Chapter 3 The Metric System Vanessa N PrasadPermaul




















































- Slides: 92

Chapter 3 The Metric System Vanessa N. Prasad-Permaul CHM 1025 Valencia Community College 1

The Metric System The English system was used primarily in the British Empire and wasn’t very standardized. The French organized a committee to devise a universal measuring system. After about 10 years, the committee designed and agreed on the metric system. The metric system offers simplicity with a single base unit for each measurement. 2

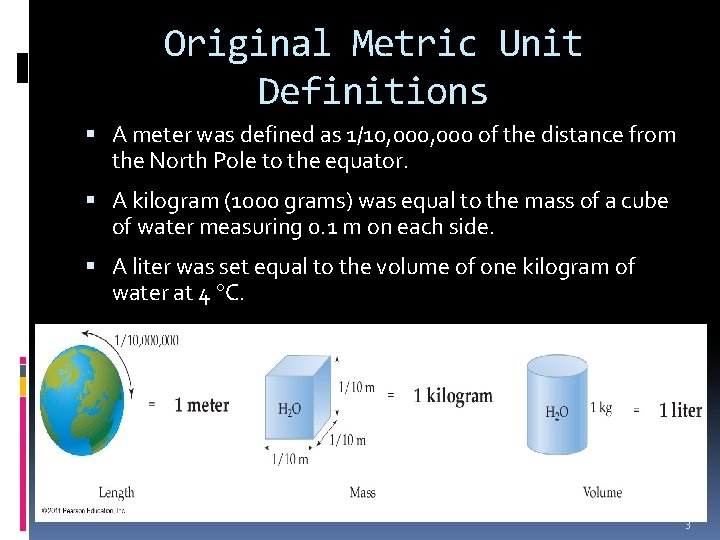
Original Metric Unit Definitions A meter was defined as 1/10, 000 of the distance from the North Pole to the equator. A kilogram (1000 grams) was equal to the mass of a cube of water measuring 0. 1 m on each side. A liter was set equal to the volume of one kilogram of water at 4 C. 3

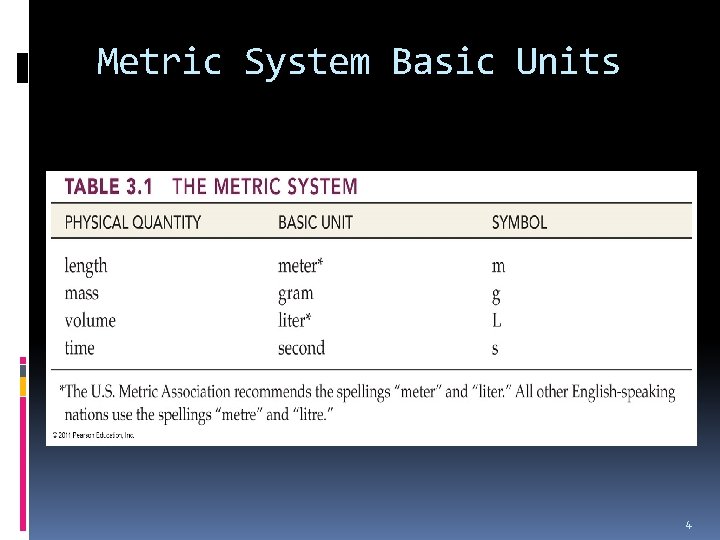
Metric System Basic Units 4

Metric System Advantage Another advantage of the metric system is that it is a decimal system. It uses prefixes to enlarge or reduce the basic units. For example: A kilometer is 1000 meters. A millimeter is 1/1000 of a meter. 5

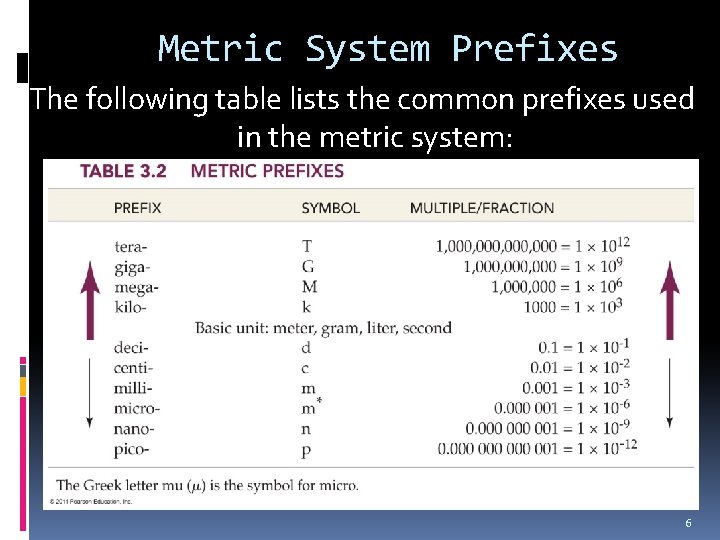
Metric System Prefixes The following table lists the common prefixes used in the metric system: 6

Metric Prefixes, Continued For example, the prefix kilo- increases a base unit by 1000: 1 kilogram is 1000 grams. The prefix milli- decreases a base unit by a factor of 1000: There are 1000 millimeters in 1 meter. 7

EXAMPLE 3. 1 Metric Basic Units and Prefixes Give the symbol for each of the following metric units and state the quantity measured by each unit: (a) gigameter (b) kilogram (c) centiliter (d) microsecond Solution We compose the symbol for each unit by combining the prefix symbol and the basic unit symbol. If we refer to Tables 3. 1 and 3. 2, we have the following: (a) Gm, length (b) kg, mass (c) c. L, volume (d) s, time

EXERCISE 3. 1 Metric Basic Units and Prefixes Practice Exercise Give the symbol for each of the following metric units and state the quantity measured by each unit: (a) nanosecond (b) microiliter (c) kilogram (d) millimeter Concept Exercise What is the basic unit of length, mass, and volume in the metric system?

Metric Symbols The names of metric units are abbreviated using symbols. Use the prefix symbol followed by the symbol for the base unit, so: Nanometer is abbreviated nm. Microgram is abbreviated g. Deciliter is abbreviated d. L. Gigasecond is abbreviated Gs. 10

Nanotechnology refers to devices and processes on the 1– 100 nm scale. For reference, a human hair is about 100, 000 nm thick! A DNA helix is a nanoscale substance, with a diameter of about 1 nm. Nanoscale hollow tubes, called carbon nanotubes, have slippery inner surfaces that allow for the easy flow of fluids. 11

Metric Equivalents We can write unit equations for the conversion between different metric units. The prefix kilo- means 1000 basic units, so 1 kilometer is 1000 meters. The unit equation is 1 km = 1000 m. Similarly, a millimeter is 1/1000 of a meter, so the unit equation is 1000 mm = 1 m. 12

Metric Unit Factors Since 1000 m = 1 km, we can write the following unit factors for converting between meters and kilometers: 1 km or 1000 m 1 km Since 1000 mm = 1 m, we can write the following unit factors: 1000 mm or 1 m 1000 mm 13

EXAMPLE 3. 2 Metric Unit Equations Complete the unit equation for each of the following exact metric equivalents: (a) 1 Mm = ? m (b) 1 kg = ? g (c) 1 L = ? d. L (d) 1 s = ? ns Solution We can refer to Table 3. 2 as necessary. (a) The prefix mega- means 1, 000 basic units; thus, 1 Mm = 1, 000 m. 1 Mm = 1 106 m (b) The prefix kilo- means 1000 basic units; thus, 1 kg = 1000 g. 1 kg = 1 103 g (c) The prefix deci- means 0. 1 of a basic unit, thus, 1 L = 10 d. L. 1 L = 1 101 d. L (d) The prefix nano- means 0. 000 001 of a basic unit, thus; 1 s = 1, 000, 000 ns. 1 s = 1 109 ns

EXERCISE 3. 2 Metric Unit Equations Practice Exercise Complete the unit equation for each of the following exact metric equivalents: (a) 1 nm = ? m (c) 1 L = ? L (b) 1 g = ? mg (d) 1 s = ? Ms

