Chapter 8 Chemical Reactions Vanessa N PrasadPermaul CHM

































- Slides: 65

Chapter 8 Chemical Reactions Vanessa N. Prasad-Permaul CHM 1025 Valencia College © 2011 Pearson Education, Inc. Chapter 8 1

Chemical and Physical Changes In a physical change, the chemical composition of the substance remains constant. Examples of physical changes are the melting of ice or the boiling of water. In a chemical change, the chemical composition of the substance changes; a chemical reaction occurs. During a chemical reaction, a new substance is formed. Chapter 8 © 2011 Pearson Education, Inc. 2

Evidence for Chemical Reactions • There are four observations that indicate a chemical reaction is taking place. 1. A gas is released. • Gas may be observed in many ways in a reaction from light fizzing to heavy bubbling. • The release of hydrogen gas from the reaction of magnesium metal with acid is shown here. Chapter 8 © 2011 Pearson Education, Inc. 3

Evidence for Chemical Reactions, Continued 2. An insoluble solid is produced. • A substance dissolves in water to give an aqueous solution. • If we add two aqueous solutions together, we may observe the production of a solid substance. • The insoluble solid formed is called a precipitate. Chapter 8 © 2011 Pearson Education, Inc. 4

Evidence for Chemical Reactions, Continued 3. A permanent color change is observed. • Many chemical reactions involve a permanent color change. • A change in color indicates that formed. a new substance has been Chapter 8 © 2011 Pearson Education, Inc. 5

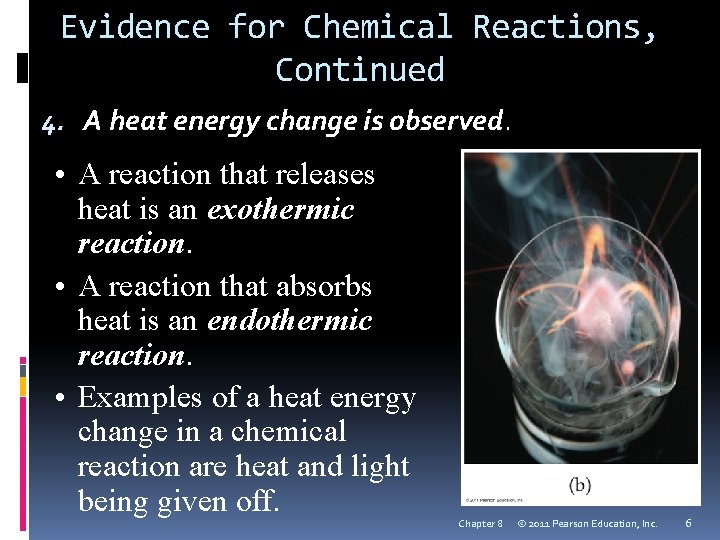
Evidence for Chemical Reactions, Continued 4. A heat energy change is observed. • A reaction that releases heat is an exothermic reaction. • A reaction that absorbs heat is an endothermic reaction. • Examples of a heat energy change in a chemical reaction are heat and light being given off. Chapter 8 © 2011 Pearson Education, Inc. 6

Example 8. 1 Evidence for a Reaction Which of the following is experimental evidence for a chemical reaction? (a) Pouring vinegar on baking soda gives foamy bubbles. (b) Mixing two solutions produces insoluble particles. (c) Mixing two colorless solutions gives a yellow solution. (d) Mixing two solutions produces a temperature increase. Practice Exercise What are four observations that a chemical reaction has occurred?

Writing Chemical Equations A chemical equation describes a chemical reaction using formulas and symbols. A general chemical equation is as follows: A+B → C+D In this equation, A and B are reactants and C and D are products. We can also add a catalyst to a reaction. A catalyst is written above the arrow and speeds up the reaction without being consumed. Chapter 8 © 2011 Pearson Education, Inc. 8

States of Matter in Equations When writing chemical equations, we usually specify the physical state of the reactants and products. A(g) + B(l) → C(s) + D(aq) In this equation, reactant A is in the gaseous state and reactant B is in the liquid state. Also, product C is in the solid state and product D is in the aqueous state. Chapter 8 © 2011 Pearson Education, Inc. 9

Chemical Equation Symbols Here are several symbols used in chemical equations: Chapter 8 © 2011 Pearson Education, Inc. 10

