CH 6 THERMOCHEMISTRY Vanessa PrasadPermaul Valencia College CHM




































- Slides: 66

CH 6: THERMOCHEMISTRY Vanessa Prasad-Permaul Valencia College CHM 1045 1

THERMOCHEMISTRY ENERGY • INTRODUCTION TO THERMODYNAMICS • KEY DEFINITIONS • FIRST LAW OF THERMODYNAMICS • HEAT, WORK & INTERNAL ENERGY • ENTHALPY • THERMODYNAMIC SYSTEM • HESS’ LAW • STANDARD ENTHALPIES OF FORMATION • ENERGY AND THE ENVIRONMENT 2

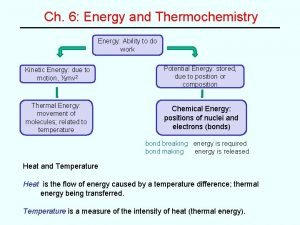
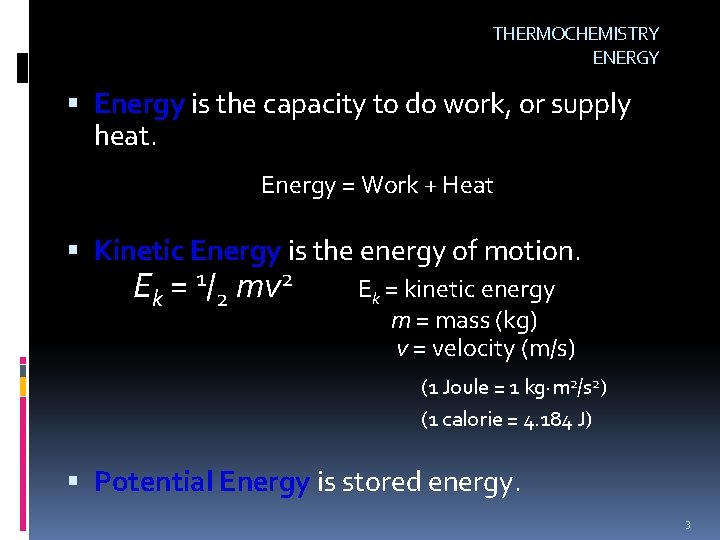
THERMOCHEMISTRY ENERGY Energy is the capacity to do work, or supply heat. Energy = Work + Heat Kinetic Energy is the energy of motion. Ek = 1/2 mv 2 Ek = kinetic energy m = mass (kg) v = velocity (m/s) (1 Joule = 1 kg m 2/s 2) (1 calorie = 4. 184 J) Potential Energy is stored energy. 3

THERMOCHEMISTRY ENERGY EXAMPLE 6. 1 A regulation baseball weighing 143 grams travels 75 miles per hour. What is the kinetic energy of this baseball in Joules? Convert to calories. Ek = ½ mv 2 m = 143 g x 1 kg 1000 g = 0. 143 kg v = 75 miles x 1 hr x 1 min x 1609. 3 m = 33. 527 m/s 1 hour 60 min 60 sec 1 mile Ek = ½ x 0. 143 kg x (33. 527 m/s)2 = 80. 3 kg. m 2/s 2 = 80. 3 J x 1 cal = 19. 2 cal 4. 184 J 4

THERMOCHEMISTRY ENERGY EXERCISE 6. 1 An electron whose mass is 9. 11 x 10 -31 kg is accelerated by a positive charge to a speed of 5. 0 x 106 m/s. What is the kinetic energy of the electron in Joules? In calories? Ek = ½ mv 2 5

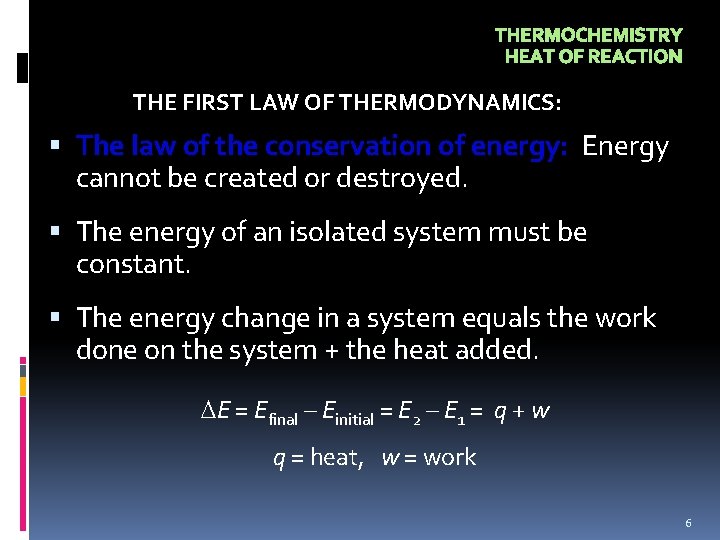
THERMOCHEMISTRY HEAT OF REACTION THE FIRST LAW OF THERMODYNAMICS: The law of the conservation of energy: Energy cannot be created or destroyed. The energy of an isolated system must be constant. The energy change in a system equals the work done on the system + the heat added. E = Efinal – Einitial = E 2 – E 1 = q + w q = heat, w = work 6

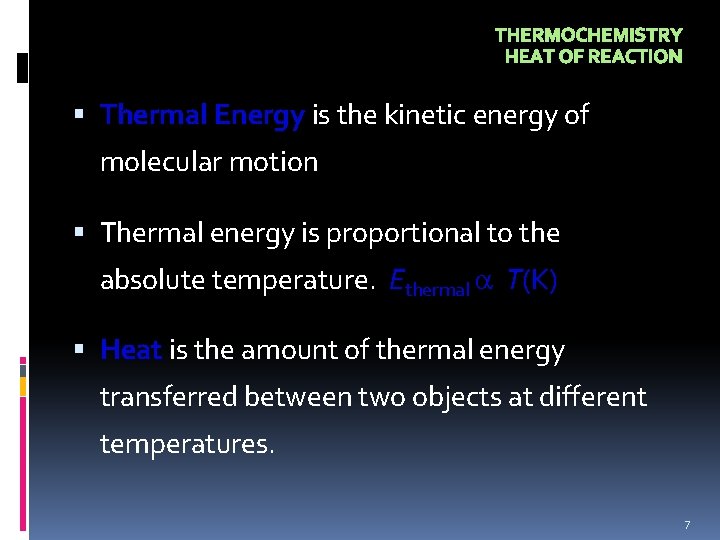
THERMOCHEMISTRY HEAT OF REACTION Thermal Energy is the kinetic energy of molecular motion Thermal energy is proportional to the absolute temperature. Ethermal T(K) Heat is the amount of thermal energy transferred between two objects at different temperatures. 7

THERMOCHEMISTRY ENTHALPY Enthalpies of Physical Change: Enthalpy is a state function, the enthalpy change from solid to vapor does not depend on the path taken between the two states. Hsubl = Hfusion + Hvap 8