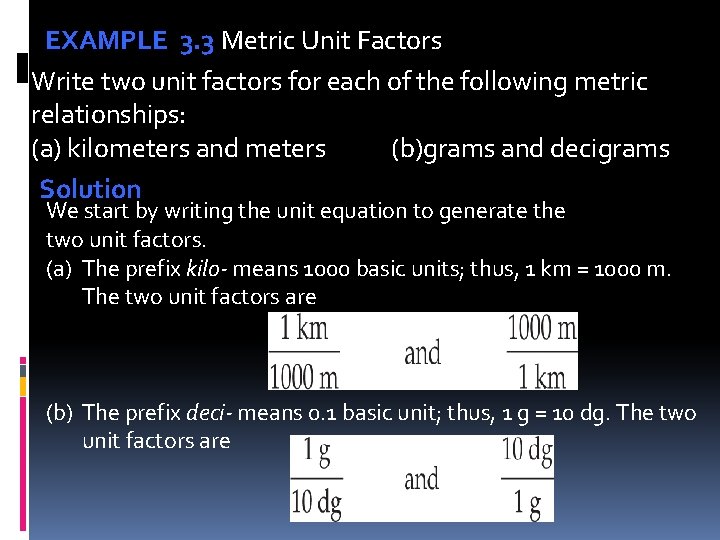
EXAMPLE 3. 3 Metric Unit Factors Write two unit factors for each of the following metric relationships: (a) kilometers and meters (b)grams and decigrams Solution We start by writing the unit equation to generate the two unit factors. (a) The prefix kilo- means 1000 basic units; thus, 1 km = 1000 m. The two unit factors are (b) The prefix deci- means 0. 1 basic unit; thus, 1 g = 10 dg. The two unit factors are

EXERCISE 3. 3 Metric Unit Factors Practice Exercise Write two unit factors for each of the following metric relationships: (a) liters and microliters (b)milliseconds and seconds

Metric–Metric Conversions We will use the unit analysis method we learned in Chapter 2 to do metric –metric conversion problems. Remember, there are three steps: 1. Write down the unit asked for in the answer. 2. Write down the given value related to the answer. 3. Apply unit factor(s) to convert the given unit to the units desired in the answer. 18

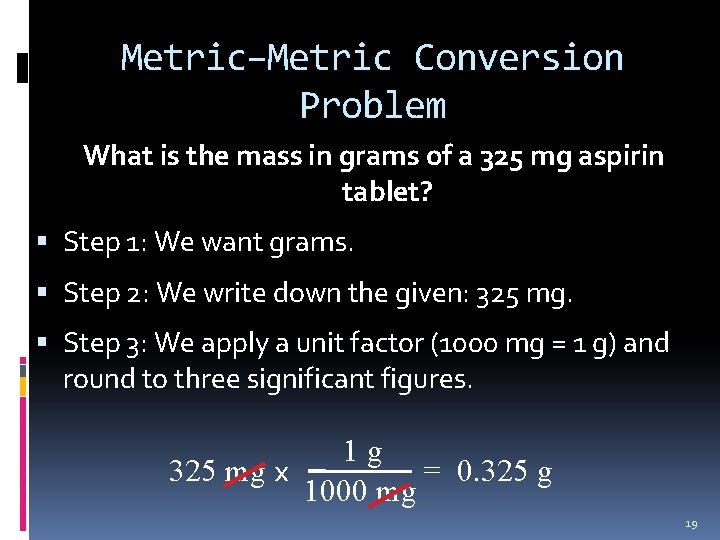
Metric–Metric Conversion Problem What is the mass in grams of a 325 mg aspirin tablet? Step 1: We want grams. Step 2: We write down the given: 325 mg. Step 3: We apply a unit factor (1000 mg = 1 g) and round to three significant figures. 1 g 325 mg x = 0. 325 g 1000 mg 19

EXAMPLE 3. 4 Metric Unit Factors Two Metric–Metric Conversions A hospital has 125 deciliters of blood plasma. What is the volume in milliliters? Step 1: We want the answer in m. L. Step 2: We have 125 d. L. Step 3: We need to first convert d. L to L and then convert L to m. L: 1 L and 1000 m. L 10 d. L 1 L 20

EXAMPLE 3. 4 Metric Unit Factors Continued… Apply both unit factors, and round the answer to three significant digits. Notice that both d. L and L units cancel, leaving us with units of m. L. 1 L x 1000 m. L 125 d. L x = 12, 500 m. L 10 d. L 1 L 21

EXERCISE 3. 4 Metric-Metric Conversions Practice Exercise A dermatology patient is treated with ultraviolet light having a wavelength of 305 nm. What is the wavelength expressed in meters? In micrometers? Concept Exercise Express the volume of a cube 1 cm on a side in milliliters.

EXAMPLE EXERCISE 3. 5 Metric–Metric Conversion Another Example The mass of the Earth’s moon is 7. 35 × 1022 kg. What is the mass expressed in megagrams, Mg? We want Mg; we have 7. 35 x 1022 kg. Convert kilograms to grams, and then grams to megagrams. 7. 35 x 1022 kg × 1000 g 1 Mg x = 5. 98 x 1019 Mg 1000000 g 1 kg 23

EXERCISE 3. 5 Metric–Metric Conversion Practice Exercise Light travels through the universe at a velocity of 3. 00 1010 cm/s. How many megameters does light travel in one second? Concept Exercise How many significant digits are in the following unit factor? 1 g/1000 mg

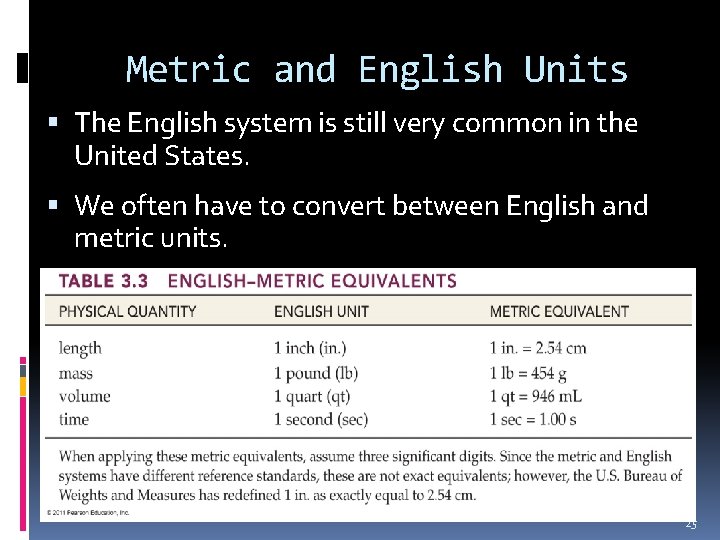
Metric and English Units The English system is still very common in the United States. We often have to convert between English and metric units. 25

Metric–English Conversion The length of an American football field, including the end zones, is 120 yards. What is the length in meters? Convert 120 yd to meters (given that 1 yd = 0. 914 m). 0. 914 m 120 yd x = 110 m 1 yd 26

EXAMPLE EXERCISE 3. 6 Metric–English Conversion English–Metric Conversion A half-gallon carton contains 64. 0 fl oz of milk. How many milliliters of milk are in a carton? We want m. L; we have 64. 0 fl oz. Use 1 qt = 32. 0 fl oz, and 1 qt = 946 m. L. 1 qt x 946 m. L 64. 0 fl oz x = 1, 890 m. L 32 fl oz 1 qt 27

EXERCISE 3. 6 Metric–English Conversion Practice Exercise A plastic bottle contains 4. 00 gallons of distilled water. How many liters of distilled water are in the bottle (given that 1 gal = 4 qt)? Concept Exercise How many significant digits are in the following unit factor? 1 qt/946 m. L