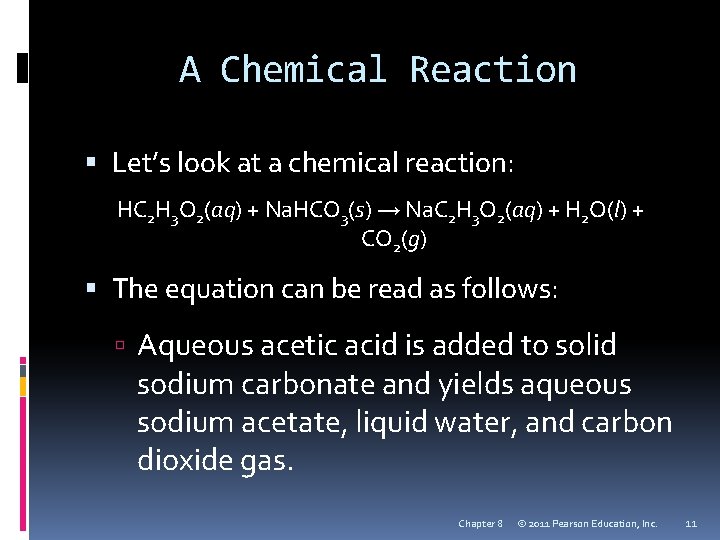
A Chemical Reaction Let’s look at a chemical reaction: HC 2 H 3 O 2(aq) + Na. HCO 3(s) → Na. C 2 H 3 O 2(aq) + H 2 O(l) + CO 2(g) The equation can be read as follows: Aqueous acetic acid is added to solid sodium carbonate and yields aqueous sodium acetate, liquid water, and carbon dioxide gas. Chapter 8 © 2011 Pearson Education, Inc. 11

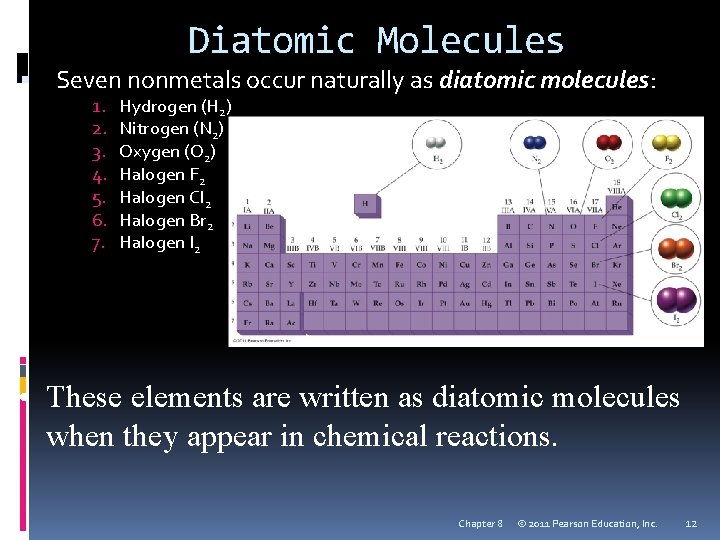
Diatomic Molecules Seven nonmetals occur naturally as diatomic molecules: 1. 2. 3. 4. 5. 6. 7. Hydrogen (H 2) Nitrogen (N 2) Oxygen (O 2) Halogen F 2 Halogen Cl 2 Halogen Br 2 Halogen I 2 • These elements are written as diatomic molecules when they appear in chemical reactions. Chapter 8 © 2011 Pearson Education, Inc. 12

Example 8. 2 Writing Chemical Equations Write a chemical equation for each of the following chemical reactions: (a) Mercury liquid and fluorine gas react to give solid mercury(II) fluoride. (b) Zinc metal reacts with sulfuric acid to give aqueous zinc sulfate and hydrogen gas. Practice Exercise Write a chemical equation for each of the following chemical reactions: (a) Aqueous solutions of sodium iodide and silver nitrate yield silver iodide precipitate and aqueous sodium nitrate. (b)Acetic acid reacts with aqueous potassium hydroxide to give aqueous potassium acetate plus water.

Balancing Chemical Equations When we write a chemical equation, the number of atoms of each element must be the same on both sides of the arrow. This is called a balanced chemical equation. We balance chemical reactions by placing a whole number coefficient in front of each substance. A coefficient multiplies all subscripts in a chemical formula: 3 H 2 O has 6 hydrogen atoms and 3 oxygen atoms. Chapter 8 © 2011 Pearson Education, Inc. 14

Guidelines for Balancing Equations Before placing coefficients in an equation, check that the formulas are correct. Never change the subscripts in a chemical formula to balance a chemical equation. Balance each element in the equation starting with the most complex formula. Balance polyatomic ions as a single unit if it appears on both sides of the equation. Chapter 8 © 2011 Pearson Education, Inc. 15

Guidelines for Balancing Equations, Continued The coefficients must be whole numbers. If you get a fraction, multiply the whole equation by the denominator to get whole numbers. [H 2(g) + ½ O 2(g) → H 2 O(l)] x 2 2 H 2(g) + O 2(g) → 2 H 2 O(l) After balancing the equation, check that there are the same number of atoms of each element (or polyatomic ion) on both sides of the equation. 2(2) = 4 H; 2 O → 2(2) = 4 H; 2 O Chapter 8 © 2011 Pearson Education, Inc. 16