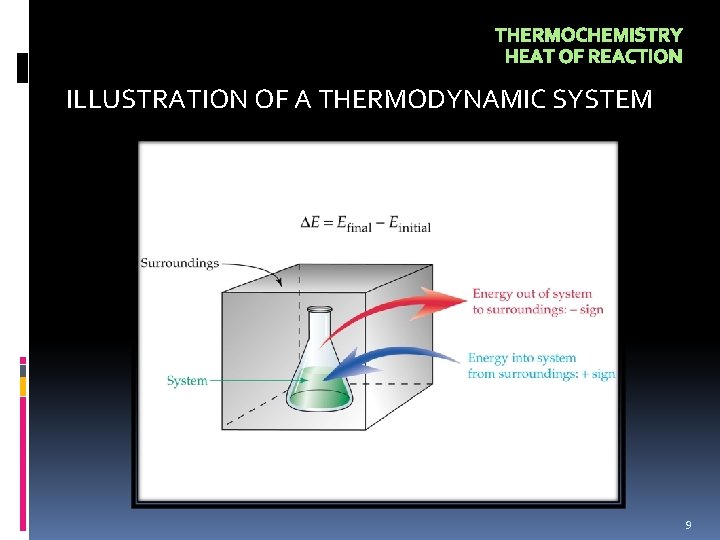
THERMOCHEMISTRY HEAT OF REACTION ILLUSTRATION OF A THERMODYNAMIC SYSTEM 9

THERMOCHEMISTRY HEAT OF REACTION In an experiment: Reactants and products are the system; everything else is the surroundings. • Energy flow from the system to the surroundings has a negative sign (loss of energy). (- E or - H) • Energy flow from the surroundings to the system has a positive sign (gain of energy). (+ E or + H) 10

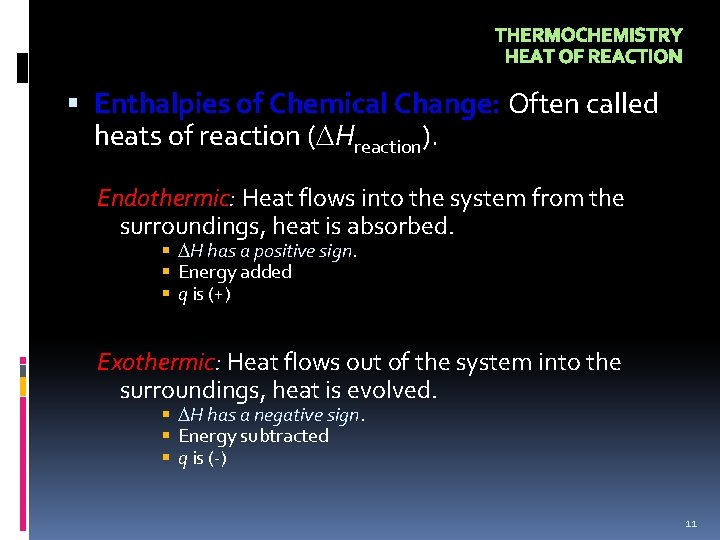
THERMOCHEMISTRY HEAT OF REACTION Enthalpies of Chemical Change: Often called heats of reaction ( Hreaction). Endothermic: Heat flows into the system from the surroundings, heat is absorbed. H has a positive sign. Energy added q is (+) Exothermic: Heat flows out of the system into the surroundings, heat is evolved. H has a negative sign. Energy subtracted q is (-) 11

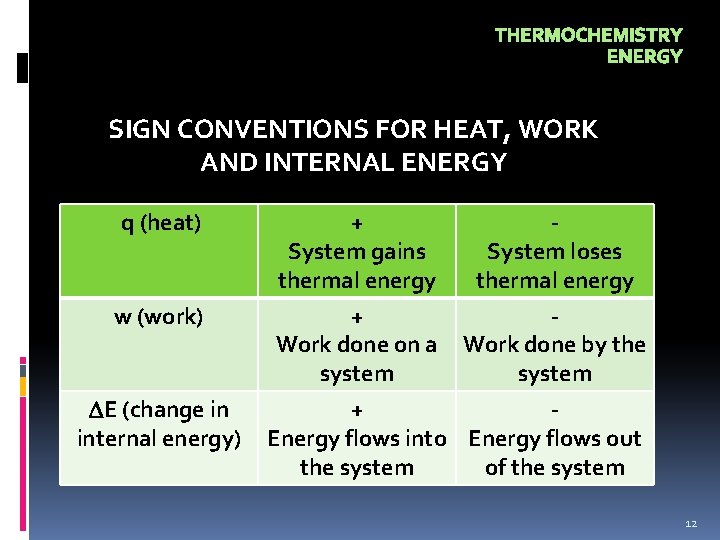
THERMOCHEMISTRY ENERGY SIGN CONVENTIONS FOR HEAT, WORK AND INTERNAL ENERGY q (heat) w (work) E (change in internal energy) + System gains thermal energy + Work done on a system System loses thermal energy Work done by the system + Energy flows into Energy flows out the system of the system 12

THERMOCHEMISTRY HEAT OF REACTION EXAMPLE 6. 2 Barium hydroxide octahydrate reacts with ammonium nitrate: Ba(OH)2. 8 H 2 O(s) + 2 NH 4 NO 3(s) 2 NH 3(g) + 10 H 2 O(l) + Ba(NO 3)2(aq) When 1 mol of barium hydroxide octahydrate reacts with 2 mol of ammonium nitrate, the reaction mixture absorbs 170. 8 k. J of heat. Is this reaction exothermic or endothermic? What is the heat of reaction (q)? Energy is absorbed so this reaction is endothermic. q is +170. 8 k. J 13 13

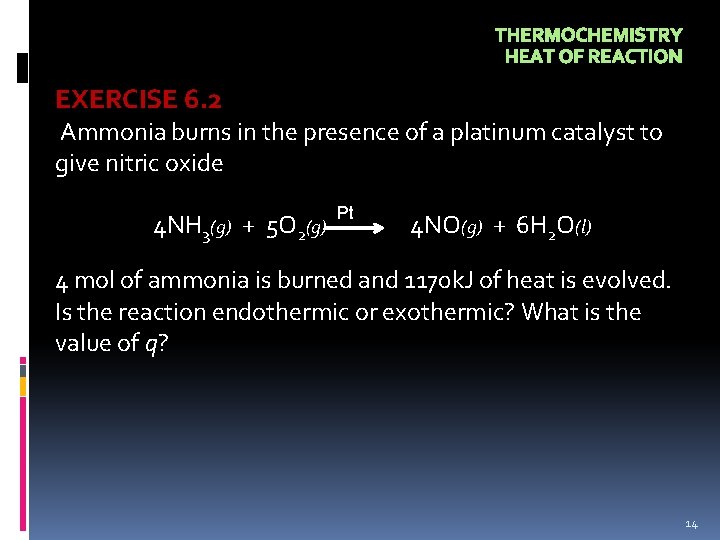
THERMOCHEMISTRY HEAT OF REACTION EXERCISE 6. 2 Ammonia burns in the presence of a platinum catalyst to give nitric oxide 4 NH 3(g) + 5 O 2(g) Pt 4 NO(g) + 6 H 2 O(l) 4 mol of ammonia is burned and 1170 k. J of heat is evolved. Is the reaction endothermic or exothermic? What is the value of q? 14

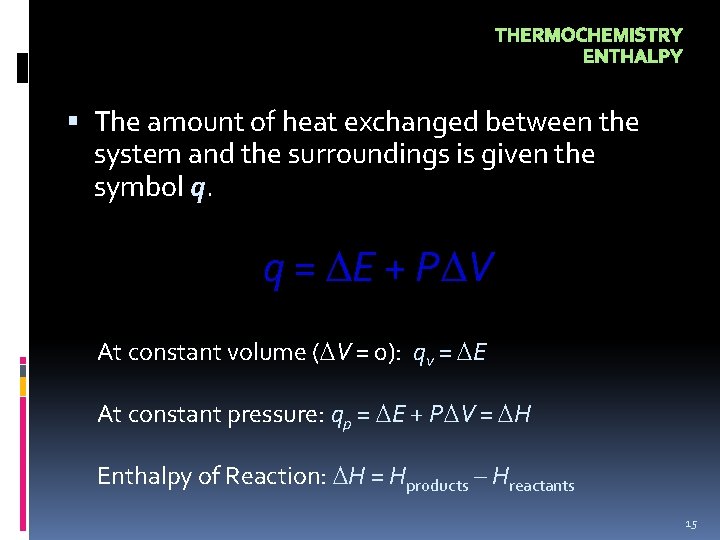
THERMOCHEMISTRY ENTHALPY The amount of heat exchanged between the system and the surroundings is given the symbol q. q = E + P V At constant volume ( V = 0): qv = E At constant pressure: qp = E + P V = H Enthalpy of Reaction: H = Hproducts – Hreactants 15

