton Vanessa meza
Vanessa meza Nihongo benkyou
Nihongo benkyou Vanessa meja
Vanessa meja Cmu slice reimbursement
Cmu slice reimbursement Vanessa ferry
Vanessa ferry Vanessa gaffar
Vanessa gaffar Jane fonda barbarella
Jane fonda barbarella Vanessa chaves miranda
Vanessa chaves miranda Vanessa aellen
Vanessa aellen It's friday night and skyler has been assigned
It's friday night and skyler has been assigned Vanessa olariu
Vanessa olariu Dr chris bryce
Dr chris bryce Vanessa weintraub
Vanessa weintraub Vanessa marceau gozsy
Vanessa marceau gozsy Vanessa le leslé
Vanessa le leslé Vanessa cardin
Vanessa cardin Vanessa e. garcia
Vanessa e. garcia Coline atterbury
Coline atterbury Vanessa magdaleno
Vanessa magdaleno Betriebsarzt lmu medizin studenten
Betriebsarzt lmu medizin studenten Network news transfer protocol
Network news transfer protocol Erika vanessa molina
Erika vanessa molina Oración que contiene sujeto morfológico
Oración que contiene sujeto morfológico Vanessa da matta o que sera amado
Vanessa da matta o que sera amado Vanessa borrego
Vanessa borrego Vanessa borrego
Vanessa borrego Vanessa riveros
Vanessa riveros Vanessa arango zuluaga
Vanessa arango zuluaga Vanessa filippone
Vanessa filippone Pizzeria vanessa
Pizzeria vanessa Dra vanessa castro
Dra vanessa castro Vanessa loi porn
Vanessa loi porn Vanessa de angelis
Vanessa de angelis Vanessa prasad permaul
Vanessa prasad permaul Vanessa prasad permaul
Vanessa prasad permaul Vanessa fat chance
Vanessa fat chance Vanessa klose
Vanessa klose Vanessa's law
Vanessa's law Odt significado
Odt significado Dr vanessa hill
Dr vanessa hill Vanessa poo
Vanessa poo Vanessa's law
Vanessa's law Vanessaroyce
Vanessaroyce Verbos impersonales
Verbos impersonales Hace 8 años la edad de danitza
Hace 8 años la edad de danitza Gloria pinedo
Gloria pinedo Vss york region
Vss york region Escala khorana
Escala khorana Vanessa witt
Vanessa witt Vanessa cifuentes
Vanessa cifuentes Vanessa malcolm
Vanessa malcolm