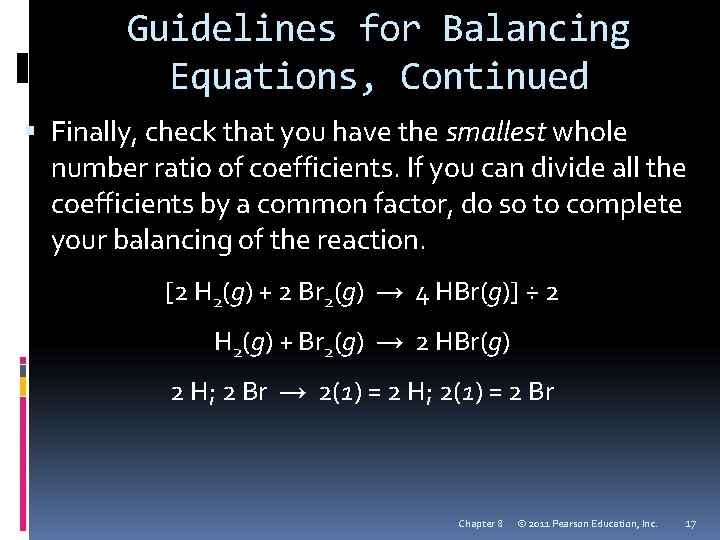
Guidelines for Balancing Equations, Continued Finally, check that you have the smallest whole number ratio of coefficients. If you can divide all the coefficients by a common factor, do so to complete your balancing of the reaction. [2 H 2(g) + 2 Br 2(g) → 4 HBr(g)] ÷ 2 H 2(g) + Br 2(g) → 2 HBr(g) 2 H; 2 Br → 2(1) = 2 H; 2(1) = 2 Br Chapter 8 © 2011 Pearson Education, Inc. 17

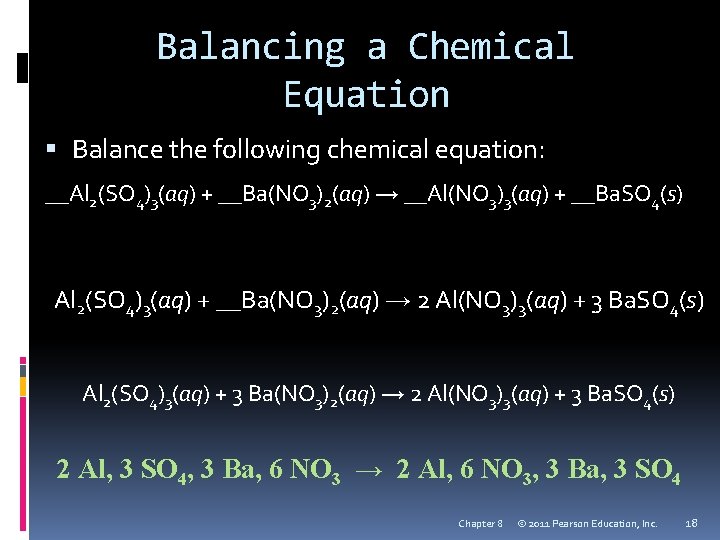
Balancing a Chemical Equation Balance the following chemical equation: __Al 2(SO 4)3(aq) + __Ba(NO 3)2(aq) → __Al(NO 3)3(aq) + __Ba. SO 4(s) Al 2(SO 4)3(aq) + __Ba(NO 3)2(aq) → 2 Al(NO 3)3(aq) + 3 Ba. SO 4(s) Al 2(SO 4)3(aq) + 3 Ba(NO 3)2(aq) → 2 Al(NO 3)3(aq) + 3 Ba. SO 4(s) 2 Al, 3 SO 4, 3 Ba, 6 NO 3 → 2 Al, 6 NO 3, 3 Ba, 3 SO 4 Chapter 8 © 2011 Pearson Education, Inc. 18