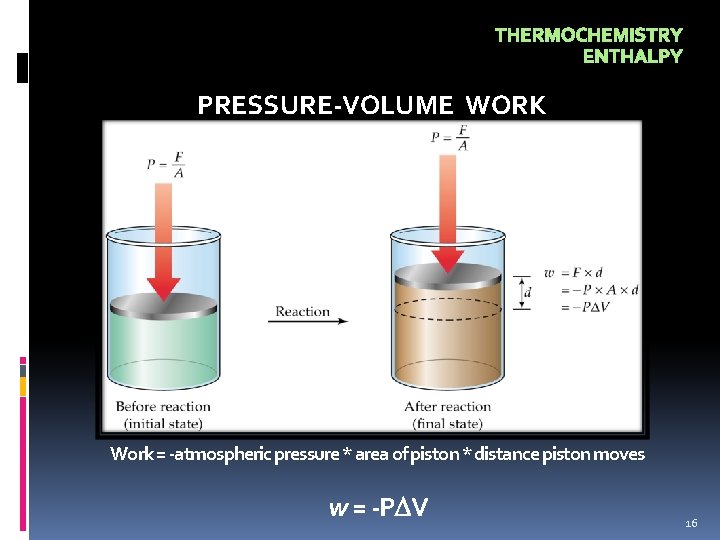
THERMOCHEMISTRY ENTHALPY PRESSURE-VOLUME WORK Work = -atmospheric pressure * area of piston * distance piston moves w = -P V 16

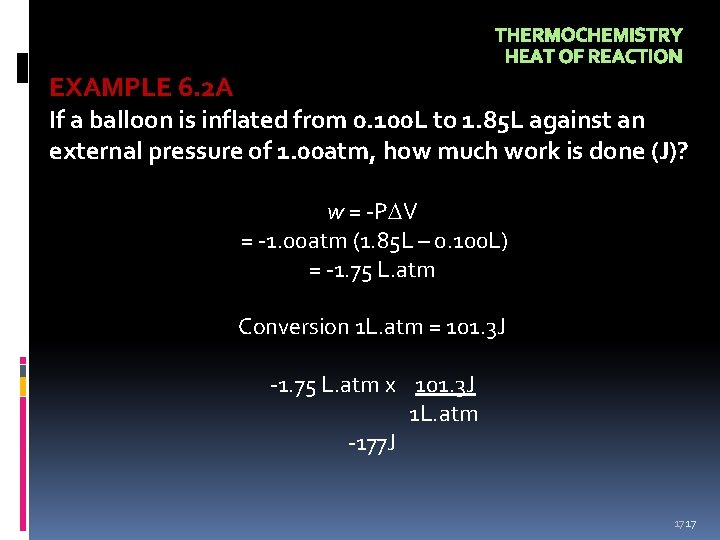
THERMOCHEMISTRY HEAT OF REACTION EXAMPLE 6. 2 A If a balloon is inflated from 0. 100 L to 1. 85 L against an external pressure of 1. 00 atm, how much work is done (J)? w = -P V = -1. 00 atm (1. 85 L – 0. 100 L) = -1. 75 L. atm Conversion 1 L. atm = 101. 3 J -1. 75 L. atm x 101. 3 J 1 L. atm -177 J 17 17

THERMOCHEMISTRY ENTHALPY EXERCISE 6. 2 A A cylinder equipped with a piston expands against an external pressure of 1. 58 atm. If the initial volume is 0. 485 L and the final volume is 1. 245 L, how much work is done (in J)? 18

THERMOCHEMISTRY THERMOCHEMICAL EQUATIONS EXAMPLE 6. 3 Aqueous sodium hydrogen carbonate reacts with hydrochloric acid to produce aqueous sodium chloride, water and carbon dioxide gas. The reaction absorbs 12. 7 k. J of heat at constant pressure for each mole of Na. HCO 3. Write thermochemical equation. Na. HCO 3(aq) + HCl Na. Cl(aq) + H 2 O(l) + CO 2(g) H = +12. 7 k. J 19

THERMOCHEMISTRY THERMOCHEMICAL EQUATIONS EXERCISE 6. 3 A propellant for rockets is obtained by mixing the liquids hydrazine (N 2 H 4) and dinitrogen tetroxide. These compounds react to give gaseous nitrogen and water vapor and 1049 k. J of heat is evolved (at constant pressure when 1 mol reacts). Write thermochemical equation for this reaction 20 20

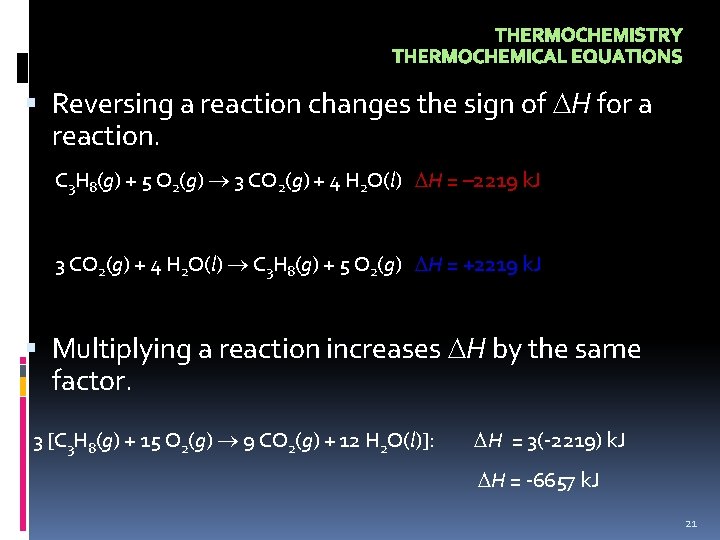
THERMOCHEMISTRY THERMOCHEMICAL EQUATIONS Reversing a reaction changes the sign of H for a reaction. C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l) H = – 2219 k. J 3 CO 2(g) + 4 H 2 O(l) C 3 H 8(g) + 5 O 2(g) H = +2219 k. J Multiplying a reaction increases H by the same factor. 3 [C 3 H 8(g) + 15 O 2(g) 9 CO 2(g) + 12 H 2 O(l)]: H = 3(-2219) k. J H = -6657 k. J 21