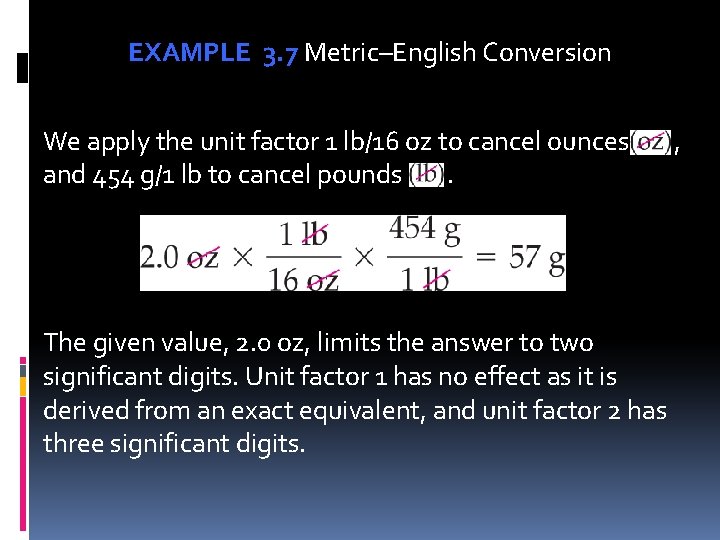
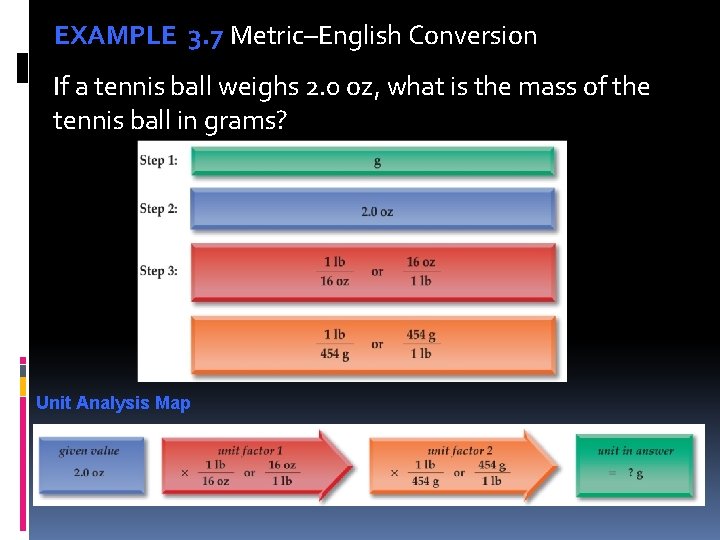
EXAMPLE 3. 7 Metric–English Conversion We apply the unit factor 1 lb/16 oz to cancel ounces , and 454 g/1 lb to cancel pounds . The given value, 2. 0 oz, limits the answer to two significant digits. Unit factor 1 has no effect as it is derived from an exact equivalent, and unit factor 2 has three significant digits.

EXAMPLE 3. 7 Metric–English Conversion If a tennis ball weighs 2. 0 oz, what is the mass of the tennis ball in grams? Unit Analysis Map

EXERCISE 3. 7 Metric–English Conversion Practice Exercise If a tennis ball has a diameter of 2. 5 inches, what is the diameter in millimeters? Concept Exercise How many significant digits are in the following unit factor? 1 kg/2. 20 lb

Compound Units Some measurements have a ratio of units. For example, the speed limit on many highways is 55 miles per hour. How would you convert this to meters per second? Convert one unit at a time using unit factors. 1. First, miles → meters 2. Next, hours → seconds 32

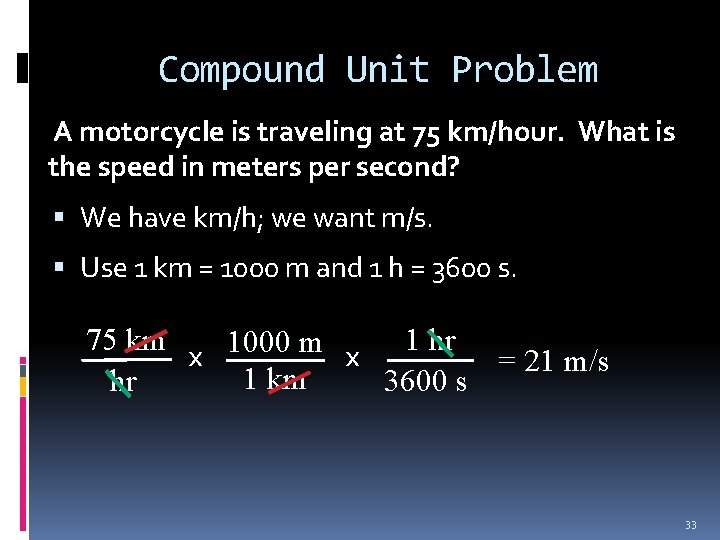
Compound Unit Problem A motorcycle is traveling at 75 km/hour. What is the speed in meters per second? We have km/h; we want m/s. Use 1 km = 1000 m and 1 h = 3600 s. 75 km x 1000 m x 1 hr = 21 m/s 1 km hr 3600 s 33

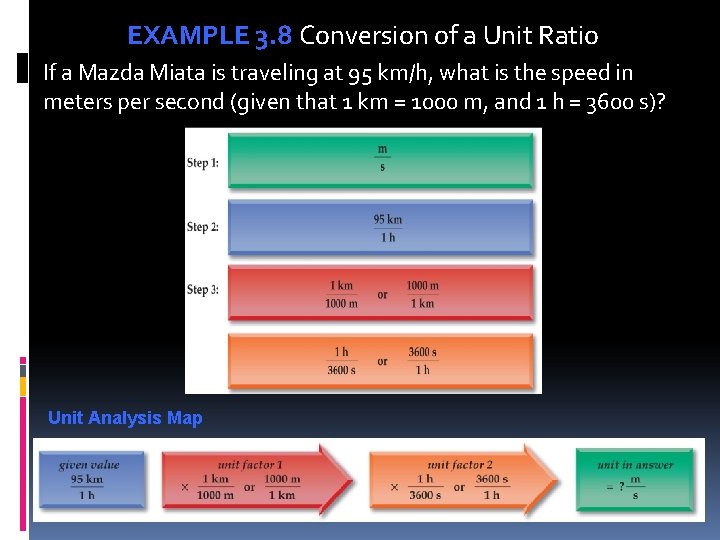
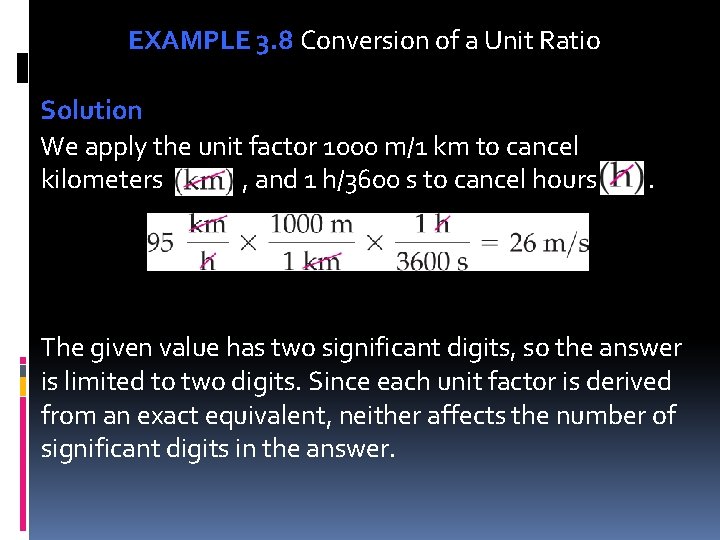
EXAMPLE 3. 8 Conversion of a Unit Ratio If a Mazda Miata is traveling at 95 km/h, what is the speed in meters per second (given that 1 km = 1000 m, and 1 h = 3600 s)? Unit Analysis Map

EXAMPLE 3. 8 Conversion of a Unit Ratio Solution We apply the unit factor 1000 m/1 km to cancel kilometers , and 1 h/3600 s to cancel hours . The given value has two significant digits, so the answer is limited to two digits. Since each unit factor is derived from an exact equivalent, neither affects the number of significant digits in the answer.