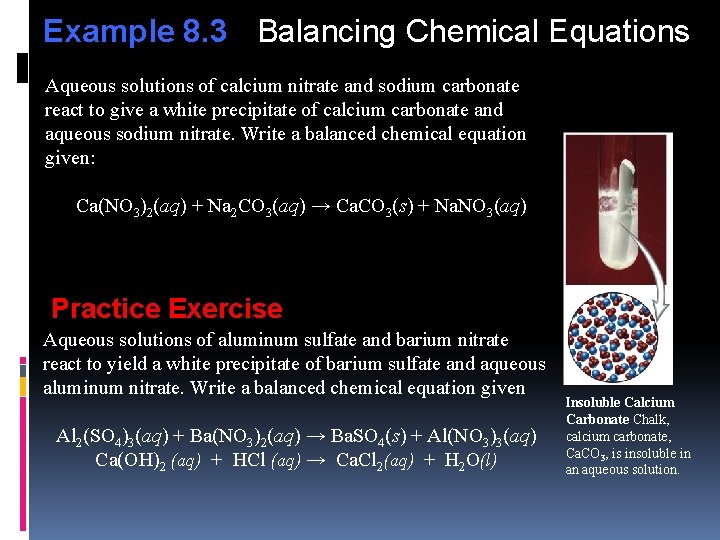
Example 8. 3 Balancing Chemical Equations Aqueous solutions of calcium nitrate and sodium carbonate react to give a white precipitate of calcium carbonate and aqueous sodium nitrate. Write a balanced chemical equation given: Ca(NO 3)2(aq) + Na 2 CO 3(aq) → Ca. CO 3(s) + Na. NO 3(aq) Practice Exercise Aqueous solutions of aluminum sulfate and barium nitrate react to yield a white precipitate of barium sulfate and aqueous aluminum nitrate. Write a balanced chemical equation given Al 2(SO 4)3(aq) + Ba(NO 3)2(aq) → Ba. SO 4(s) + Al(NO 3)3(aq) Ca(OH)2 (aq) + HCl (aq) → Ca. Cl 2(aq) + H 2 O(l) Insoluble Calcium Carbonate Chalk, calcium carbonate, Ca. CO 3, is insoluble in an aqueous solution.

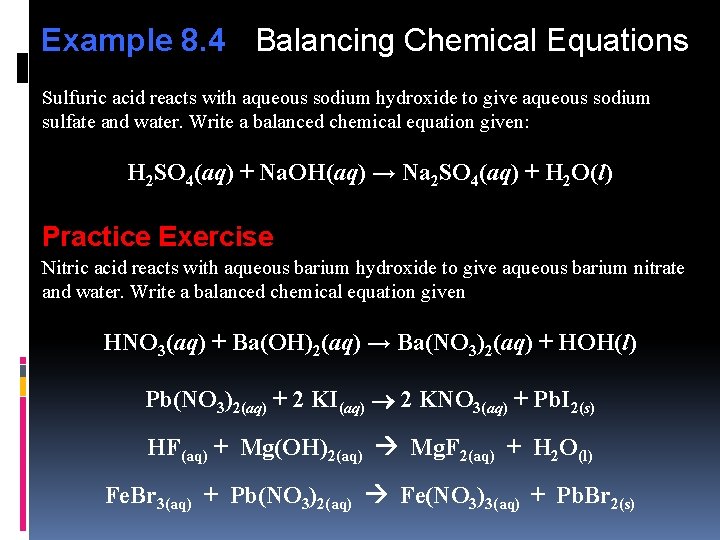
Example 8. 4 Balancing Chemical Equations Sulfuric acid reacts with aqueous sodium hydroxide to give aqueous sodium sulfate and water. Write a balanced chemical equation given: H 2 SO 4(aq) + Na. OH(aq) → Na 2 SO 4(aq) + H 2 O(l) Practice Exercise Nitric acid reacts with aqueous barium hydroxide to give aqueous barium nitrate and water. Write a balanced chemical equation given HNO 3(aq) + Ba(OH)2(aq) → Ba(NO 3)2(aq) + HOH(l) Pb(NO 3)2(aq) + 2 KI(aq) 2 KNO 3(aq) + Pb. I 2(s) HF(aq) + Mg(OH)2(aq) Mg. F 2(aq) + H 2 O(l) Fe. Br 3(aq) + Pb(NO 3)2(aq) Fe(NO 3)3(aq) + Pb. Br 2(s)

Classifying Chemical Reactions We can place chemical reactions into five categories: 1. Combination reactions 2. Decomposition reactions 3. Single-replacement reactions 4. Double-replacement reactions 5. Neutralization reactions Chapter 8 © 2011 Pearson Education, Inc. 21

Example 8. 5 Classifying Chemical Reactions Classify each of the following reactions as combination, decomposition, singlereplacement, double-replacement, or neutralization. (a) Copper metal heated with oxygen gas produces solid copper(II) oxide. (b) Heating powdered iron(III) carbonate produces solid iron(III) oxide and carbon dioxidegas. (c) Aluminum metal reacts with aqueous manganese(II) sulfate to give aqueous aluminum sulfate and manganese metal. 2 Al(s) + 3 Mn. SO 4(aq) → Al 2(SO 4)3(aq) + 3 Mn(s) (d) Aqueous sodium chromate reacts with aqueous barium chloride to give insoluble barium chromate and aqueous sodium chloride. Na 2 Cr. O 4(aq) + Ba. Cl 2(aq) → Ba. Cr. O 4(s) + 2 Na. Cl(aq) (e) Nitric acid reacts with aqueous potassium hydroxide to give aqueous potassium nitrate and water. HNO 3(aq) + KOH(aq) → KNO 3(aq) + H 2 O(l)