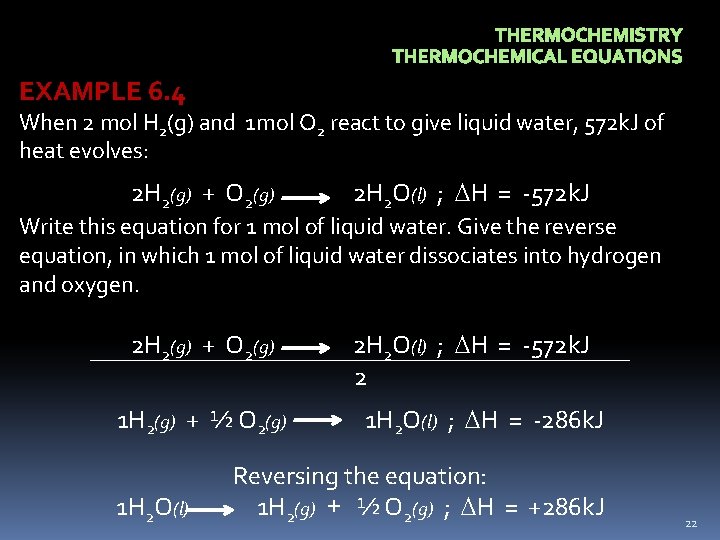
THERMOCHEMISTRY THERMOCHEMICAL EQUATIONS EXAMPLE 6. 4 When 2 mol H 2(g) and 1 mol O 2 react to give liquid water, 572 k. J of heat evolves: 2 H 2(g) + O 2(g) 2 H 2 O(l) ; H = -572 k. J 2 Write this equation for 1 mol of liquid water. Give the reverse equation, in which 1 mol of liquid water dissociates into hydrogen and oxygen. 1 H 2(g) + ½ O 2(g) 1 H 2 O(l) ; H = -286 k. J Reversing the equation: 1 H 2(g) + ½ O 2(g) ; H = +286 k. J 22

THERMOCHEMISTRY THERMOCHEMICAL EQUATIONS EXERCISE 6. 4 Write thermochemical equation for the reaction described in Exercise 6. 3 for the case involving 1 mol N 2 H 4. Write the reverse reaction. 23

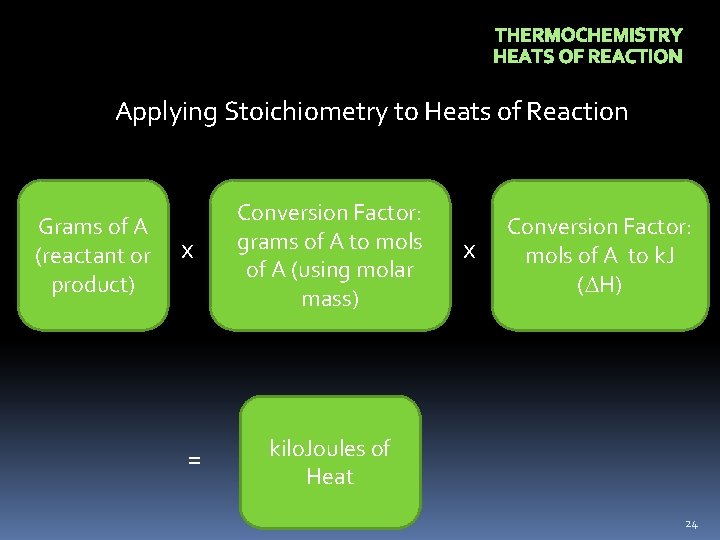
THERMOCHEMISTRY HEATS OF REACTION Applying Stoichiometry to Heats of Reaction Grams of A (reactant or product) x Conversion Factor: grams of A to mols of A (using molar mass) = kilo. Joules of Heat x Conversion Factor: mols of A to k. J ( H) 24

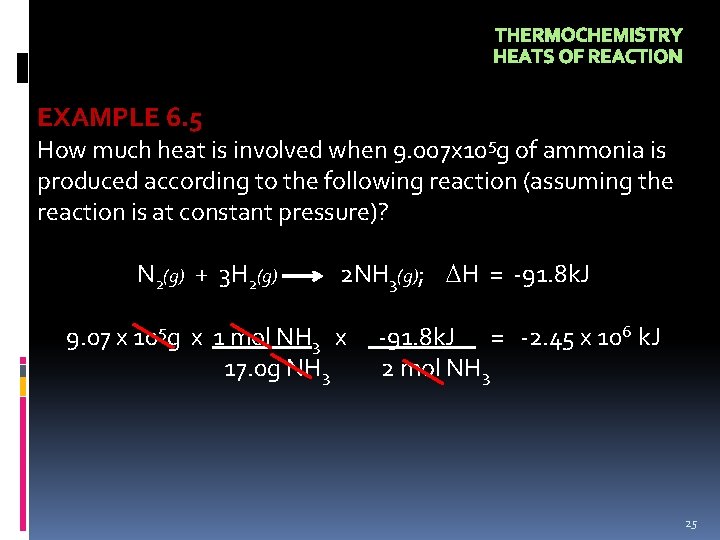
THERMOCHEMISTRY HEATS OF REACTION EXAMPLE 6. 5 How much heat is involved when 9. 007 x 105 g of ammonia is produced according to the following reaction (assuming the reaction is at constant pressure)? N 2(g) + 3 H 2(g) 2 NH 3(g); H = -91. 8 k. J 9. 07 x 105 g x 1 mol NH 3 x 17. 0 g NH 3 -91. 8 k. J = -2. 45 x 106 k. J 2 mol NH 3 25

THERMOCHEMISTRY HEATS OF REACTION EXERCISE 6. 5 How much heat evolves when 10. 0 g of hydrazine reacts according to the reaction described in EXERCISE 6. 3? 26

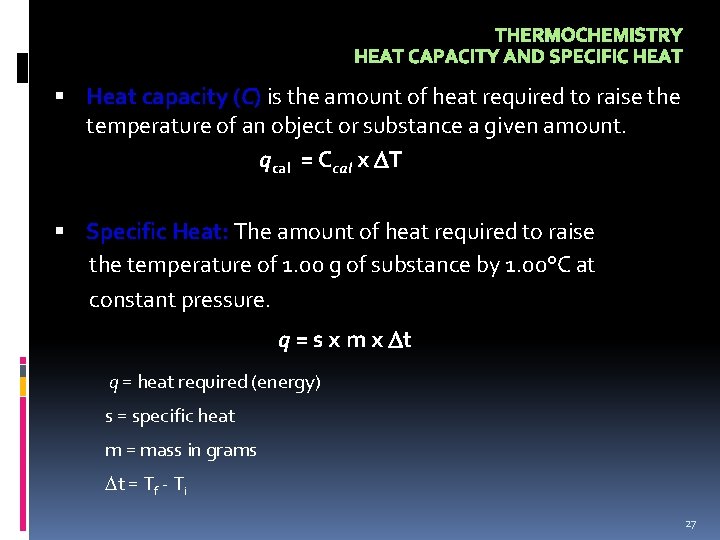
THERMOCHEMISTRY HEAT CAPACITY AND SPECIFIC HEAT Heat capacity (C) is the amount of heat required to raise the temperature of an object or substance a given amount. qcal = Ccal x T Specific Heat: The amount of heat required to raise the temperature of 1. 00 g of substance by 1. 00°C at constant pressure. q = s x m x t q = heat required (energy) s = specific heat m = mass in grams t = Tf - Ti 27

THERMOCHEMISTRY HEATS OF REACTION SPECIFIC HEATS AND MOLAR CAPACITIES FOR SOME COMMON SUBSTANCES @ 25 o. C 28

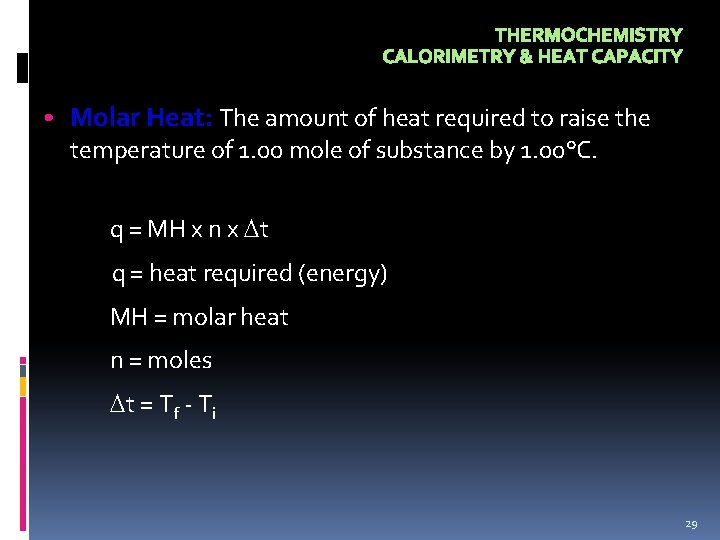
THERMOCHEMISTRY CALORIMETRY & HEAT CAPACITY • Molar Heat: The amount of heat required to raise the temperature of 1. 00 mole of substance by 1. 00°C. q = MH x n x t q = heat required (energy) MH = molar heat n = moles t = Tf - Ti 29