EXERCISE 3. 8 Conversion of a Unit Ratio Practice Exercise If a runner completes a 10 K race in 32. 50 minutes (min), what is the 10. 0 km pace in miles per hour (given that 1 mi = 1. 61 km)? Concept Exercise Which speed is faster: 65 mi/h or 65 km/h?

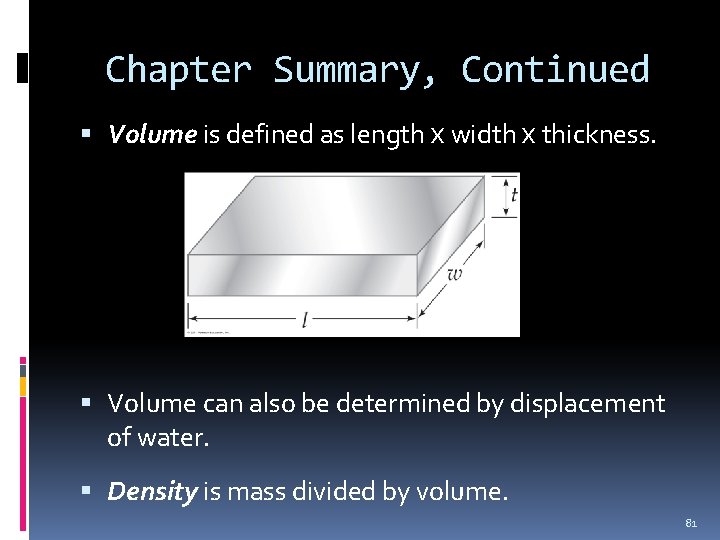
Volume by Calculation The volume of an object is calculated by multiplying the length (l) by the width (w) by the thickness (t). volume = l x w x t All three measurements must be in the same units. If an object measures 3 cm by 2 cm by 1 cm, the volume is 6 cm 3 (cm 3 is cubic centimeters). 37

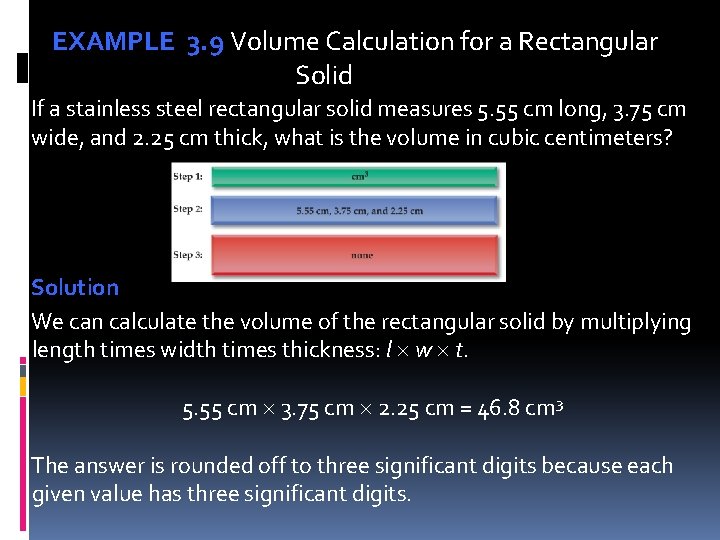
EXAMPLE 3. 9 Volume Calculation for a Rectangular Solid If a stainless steel rectangular solid measures 5. 55 cm long, 3. 75 cm wide, and 2. 25 cm thick, what is the volume in cubic centimeters? Solution We can calculate the volume of the rectangular solid by multiplying length times width times thickness: l w t. 5. 55 cm 3. 75 cm 2. 25 cm = 46. 8 cm 3 The answer is rounded off to three significant digits because each given value has three significant digits.

EXERCISE 3. 9 Volume Calculation for a Rectangular Solid Practice Exercise If a rectangular brass solid measures 52. 0 mm by 25. 0 mm by 15. 0 mm, what is the volume in cubic millimeters? Concept Exercise Express the volume of a cube 10 cm on a side in liters.

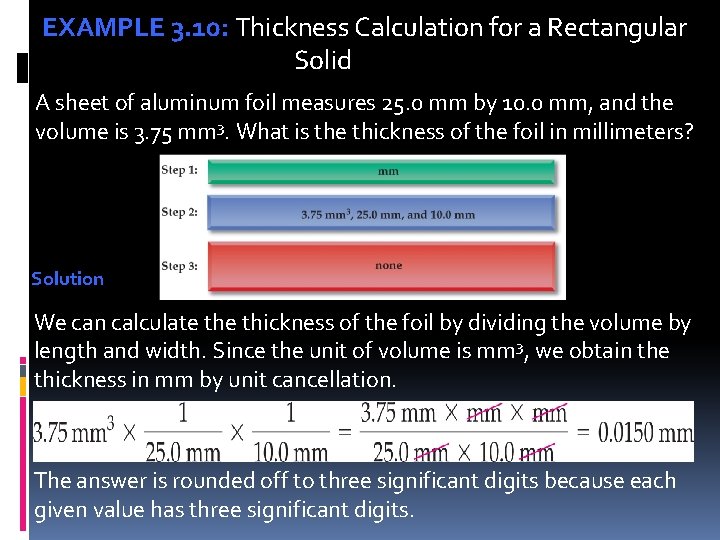
EXAMPLE 3. 10: Thickness Calculation for a Rectangular Solid A sheet of aluminum foil measures 25. 0 mm by 10. 0 mm, and the volume is 3. 75 mm 3. What is the thickness of the foil in millimeters? Solution We can calculate thickness of the foil by dividing the volume by length and width. Since the unit of volume is mm 3, we obtain the thickness in mm by unit cancellation. The answer is rounded off to three significant digits because each given value has three significant digits.

EXERCISE 3. 10: Thickness Calculation for a Rectangular Solid Practice Exercise A sheet of aluminum foil measures 35. 0 cm by 25. 0 cm, and the volume is 1. 36 cm 3. What is the thickness of the foil in centimeters? Aluminum foil : A thin sheet of aluminum foil. Concept Exercise Which of the following is the greatest thickness? 1 mm, 0. 1 cm, or 0. 001 m

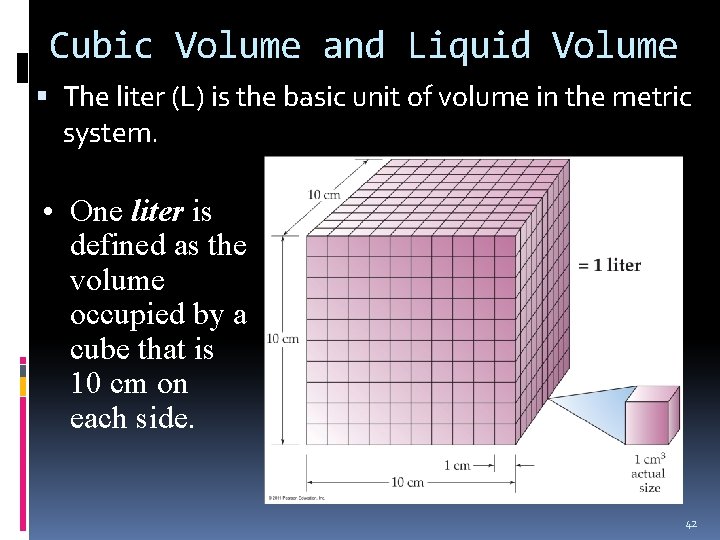
Cubic Volume and Liquid Volume The liter (L) is the basic unit of volume in the metric system. • One liter is defined as the volume occupied by a cube that is 10 cm on each side. 42

Cubic and Liquid Volume Units 1 liter is equal to 1000 cubic centimeters. 10 cm x 10 cm = 1000 cm 3 = 1 L = 1000 m. L. Therefore, 1 cm 3 = 1 m. L. 43

EXAMPLE 3. 11 Metric–English Volume Conversion Cubic–Liquid Volume Conversion An automobile engine displaces a volume of 498 cm 3 in each cylinder. What is the displacement of a cylinder in cubic inches, in 3? We want in 3; we have 498 cm 3. Use 1 in = 2. 54 cm three times. 498 cm 3 x 1 in = 30. 4 in 3 2. 54 cm 44