Exercise 8. 5 Classifying Chemical Reactions Practice Exercise Classify the following types of reactions as combination, decomposition, single-replacement, double-replacement, or neutralization: (a) 2 Sr(s) + O 2(g) → 2 Sr. O(s) (b) Ni(s) + Cu. SO 4(aq) → Ni. SO 4(aq) + Cu(s) (c) HC 2 H 3 O 2(aq) + Na. OH(aq) → Na. C 2 H 3 O 2(aq) + H 2 O(l) (d) Zn(HCO 3)2(s) → Zn. CO 3(s) + H 2 O(g) + CO 2(g) (e) Ag. NO 3(aq) + KCl(aq) → Ag. Cl(s) + KNO 3(aq)

Combination Reactions A combination reaction is a reaction in which two simpler substances are combined into a more complex compound. Combination reactions are also called synthesis reactions. We will look at three combination reactions: 1. The reaction of a metal with oxygen 2. The reaction of a nonmetal with oxygen 3. The reaction of a metal and a nonmetal Chapter 8 © 2011 Pearson Education, Inc. 24

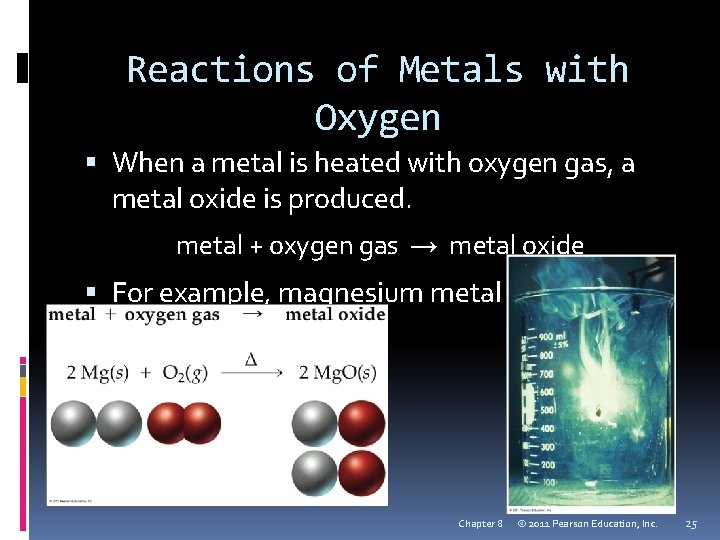
Reactions of Metals with Oxygen When a metal is heated with oxygen gas, a metal oxide is produced. metal + oxygen gas → metal oxide For example, magnesium metal produces magnesium oxide. Chapter 8 © 2011 Pearson Education, Inc. 25

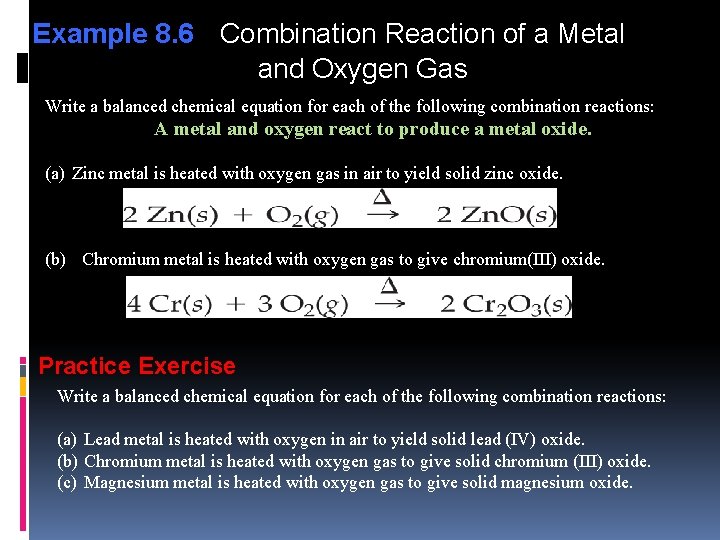
Example 8. 6 Combination Reaction of a Metal and Oxygen Gas Write a balanced chemical equation for each of the following combination reactions: A metal and oxygen react to produce a metal oxide. (a) Zinc metal is heated with oxygen gas in air to yield solid zinc oxide. (b) Chromium metal is heated with oxygen gas to give chromium(III) oxide. Practice Exercise Write a balanced chemical equation for each of the following combination reactions: (a) Lead metal is heated with oxygen in air to yield solid lead (IV) oxide. (b) Chromium metal is heated with oxygen gas to give solid chromium (III) oxide. (c) Magnesium metal is heated with oxygen gas to give solid magnesium oxide.