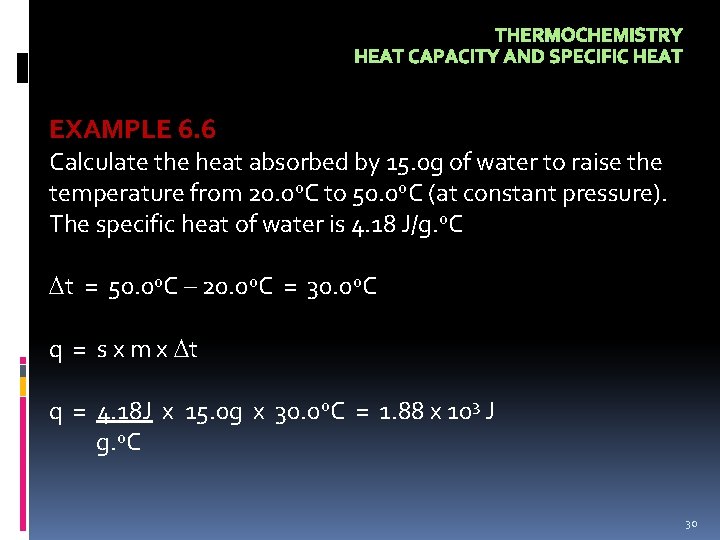
THERMOCHEMISTRY HEAT CAPACITY AND SPECIFIC HEAT EXAMPLE 6. 6 Calculate the heat absorbed by 15. 0 g of water to raise the temperature from 20. 0 o. C to 50. 0 o. C (at constant pressure). The specific heat of water is 4. 18 J/g. o. C t = 50. 0 o. C – 20. 0 o. C = 30. 0 o. C q = s x m x t q = 4. 18 J x 15. 0 g x 30. 0 o. C = 1. 88 x 103 J g. o. C 30

THERMOCHEMISTRY HEAT CAPACITY AND SPECIFIC HEAT EXERCISE 6. 6 Iron metal has a specific heat of 0. 449 J/(g. o. C). How much heat is transferred to a 5. 00 g piece of iron, initially at 20. 0 o. C when placed in a pot of boiling water? (Assume that the temperature of the water is 100. 0 o. C and the water remains at this temperature, which is the final temperature of the iron). 31

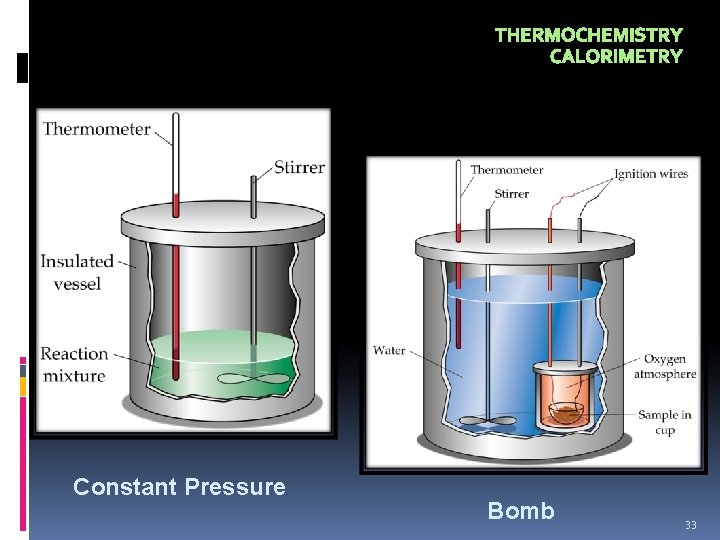
THERMOCHEMISTRY CALORIMETRY Calorimetry is the science of measuring heat changes (q) for chemical reactions. There are two types of calorimeters: • Bomb Calorimetry: A bomb calorimeter measures the heat change at constant volume such that q = E. • Constant Pressure Calorimetry: A constant pressure calorimeter measures the heat change at constant pressure such that q = H. 32

THERMOCHEMISTRY CALORIMETRY Constant Pressure Bomb 33

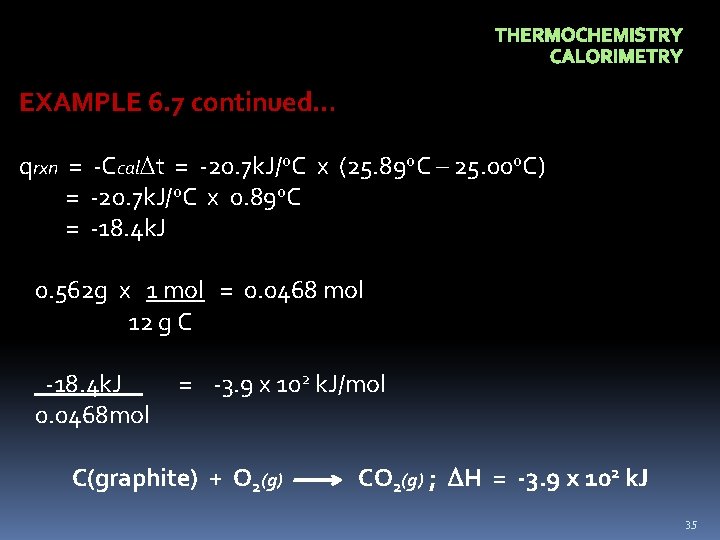
THERMOCHEMISTRY CALORIMETRY EXAMPLE 6. 7 Suppose 0. 562 g of graphite is placed in a calorimeter with an excess of oxygen at 25. 00 o. C and 1 atm pressure. Excess O 2 ensures that all carbon burns to form CO 2. The graphite is ignited, and it burns according to the following equation C(graphite) + O 2(g) CO 2(g) On reaction, the calorimeter temperature rises from 25. 00 o. C to 25. 89 o. C. The heat capacity of the calorimeter and it’s contents was determined in a separate experiment to be 20. 7 k. J/o. C. What is the heat of reaction at 25. 00 o. C and 1 atm pressure? Express in a thermochemical equation. 34

THERMOCHEMISTRY CALORIMETRY EXAMPLE 6. 7 continued… qrxn = -Ccal t = -20. 7 k. J/o. C x (25. 89 o. C – 25. 00 o. C) = -20. 7 k. J/o. C x 0. 89 o. C = -18. 4 k. J 0. 562 g x 1 mol = 0. 0468 mol 12 g C -18. 4 k. J 0. 0468 mol = -3. 9 x 102 k. J/mol C(graphite) + O 2(g) CO 2(g) ; H = -3. 9 x 102 k. J 35