EXERCISE 3. 11: Metric–English Volume Conversion Practice Exercise Given that an SUV has a 304 in. 3 engine, express the engine volume in liters. Concept Exercise Which of the following is the greater volume? 500 m. L or 500 cm 3

Volume by Displacement If a solid has an irregular shape, its volume cannot be determined by measuring its dimensions. You can determine its volume indirectly by measuring the amount of water it displaces. This technique is called volume by displacement. Volume by displacement can also be used to determine the volume of a gas. 46

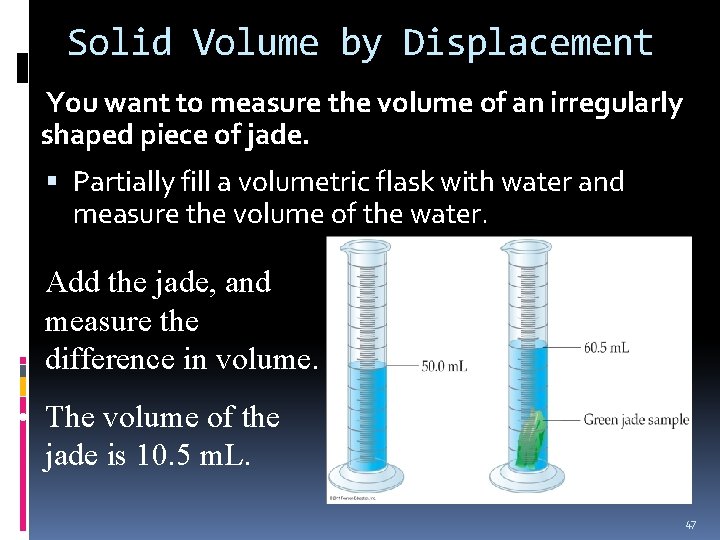
Solid Volume by Displacement You want to measure the volume of an irregularly shaped piece of jade. Partially fill a volumetric flask with water and measure the volume of the water. • Add the jade, and measure the difference in volume. • The volume of the jade is 10. 5 m. L. 47

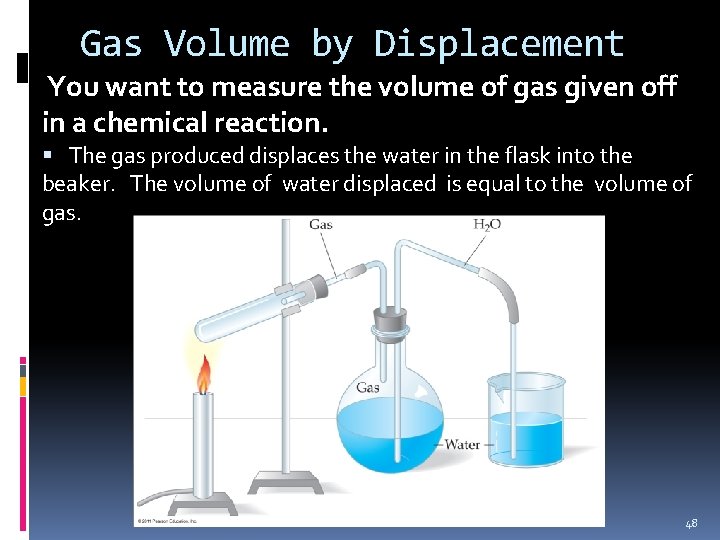
Gas Volume by Displacement You want to measure the volume of gas given off in a chemical reaction. The gas produced displaces the water in the flask into the beaker. The volume of water displaced is equal to the volume of gas. 48

EXAMPLE 3. 12: Volume by Displacement A quartz stone weighing 30. 475 g is dropped into a graduated cylinder. If the water level increases from 25. 0 m. L to 36. 5 m. L, what is the volume of the quartz stone? Solution We can calculate the displaced volume in milliliters by subtracting the initial volume from the final volume. 36. 5 m. L – 25. 0 m. L = 11. 5 m. L

EXERCISE 3. 12: Volume by Displacement Practice Exercise Hydrogen peroxide decomposes to give oxygen gas, which displaces a volume of water into a beaker. If the water level in the beaker increases from 50. 0 m. L to 105. 5 m. L, what is the volume of oxygen gas? Concept Exercise Which of the following has the greater volume? 1 m. L or 1 cm 3

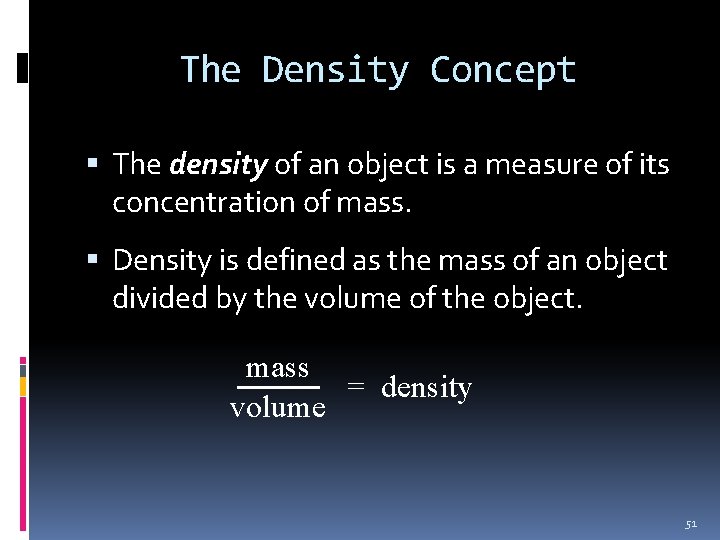
The Density Concept The density of an object is a measure of its concentration of mass. Density is defined as the mass of an object divided by the volume of the object. mass = density volume 51

Density is expressed in different units. It is usually grams per milliliter (g/m. L) for liquids, grams per cubic centimeter (g/cm 3) for solids, and grams per liter (g/L) for gases. 52

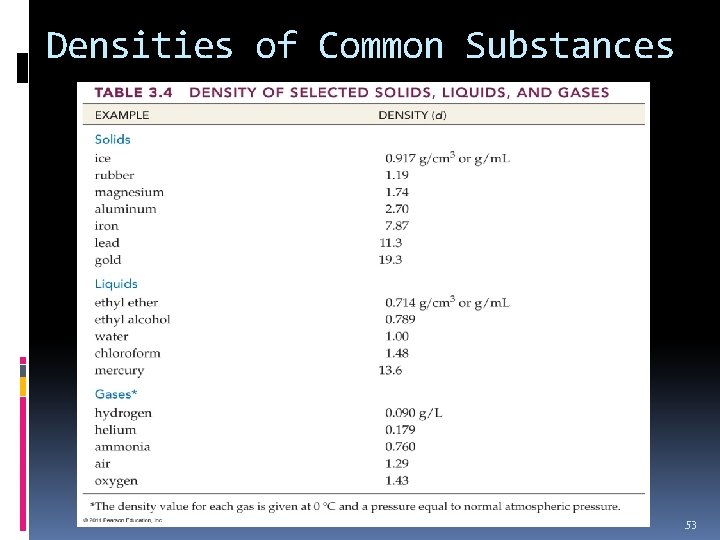
Densities of Common Substances 53

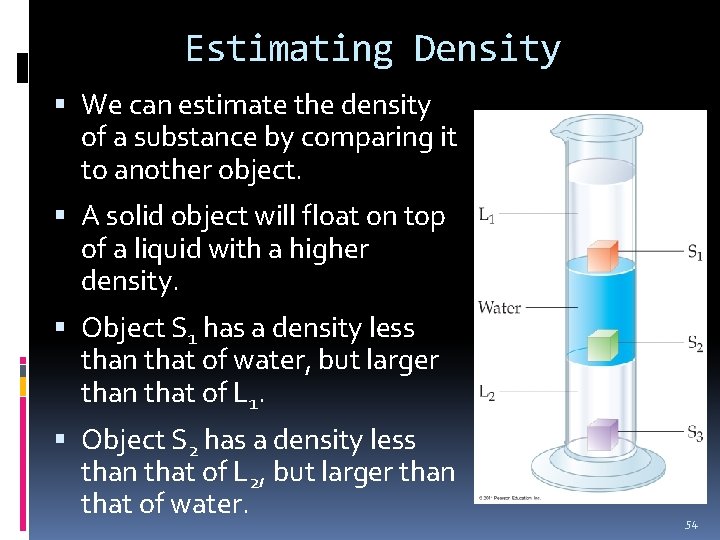
Estimating Density We can estimate the density of a substance by comparing it to another object. A solid object will float on top of a liquid with a higher density. Object S 1 has a density less than that of water, but larger than that of L 1. Object S 2 has a density less than that of L 2, but larger than that of water. 54