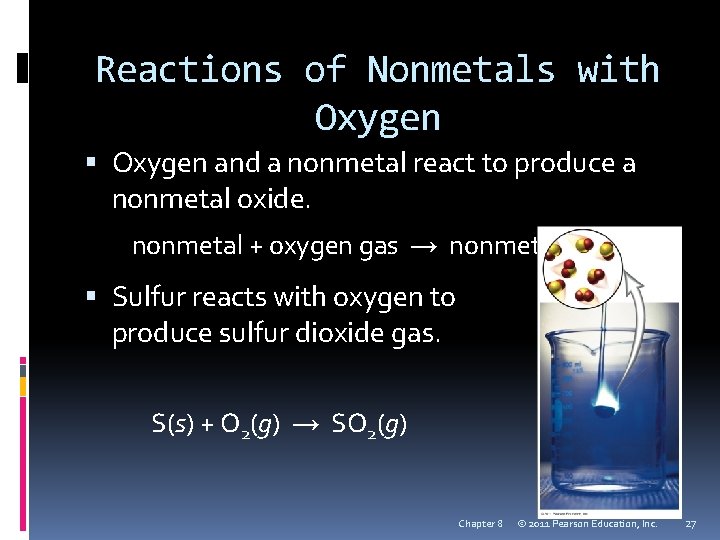
Reactions of Nonmetals with Oxygen and a nonmetal react to produce a nonmetal oxide. nonmetal + oxygen gas → nonmetal oxide Sulfur reacts with oxygen to produce sulfur dioxide gas. S(s) + O 2(g) → SO 2(g) Chapter 8 © 2011 Pearson Education, Inc. 27

Example 8. 7 Combination Reaction of a Nonmetal and Oxygen Gas Write a balanced chemical equation for each of the following combination reactions: A nonmetal and oxygen combine to produce a nonmetal oxide. (a) Carbon is heated with oxygen gas to produce carbon dioxide gas. (b) Phosphorus and oxygen gas react to give solid diphosphorus pentaoxide. 4 P(s) + 5 O 2(g) → 2 P 2 O 5(s) Practice Exercise Write a balanced chemical equation for each of the following combination reactions: (a) Nitrogen gas is heated with oxygen to give dinitrogen trioxide gas. (b) Bromine gas is heated with oxygen to give dibromine monoxide gas. (c) Carbon solid is heated with oxygen to give carbon monoxide gas

Metal + Nonmetal Reactions A metal and a nonmetal react in a combination reaction to give an ionic compound. metal + nonmetal → ionic compound Sodium reacts with chlorine gas to produce sodium chloride. 2 Na(s) + Cl 2(g) → 2 Na. Cl(s) When a main group metal reacts with a nonmetal, the formula of the ionic compound is predictable. If the compound contains a transition metal, the formula is not predictable. Chapter 8 © 2011 Pearson Education, Inc. 29

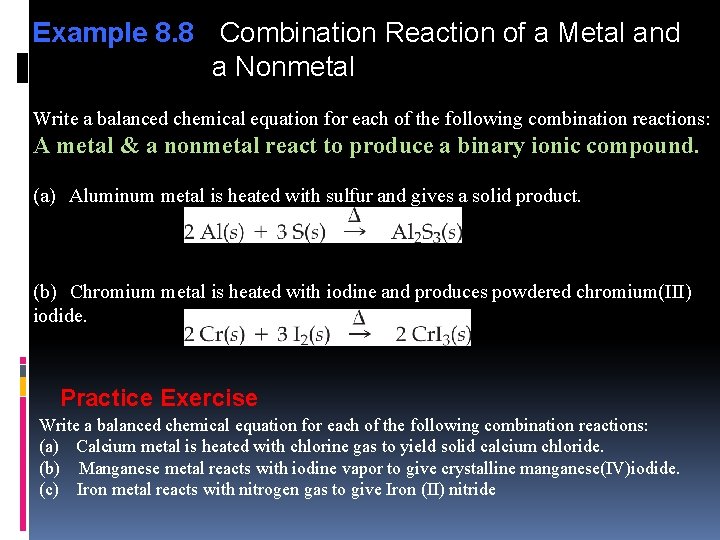
Example 8. 8 Combination Reaction of a Metal and a Nonmetal Write a balanced chemical equation for each of the following combination reactions: A metal & a nonmetal react to produce a binary ionic compound. (a) Aluminum metal is heated with sulfur and gives a solid product. (b) Chromium metal is heated with iodine and produces powdered chromium(III) iodide. Practice Exercise Write a balanced chemical equation for each of the following combination reactions: (a) Calcium metal is heated with chlorine gas to yield solid calcium chloride. (b) Manganese metal reacts with iodine vapor to give crystalline manganese(IV)iodide. (c) Iron metal reacts with nitrogen gas to give Iron (II) nitride

Decomposition Reactions In a decomposition reaction, a single compound is broken down into simpler substances. Heat or light is usually required to start a decomposition reaction. Ionic compounds containing oxygen often decompose into a metal and oxygen gas. For example, heating solid mercury(II) oxide produces mercury metal and oxygen gas. 2 Hg. O(s) → 2 Hg(l) + O 2(g) Chapter 8 © 2011 Pearson Education, Inc. 31