THERMOCHEMISTRY CALORIMETRY EXERCISE 6. 7 Suppose 33 m. L of 0. 120 M HCl is added to 42 m. L of a solution containing excess sodium hydroxide in a coffee cup calorimeter. The solution temperature, originally at 25 o. C, rises to 31. 8 o. C. Give the enthalpy change H for the reaction: HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) Assume that the heat capacity and the density of the final solution in the cup are those of water. s = 4. 184 k. J/g. o. C d = 1. 000 g/m. L Express the answer as a thermochemical equation. 36

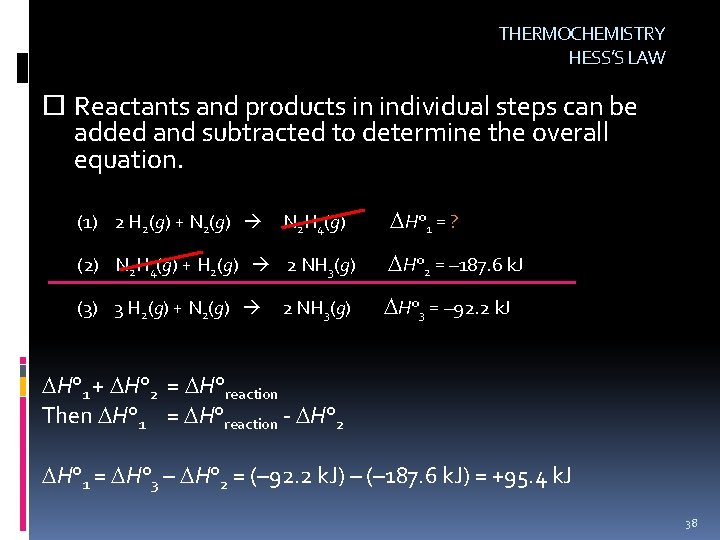
THERMOCHEMISTRY HESS’S LAW Hess’s Law: The overall enthalpy change for a reaction is equal to the sum of the enthalpy changes for the individual steps in the reaction. (not a physical change, chemical change) 3 H 2(g) + N 2(g) 2 NH 3(g) H° = – 92. 2 k. J 37

THERMOCHEMISTRY HESS’S LAW Reactants and products in individual steps can be added and subtracted to determine the overall equation. (1) 2 H 2(g) + N 2(g) N 2 H 4(g) H° 1 = ? (2) N 2 H 4(g) + H 2(g) 2 NH 3(g) H° 2 = – 187. 6 k. J (3) 3 H 2(g) + N 2(g) H° 3 = – 92. 2 k. J 2 NH 3(g) H° 1 + H° 2 = H°reaction Then H° 1 = H°reaction - H° 2 H° 1 = H° 3 – H° 2 = (– 92. 2 k. J) – (– 187. 6 k. J) = +95. 4 k. J 38

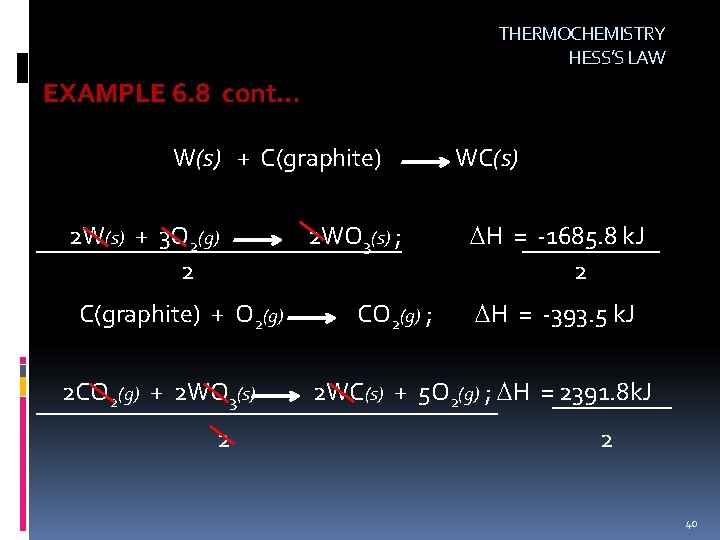
THERMOCHEMISTRY HESS’S LAW EXAMPLE 6. 8 What is the enthalpy of reaction, H, for the formation of tungsten carbide (WC) from the elements? W(s) + C(graphite) 2 W(s) + 3 O 2(g) 2 WO 3(s) ; C(graphite) + O 2(g) 2 WC(s) + 5 O 2(g) CO 2(g) ; WC(s) H = -1685. 8 k. J H = -393. 5 k. J 2 WO 3(s) + 2 CO 2(g) ; H = -2391. 8 k. J LAST EQUATION NEEDS TO BE REVERSED 2 CO 2(g) + 2 WO 3(s) 2 WC(s) + 5 O 2(g) ; H = 2391. 8 k. J 39

THERMOCHEMISTRY HESS’S LAW EXAMPLE 6. 8 cont… W(s) + C(graphite) 2 W(s) + 3 O 2(g) 2 C(graphite) + O 2(g) 2 CO 2(g) + 2 WO 3(s) 2 2 WO 3(s) ; CO 2(g) ; WC(s) H = -1685. 8 k. J 2 H = -393. 5 k. J 2 WC(s) + 5 O 2(g) ; H = 2391. 8 k. J 2 40

THERMOCHEMISTRY HESS’S LAW EXAMPLE 6. 8 cont… W(s) + C(graphite) W(s) + 3/2 O 2(g) C(graphite) + O 2(g) CO 2(g) + WO 3(s) W(s) + C(graphite) WC(s) H = -842. 9 k. J WO 3(s) ; CO 2(g) ; WC(s) + WC(s) H = -393. 5 k. J 5/2 O 2(g) ; H = 1195. 9 k. J H = -40. 5 k. J 41

THERMOCHEMISTRY HESS’S LAW EXERCISE 6. 8 Manganese metal can be obtained by the reaction of manganese dioxide and aluminum 4 Al(s) + 3 Mn. O 2(s) 2 Al 2 O 3(s) + 3 Mn(s) What is the H for this reaction? Use the following data: 2 Al(s) + 3/2 O 2(g) Al 2 O 3(s) ; H = − 1676 k. J Mn(s) + O 2(g) Mn. O 2(s) ; H = − 520 k. J 42

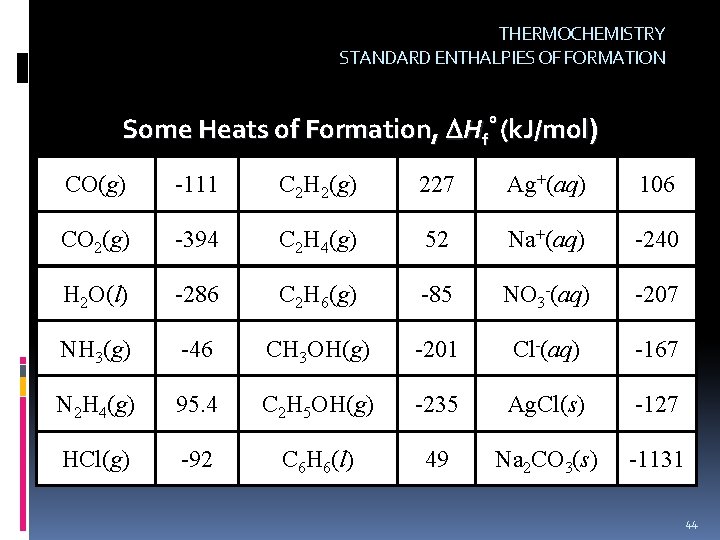
THERMOCHEMISTRY STANDARD ENTHALPIES OF FORMATION Standard Heats of Formation ( H°f): The enthalpy change for the formation of 1 mole of substance in its standard state from its constituent elements in their standard states. The standard heat of formation for any element in its standard state is defined as being ZERO. H°f = 0 for an element in its standard state 43