EXAMPLE EXERCISE 3. 13 Density Calculation Calculating Density What is the density of a platinum nugget that has a mass of 224. 50 g and a volume of 10. 0 cm 3 ? Recall, density is mass/volume. 224. 50 g 10. 0 cm 3 = 22. 5 g/cm 3 55

EXERCISE 3. 13: Density Calculation Practice Exercise Carbon tetrachloride is a solvent used for degreasing electronic parts. If 40. 0 m. L of carbon tetrachloride has a mass of 39. 75 g, what is the density of the liquid? Concept Exercise Which of the following has the greater density: ice or water?

EXAMPLE EXERCISE 3. 14 Density as a Unit Factor We can use density as a unit factor for conversions between mass and volume. An automobile battery contains 1275 m. L of acid. If the density of battery acid is 1. 84 g/m. L, how many grams of acid are in an automobile battery? – We have 1275 m. L; we want grams: 1275 m. L x 1. 84 g = 2350 g m. L 57

EXERCISE 3. 14: Density as a Unit Factor Practice Exercise The most abundant gases in our atmosphere are nitrogen, oxygen, and argon. What is the volume of 1. 00 kg of air? (Assume the density of air is 1. 29 g/L. ) Concept Exercise Which of the following is the greater density? 1 g/m. L or 1 kg/L

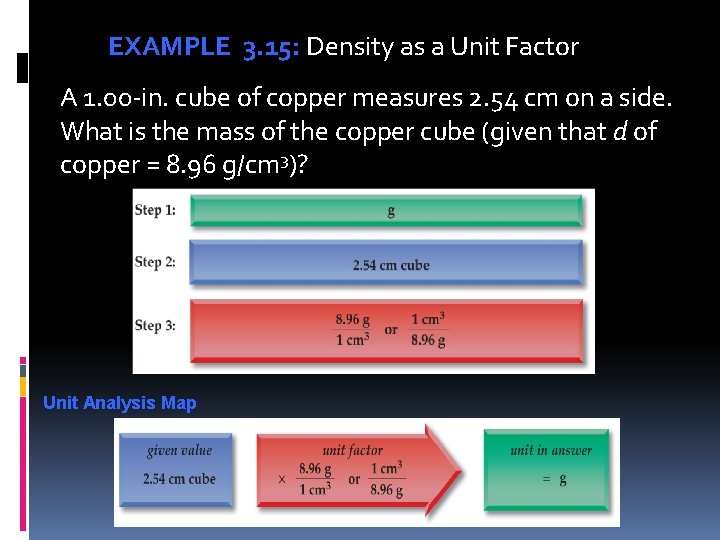
EXAMPLE 3. 15: Density as a Unit Factor A 1. 00 -in. cube of copper measures 2. 54 cm on a side. What is the mass of the copper cube (given that d of copper = 8. 96 g/cm 3)? Unit Analysis Map

EXAMPLE 3. 15: Density as a Unit Factor Solution First, we find the volume of the copper cube. We obtain the volume of the cube, 16. 4 cm 3, by multiplying (2. 54 cm). We use the given density, 8. 96 g/1 cm 3, as a unit factor to cancel cubic centimeters , which appears in the denominator. The given value and unit factor each has three significant digits, so the answer is rounded off to three significant digits. Copper Metal A 1. 00 -inch cube of copper metal.

EXERCISE 3. 15: Density as a Unit Factor Practice Exercise A cube of silver is 8. 00 cm on a side and has a mass of 1312. 5 g. What is the density of silver? Concept Exercise If some humans float in water and other sink, what is the approximate density of the human body?

Critical Thinking: Gasoline The density of gasoline is 730. g/L at 0 ºC (32 ºF) and 713 g/L at 25 ºC (77 ºF). What is the mass difference of 1. 00 gallon of gasoline at these two temperatures (1 gal = 3. 784 L)? 3. 784 L x 730. g At 0 ºC: 1. 00 gal x = 2760 g L 1 gal 3. 784 L x 713 g At 25 ºC: 1. 00 gal x = 2700 g L 1 gal The difference is about 60 grams (about 2 %). 62

Temperature is a measure of the average kinetic energy of the individual particles in a sample. There are three temperature scales: 1. Celsius 2. Fahrenheit 3. Kelvin is the absolute temperature scale. 63

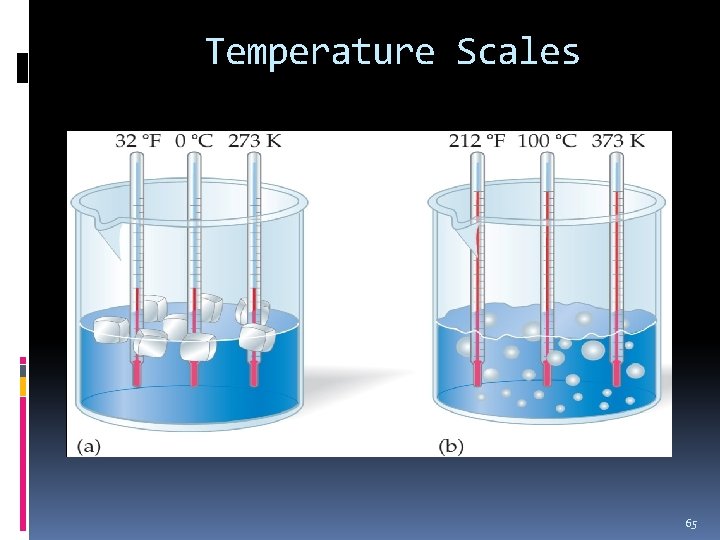
Temperature Scales On the Fahrenheit scale, water freezes at 32 °F and boils at 212 °F. On the Celsius scale, water freezes at 0 °C and boils at 100 °C. These are the reference points for the Celsius scale. Water freezes at 273 K and boils at 373 K on the Kelvin scale. 64

Temperature Scales 65

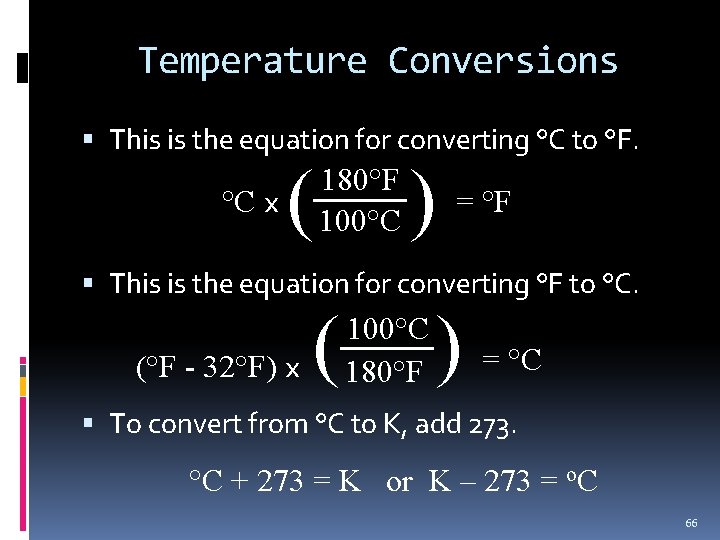
Temperature Conversions This is the equation for converting °C to °F. °C x ( ) 180°F 100°C = °F This is the equation for converting °F to °C. (°F - 32°F) x ( ) 100°C 180°F = °C To convert from °C to K, add 273. °C + 273 = K or K – 273 = o. C 66