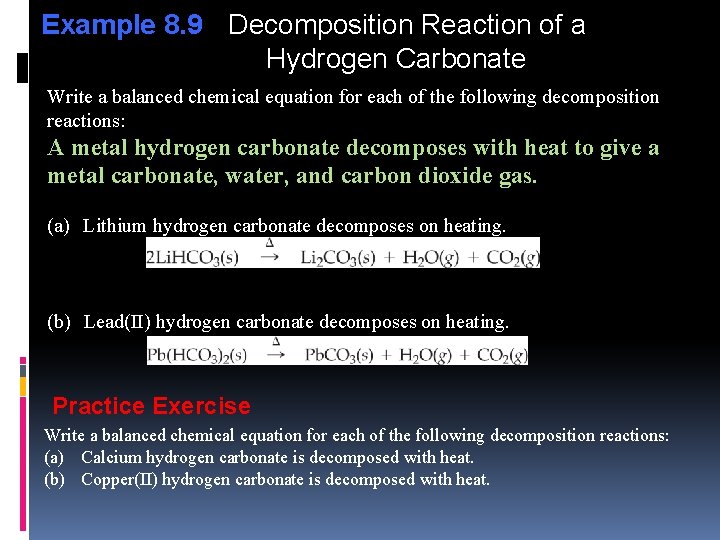
Example 8. 9 Decomposition Reaction of a Hydrogen Carbonate Write a balanced chemical equation for each of the following decomposition reactions: A metal hydrogen carbonate decomposes with heat to give a metal carbonate, water, and carbon dioxide gas. (a) Lithium hydrogen carbonate decomposes on heating. (b) Lead(II) hydrogen carbonate decomposes on heating. Practice Exercise Write a balanced chemical equation for each of the following decomposition reactions: (a) Calcium hydrogen carbonate is decomposed with heat. (b) Copper(II) hydrogen carbonate is decomposed with heat.

Carbonate Decompositions Metal hydrogen carbonates decompose to give a metal carbonate, water, and carbon dioxide. For example, nickel(II) hydrogen carbonate decomposes as follows: Ni(HCO 3)2(s) → Ni. CO 3(s) + H 2 O(l) + CO 2(g) Metal carbonates decompose to give a metal oxide and carbon dioxide gas. For example, calcium carbonate decomposes as follows: Ca. CO 3(s) → Ca. O(s) + CO 2(g) Chapter 8 © 2011 Pearson Education, Inc. 33

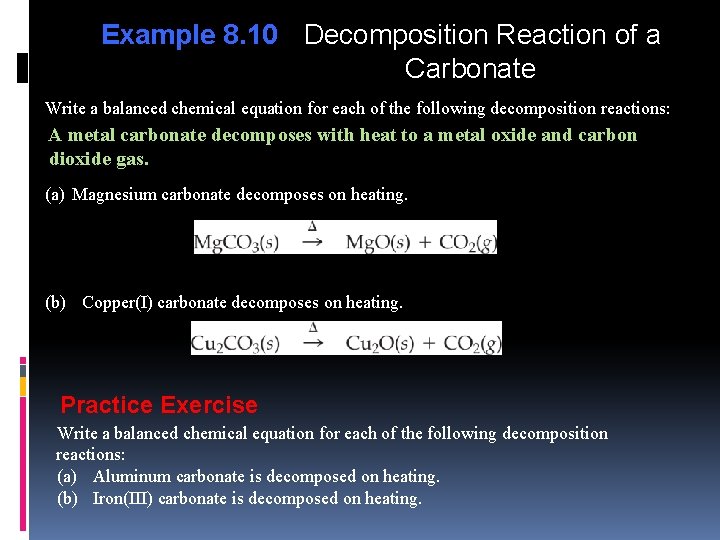
Example 8. 10 Decomposition Reaction of a Carbonate Write a balanced chemical equation for each of the following decomposition reactions: A metal carbonate decomposes with heat to a metal oxide and carbon dioxide gas. (a) Magnesium carbonate decomposes on heating. (b) Copper(I) carbonate decomposes on heating. Practice Exercise Write a balanced chemical equation for each of the following decomposition reactions: (a) Aluminum carbonate is decomposed on heating. (b) Iron(III) carbonate is decomposed on heating.

Example 8. 11 Decomposition Reaction Producing Oxygen Gas Write a balanced chemical equation for each of the following decomposition reactions: (a) Solid potassium chlorate decomposes upon heating to give solid potassium chloride and oxygen gas. (b) Solid sodium nitrate decomposes upon heating to give solid sodium nitrite and oxygen gas. Practice Exercise Write a balanced chemical equation for each of the following decomposition reactions: (a) Solid lead(IV) oxide decomposes upon heating to give solid lead(II) oxide and oxygen gas. (b) Aqueous hydrogen peroxide, H 2 O 2, decomposes upon heating to give water and oxygen gas.