THERMOCHEMISTRY STANDARD ENTHALPIES OF FORMATION Some Heats of Formation, Hf° (k. J/mol) CO(g) -111 C 2 H 2(g) 227 Ag+(aq) 106 CO 2(g) -394 C 2 H 4(g) 52 Na+(aq) -240 H 2 O(l) -286 C 2 H 6(g) -85 NO 3 -(aq) -207 NH 3(g) -46 CH 3 OH(g) -201 Cl-(aq) -167 N 2 H 4(g) 95. 4 C 2 H 5 OH(g) -235 Ag. Cl(s) -127 HCl(g) -92 C 6 H 6(l) 49 Na 2 CO 3(s) -1131 44

THERMOCHEMISTRY STANDARD ENTHALPIES OF FORMATION Thermodynamic Standard State: Most stable form of a substance at 1 atm pressure and 25°C; 1 M concentration for all substances in solution. These are indicated by a superscript ° to the symbol of the quantity reported. Standard enthalpy change is indicated by the symbol H°. 45

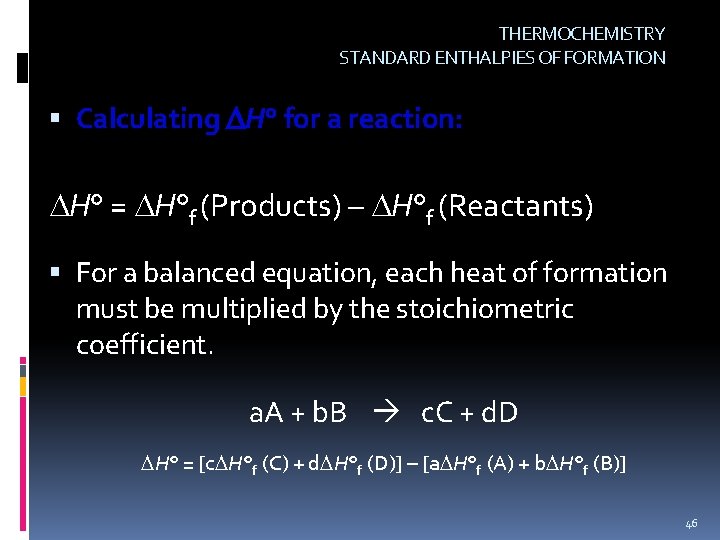
THERMOCHEMISTRY STANDARD ENTHALPIES OF FORMATION Calculating H° for a reaction: H° = H°f (Products) – H°f (Reactants) For a balanced equation, each heat of formation must be multiplied by the stoichiometric coefficient. a. A + b. B c. C + d. D H° = [c H°f (C) + d H°f (D)] – [a H°f (A) + b H°f (B)] 46

THERMOCHEMISTRY STANDARD ENTHALPIES OF FORMATION EXAMPLE 6. 9 Use the values of Hof to calculate the heat of vaporization Hovap of carbon disulfide at 25 o. C. The vaporization process is: CS 2(l) CS 2(g) Hof = 89. 7 k. J/mol Hof = 116. 9 k. J/mol Hovap = Sn Hof(products) – Sm Hof (reactants) Hof[CS 2(g)] - Hof [CS 2(l)] 116. 9 k. J - 89. 7 k. J = 27. 2 k. J 47

THERMOCHEMISTRY STANDARD ENTHALPIES OF FORMATION EXERCISE 6. 9 Calculate the heat of vaporization, Hovap of water using standard enthalpies of formation 48

THERMOCHEMISTRY STANDARD ENTHALPIES OF FORMATION EXAMPLE 6. 10 Large quantities of ammonia are used to prepared nitric acid. The first consists of the catalytic oxidation of ammonia to nitric oxide 4 NH 3(g) + 5 O 2(g) 45. 9 k. J 0 k. J 4 NO(g) + 6 H 2 O(g) 90. 3 6 k. J -241. 8 k. J What is the standard enthalpy change for this reaction? Hovap = Sn Hof(products) – Sm Hof (reactants) = [4(90. 3) + 6(-241. 8)]k. J - [4(-45. 9) + 5(0)]k. J = -906 k. J 49
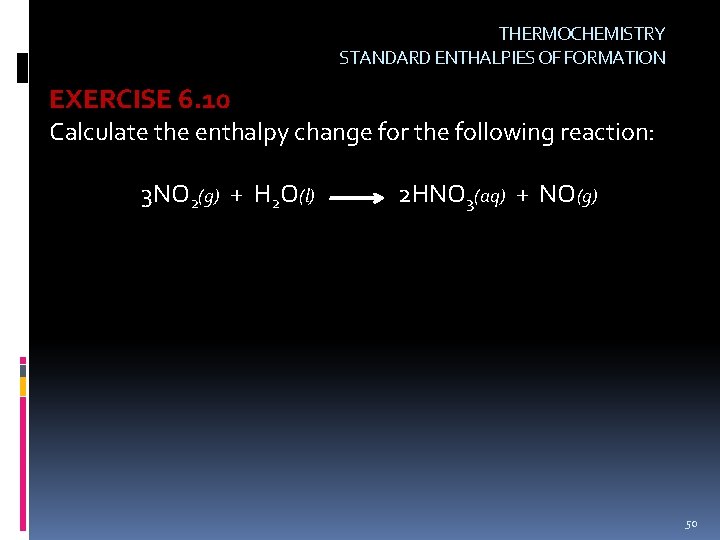
THERMOCHEMISTRY STANDARD ENTHALPIES OF FORMATION EXERCISE 6. 10 Calculate the enthalpy change for the following reaction: 3 NO 2(g) + H 2 O(l) 2 HNO 3(aq) + NO(g) 50

THERMOCHEMISTRY STANDARD ENTHALPIES OF FORMATION EXERCISE 6. 11 Calculate the standard enthalpy change for the reaction of an aqueous solution of barium hydroxide with an aqueous solution of ammonium nitrate at 25 o. C. 51

THERMOCHEMISTRY FUELS is any substance that is burned or react to provide heat and other forms of energy. ØFOODS AS FUELS ØFOSSIL FUELS ØCOAL GASIFICATION AND LIQUEFICATION ØROCKET FUELS 52

THERMOCHEMISTRY FUELS Sources of energy consumed in the United States. 53

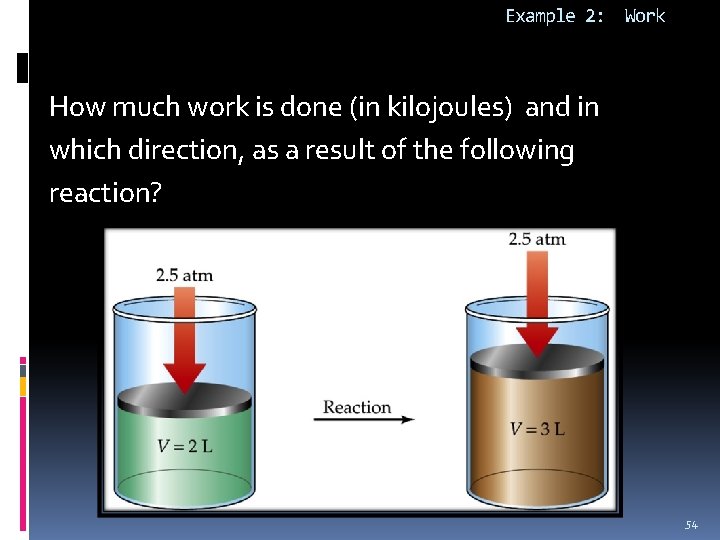
Example 2: Work How much work is done (in kilojoules) and in which direction, as a result of the following reaction? 54