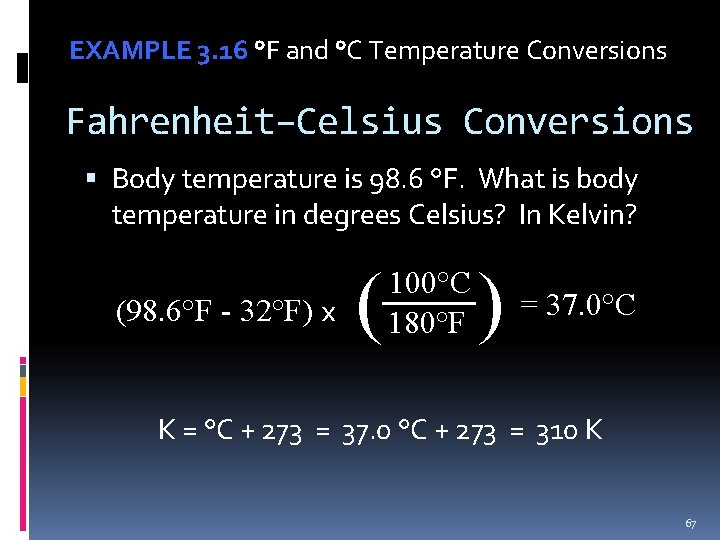
EXAMPLE 3. 16 °F and °C Temperature Conversions Fahrenheit–Celsius Conversions Body temperature is 98. 6 °F. What is body temperature in degrees Celsius? In Kelvin? (98. 6°F - 32°F) x ( ) 100°C 180°F = 37. 0°C K = °C + 273 = 37. 0 °C + 273 = 310 K 67

EXAMPLE 3. 16: °F and °C Temperature Conversions Practice Exercise The average surface temperature of Mars is – 55 °C. What is the average temperature in degrees Fahrenheit? Australian Stamp The cartoon illustrates that 38 °C is approximately equal to 100 °F Concept Exercise What is the relationship between the Celsius and centigrade temperature scales?

EXAMPLE 3. 17: °C and K Temperature Conversions Dermatologists use liquid nitrogen to freeze skin tissue. If the Celsius temperature of liquid nitrogen is – 196 °C, what is the Kelvin temperature? Solution Given the Celsius temperature, we add 273 units to find the corresponding Kelvin temperature. – 1. 96 ° C + 273 = 77 k Liquid Nitrogen Although nitrogen is normally a gas, it liquefies at – 196 °C. When liquid nitrogen is poured from a Thermos, it is cold enough to freeze the moisture in air and form a white mist.

EXERCISE 3. 17: °C and K Temperature Conversions Practice Exercise The secret to “fire-walking” is to first walk barefoot through damp grass and then step lively on the red-hot coals. If the bed of coals is 1475 K, what is the Celsius temperature? Concept Exercise Which of the following temperatures does not exist? – 100 °F, – 100 °C, – 100 K

Heat is the flow of energy from an object of higher temperature to an object of lower temperature. Heat measures the total energy of a system. Temperature measures the average energy of particles in a system. Heat is often expressed in terms of joules (J) or calories (cal). 71

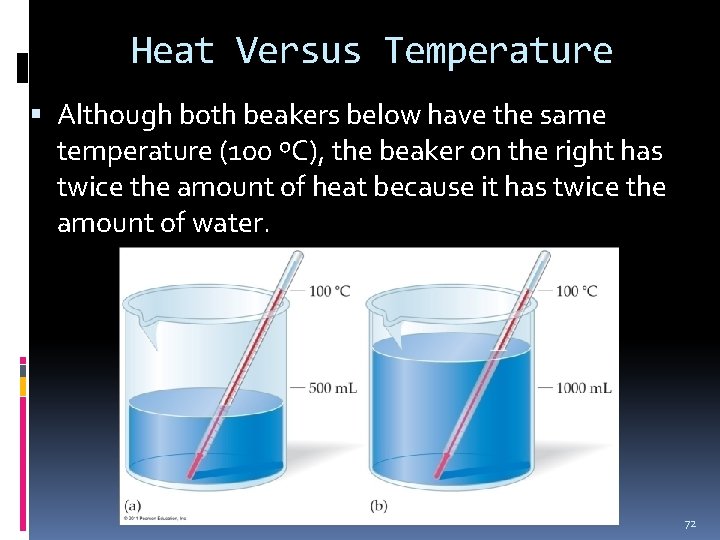
Heat Versus Temperature Although both beakers below have the same temperature (100 ºC), the beaker on the right has twice the amount of heat because it has twice the amount of water. 72

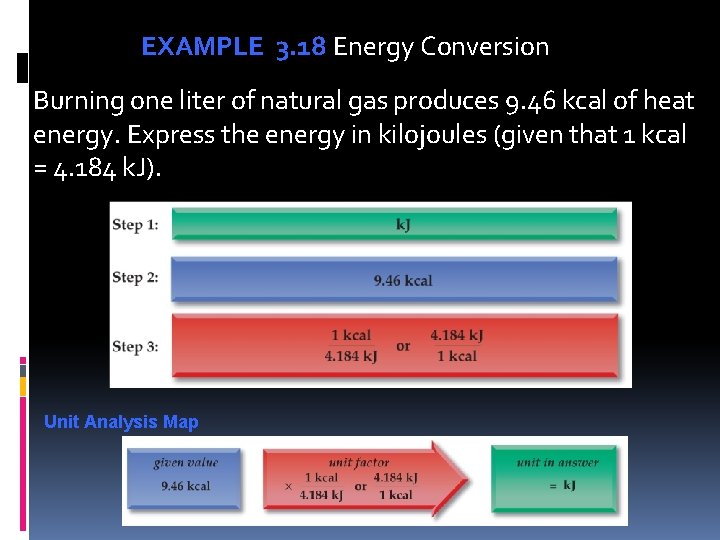
EXAMPLE 3. 18 Energy Conversion Burning one liter of natural gas produces 9. 46 kcal of heat energy. Express the energy in kilojoules (given that 1 kcal = 4. 184 k. J). Unit Analysis Map

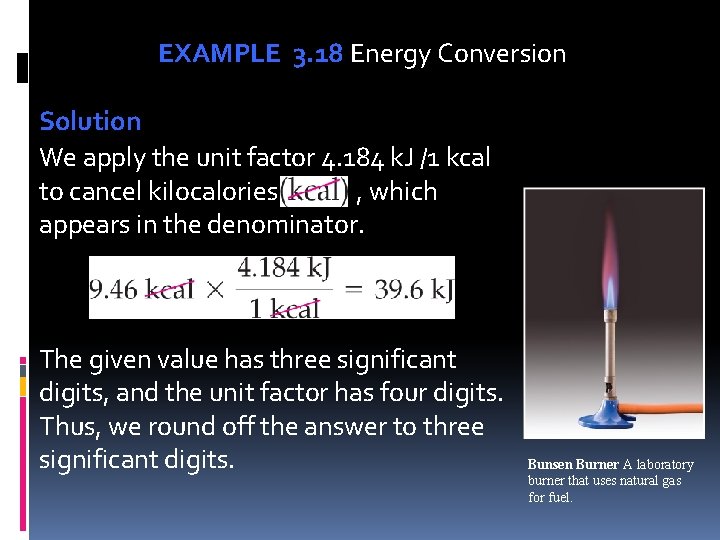
EXAMPLE 3. 18 Energy Conversion Solution We apply the unit factor 4. 184 k. J /1 kcal to cancel kilocalories , which appears in the denominator. The given value has three significant digits, and the unit factor has four digits. Thus, we round off the answer to three significant digits. Bunsen Burner A laboratory burner that uses natural gas for fuel.