Example 3: Work The explosion of 2. 00 mol of solid TNT with a volume of approximately 0. 274 L produces gases with a volume of 489 L at room temperature. How much PV (in kilojoules) work is done during the explosion? Assume P = 1 atm, T = 25°C. 2 C 7 H 5 N 3 O 6(s) 12 CO(g) + 5 H 2(g) + 3 N 2(g) + 2 C(s) 55

Example 5: Is an endothermic reaction a favorable process thermodynamically speaking? 1) Yes 2) No 56

Example 6: Hess’s Law The industrial degreasing solvent methylene chloride (CH 2 Cl 2, dichloromethane) is prepared from methane by reaction with chlorine: CH 4(g) + 2 Cl 2(g) CH 2 Cl 2(g) + 2 HCl(g) Use the following data to calculate H° (in kilojoules) for the above reaction: CH 4(g) + Cl 2(g) CH 3 Cl(g) + HCl(g) H° = – 98. 3 k. J CH 3 Cl(g) + Cl 2(g) CH 2 Cl 2(g) + HCl(g) H° = – 104 k. J 57

Example 7: Standard heat of formation Calculate H° (in kilojoules) for the reaction of ammonia with O 2 to yield nitric oxide (NO) and H 2 O(g), a step in the Ostwald process for the commercial production of nitric acid. 58

Example 8: Standard heat of formation Calculate H° (in kilojoules) for the photosynthesis of glucose and O 2 from CO 2 and liquid water, a reaction carried out by all green plants. 59

Example 9: Which of the following would indicate an endothermic reaction? Why? 1. - H 2. + H 60

Example 10: Specific Heat What is the specific heat of lead if it takes 96 J to raise the temperature of a 75 g block by 10. 0°C? 61

Example 11: Specific Heat How much energy (in J) does it take to increase the temperature of 12. 8 g of Gold from 56 C to 85 C? 62

Example 12: Molar Heat How much energy (in J) does it take to increase the temperature of 1. 45 x 104 moles of water from 69 C to 94 C? 63

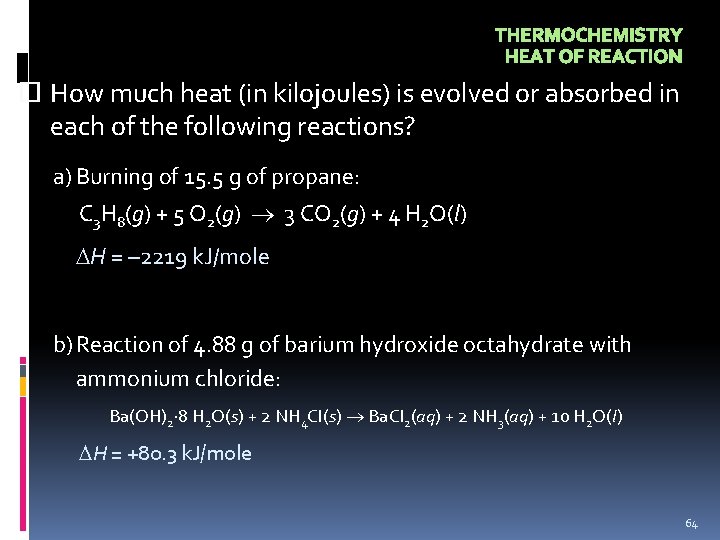
THERMOCHEMISTRY HEAT OF REACTION How much heat (in kilojoules) is evolved or absorbed in each of the following reactions? a) Burning of 15. 5 g of propane: C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l) H = – 2219 k. J/mole b)Reaction of 4. 88 g of barium hydroxide octahydrate with ammonium chloride: Ba(OH)2· 8 H 2 O(s) + 2 NH 4 Cl(s) Ba. Cl 2(aq) + 2 NH 3(aq) + 10 H 2 O(l) H = +80. 3 k. J/mole 64

Heat of Phase Transitions from H f Calculate the heat of vaporization, H vap of water, using standard enthalpies of formation H 2 O(g) H 2 O(l) H f -241. 8 k. J/mol -285. 8 k. J/mol 65

66
 Dual enrollment valencia
Dual enrollment valencia Contoh mesin non konvensional
Contoh mesin non konvensional Joenschem
Joenschem Chm 201
Chm 201 Chm tool
Chm tool Chemical milling layout
Chemical milling layout Chm 1045
Chm 1045 Chm logo
Chm logo Chem 130 final exam
Chem 130 final exam Chm 111
Chm 111 Chemistry 151 final exam
Chemistry 151 final exam Chm 241
Chm 241 Energy changes
Energy changes Thermochemistry equations
Thermochemistry equations Ap chemistry thermochemistry frq
Ap chemistry thermochemistry frq Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Chapter 6 thermochemistry
Chapter 6 thermochemistry Thermochemistry
Thermochemistry Ap chemistry unit 9
Ap chemistry unit 9 Thermochemistry is concerned with the
Thermochemistry is concerned with the Study of energy and its transformations
Study of energy and its transformations Chapter 17 thermochemistry practice problems answers
Chapter 17 thermochemistry practice problems answers Thermochemical equation examples
Thermochemical equation examples Internal thermal energy
Internal thermal energy Thermochemistry is the study of
Thermochemistry is the study of Bomb calorimetry equation
Bomb calorimetry equation What is thermochemistry
What is thermochemistry Thermochemistry cartoon
Thermochemistry cartoon Thermochemistry is the study of *
Thermochemistry is the study of * Thermochemistry is study of
Thermochemistry is study of Thermochemistry is study of: *
Thermochemistry is study of: * Thermochemistry exam review
Thermochemistry exam review Thermochemistry is the study of
Thermochemistry is the study of Kinetic energy thermochemistry
Kinetic energy thermochemistry Thermochemistry is the study of
Thermochemistry is the study of Chapter 17 thermochemistry answer key
Chapter 17 thermochemistry answer key Kirchhoff's law enthalpy example
Kirchhoff's law enthalpy example How to calculate the heat absorbed
How to calculate the heat absorbed Chapter 17 thermochemistry
Chapter 17 thermochemistry Thermochemical equation
Thermochemical equation Thermochemistry video
Thermochemistry video Chapter 17 thermochemistry
Chapter 17 thermochemistry General chemistry thermochemistry
General chemistry thermochemistry Thermochemistry equations
Thermochemistry equations Nn
Nn Hess law constant heat summation
Hess law constant heat summation Energy diagram thermochemistry
Energy diagram thermochemistry Thermochemistry
Thermochemistry Contoh entalpi pengatoman standar
Contoh entalpi pengatoman standar Ap chemistry thermodynamics
Ap chemistry thermodynamics Chapter 17 thermochemistry
Chapter 17 thermochemistry Why are chemists interested in studying thermochemistry?
Why are chemists interested in studying thermochemistry? Thermochemistry vocabulary
Thermochemistry vocabulary Cartoon thermochemistry
Cartoon thermochemistry Thermochemistry conversions
Thermochemistry conversions Thermochemistry concepts
Thermochemistry concepts Thermochemistry
Thermochemistry Bab 5
Bab 5 Symbolic border one pager
Symbolic border one pager Thermochemistry
Thermochemistry Coffee cup calorimeter
Coffee cup calorimeter Thermochemistry review
Thermochemistry review Electrones de valencia de francio
Electrones de valencia de francio Sala de congresos valencia
Sala de congresos valencia Fundasababe valencia
Fundasababe valencia Karen tatiana valencia rivero
Karen tatiana valencia rivero Regla del octeto ejemplos
Regla del octeto ejemplos