EXERCISE 3. 18 Energy Conversion Practice Exercise Burning one gram of gasoline produces 51. 3 k. J of energy. Express the heat energy in kilocalories (given that 1 kcal = 4. 184 k. J). Concept Exercise If an aerosol can feels cold after releasing the spray, is heat flowing from the can or from your hand?

Specific Heat The specific heat of a substance is the amount of heat required to bring about a change in temperature. It is expressed with units of calories per gram per degree Celsius. The larger the specific heat, the more heat is required to raise the temperature of the substance. 76

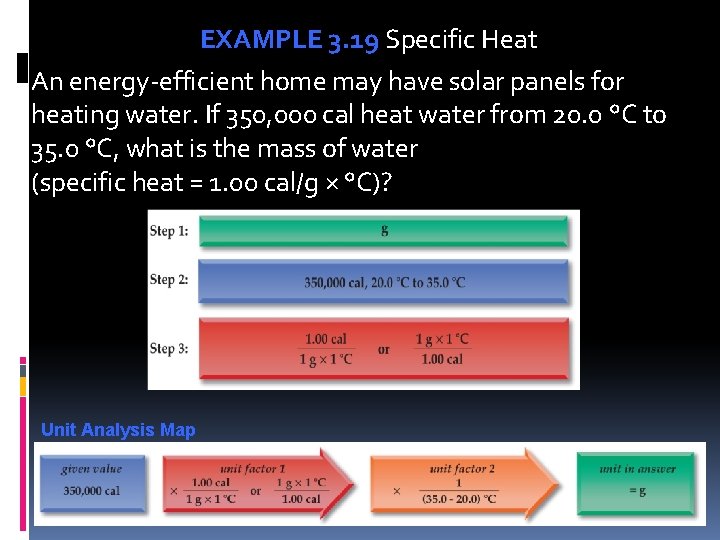
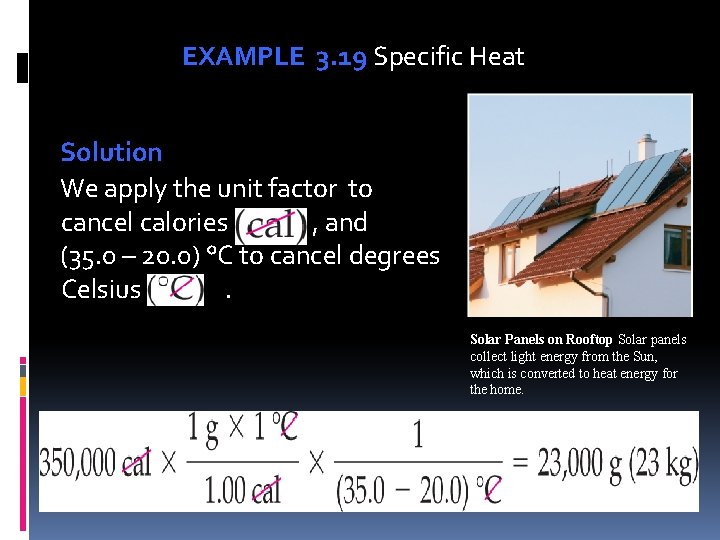
EXAMPLE 3. 19 Specific Heat An energy-efficient home may have solar panels for heating water. If 350, 000 cal heat water from 20. 0 °C to 35. 0 °C, what is the mass of water (specific heat = 1. 00 cal/g × °C)? Unit Analysis Map

EXAMPLE 3. 19 Specific Heat Solution We apply the unit factor to cancel calories , and (35. 0 – 20. 0) °C to cancel degrees Celsius . Solar Panels on Rooftop Solar panels collect light energy from the Sun, which is converted to heat energy for the home.

EXERCISE 3. 19 Specific Heat Practice Exercise A 725 g steel horseshoe is heated to 425 °C and dropped into a bucket of cold water. If the horseshoe cools to 20 °C and the specific heat of steel is 0. 11 cal/g °C, how much heat is released? Concept Exercise If the crust of an apple pie cooks faster than the filling, which has the higher specific heat: the crust or apple filling?

Chapter Summary The basic units in the metric system are grams for mass, liters for volume, and meters for distance. The base units are modified using prefixes to reduce or enlarge the base units by factors of ten. We can use unit factors to convert between metric units. We can convert between metric and English units using unit factors. 80

Chapter Summary, Continued Volume is defined as length x width x thickness. Volume can also be determined by displacement of water. Density is mass divided by volume. 81

Chapter Summary, Continued Temperature is a measure of the average energy of the particles in a sample. Heat is a measure of the total energy of a substance. Specific heat is a measure of how much heat is required to raise the temperature of a substance. 82

Give the symbol for microliter. a. b. c. d. m. L mi. L ML μL

Give the symbol for microliter. a. b. c. d. m. L mi. L ML μL

2. 00 microliters = ? liters a. b. c. d. 2. 00 × 10– 9 L 2. 00 × 10– 6 L 2. 00 × 10– 3 L 2. 00 × 103 L

2. 00 microliters = ? liters a. b. c. d. 2. 00 × 10– 9 L 2. 00 × 10– 6 L 2. 00 × 10– 3 L 2. 00 × 103 L

Given that 1 yd = 0. 914 m and 1 mi = 1760 yd, which Olympic track event covers a distance that is closest to a half mile? a. b. c. d. 200 m 400 m 800 m 1000 m

Given that 1 yd = 0. 914 m and 1 mi = 1760 yd, which Olympic track event covers a distance that is closest to a half mile? a. b. c. d. 200 m 400 m 800 m 1000 m

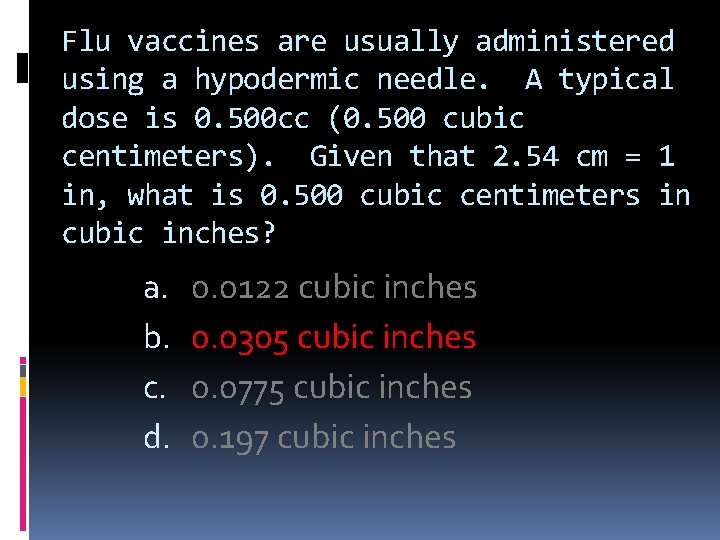
Flu vaccines are usually administered using a hypodermic needle. A typical dose is 0. 500 cc (0. 500 cubic centimeters). Given that 2. 54 cm = 1 in, what is 0. 500 cubic centimeters in cubic inches? a. b. c. d. 0. 0122 cubic inches 0. 0305 cubic inches 0. 0775 cubic inches 0. 197 cubic inches

Flu vaccines are usually administered using a hypodermic needle. A typical dose is 0. 500 cc (0. 500 cubic centimeters). Given that 2. 54 cm = 1 in, what is 0. 500 cubic centimeters in cubic inches? a. b. c. d. 0. 0122 cubic inches 0. 0305 cubic inches 0. 0775 cubic inches 0. 197 cubic inches

When added to a graduated cylinder containing 4. 00 m. L of water, 5. 00 g of which metal will displace the greatest volume of water? a. b. c. d. iron, d = 7. 87 g/cm 3 lead, d = 11. 3 g/cm 3 gold d = 19. 3 g/cm 3 5. 00 g of any of these metals will displace the same volume of water

When added to a graduated cylinder containing 4. 00 m. L of water, 5. 00 g of which metal will displace the greatest volume of water? a. b. c. d. iron, d = 7. 87 g/cm 3 lead, d = 11. 3 g/cm 3 gold d = 19. 3 g/cm 3 5. 00 g of any of these metals will displace the same volume of water