Activity Series Concept When a metal undergoes a replacement reaction, it displaces another metal from a compound or aqueous solution. The metal that displaces the other metal does so because it is more active. The activity of a metal is a measure of its ability to compete in a replacement reaction. In an activity series, a sequence of metals is arranged according to its ability to undergo a reaction. Chapter 8 © 2011 Pearson Education, Inc. 36

Activity Series Metals that are most reactive appear first in the activity series. Metals that are least reactive appear last in the activity series. The relative activity series is: Li > K > Ba > Sr > Ca > Na > Mg > Al > Mn > Zn > Fe > Cd > Co > Ni > Sn > Pb > (H) > Cu > Ag > Hg > Au Chapter 8 © 2011 Pearson Education, Inc. 37

Active Metals A few metals are active enough to react directly with water. These are called active metals. The active metals are Li, Na, K, Rb, Cs, Ca, Sr, and Ba. They react with water to produce a metal hydroxide and hydrogen gas. 2 Na(s) + 2 H 2 O(l) → 2 Na. OH(aq) + H 2(g) Ca(s) + 2 H 2 O(l) → Ca(OH)2(aq) + H 2(g) Chapter 8 © 2011 Pearson Education, Inc. 38

Aqueous Acid Displacements Metals that precede (H) in the activity series react with acids, and those that follow (H) do not react with acids. More active metals react with acid to produce hydrogen gas and an ionic compound. Fe(s) + 2 HCl(aq) → Fe. Cl 2(aq) + H 2(g) Metals less active than (H) show no reaction. Au(s) + H 2 SO 4(aq) → NR Chapter 8 © 2011 Pearson Education, Inc. 39

Example 8. 12 Predictions Based on the Activity Series Predict whether or not a reaction occurs for each of the following: (a) Aluminum foil is added to an iron(II) sulfate solution. (b) Iron wire is added to an aluminum sulfate solution. (c) Manganese metal is added to acetic acid. (d) Magnesium metal is added to water. Solution We refer to the activity series for the reactivity of each of the metals. (a) Aluminum precedes iron in the activity series: Al > Fe. Thus, a reaction occurs, and Al displaces Fe from the solution. (b) Conversely, iron follows aluminum in the activity series: Al > Fe. Thus, there is no reaction. (c) Manganese precedes hydrogen in the activity series: Mn > (H). Thus, a reaction occurs, and Mn produces H 2 gas that bubbles from the solution. (d) Magnesium is not an active metal. Therefore, magnesium does not react with water, and there is no reaction.

Exercise 8. 12 Predictions Based on the Activity Series Practice Exercise Predict whether or not a reaction occurs for each of the following: (a) A zinc granule is dropped into hydrochloric acid. (b) A chromium strip is put into water. (c) A gold ring is dropped into sulfuric acid. (d) Iron wire is placed in copper sulfate. (e) A cadmium foil is put into a lead(II) nitrate solution. (f) Solid sodium is placed in water. Gold in Acid A gold ring gives no reaction in an aqueous sulfuric acid, H 2 SO 4, solution.

Single-Replacement Reactions A single-replacement reaction is a reaction in which a more active metal displaces another less active metal in a compound. If a metal precedes another in the activity series, it will undergo a single-replacement reaction. Fe(s) + Cu. SO 4(aq) → Fe. SO 4(aq) + Cu(s) Chapter 8 © 2011 Pearson Education, Inc. 42

Example 8. 13 Single-Replacement Reactions Write a balanced chemical equation for each of the following single-replacement reactions. (a) Nickel metal is placed in a tin(II) sulfate solution. (b) Cobalt metal is put in a cadmium nitrate solution. (c) Manganese chips are added to sulfuric acid. (d) A chunk of potassium is dropped into water. (a) Nickel is above tin in the activity series: Ni > Sn. Ni(s) + Sn. SO 4(aq) → Ni. SO 4(aq) + Sn(s) (b) Cobalt is below cadmium in the series: Cd > Co. Co(s) + Cd(NO 3)2(aq) → NR (c) Manganese is above hydrogen in the series: Mn > (H). Mn(s) + H 2 SO 4(aq) → Mn. SO 4(aq) + H 2(g) (d) Potassium is a Group IA/1 active metal. Therefore, a reaction occurs, and K releases H 2 gas from the solution. 2 K(s) + 2 H 2 O(l) → 2 KOH(aq) + H 2(g)

Exercise 8. 13 Single-Replacement Reactions Practice Exercise Write a balanced chemical equation for each of the following single-replacement reactions: (a) A small piece of strontium metal is dropped into water. (b) A chunk of cadmium metal is dropped into hydrochloric acid. (c) Gold metal is placed in a silver nitrate solution. (d) Nickel metal is placed in a silver nitrate solution. (e) Calcium metal is placed in water. (f) Copper wire is placed in silver nitrate.

Solubility Rules Not all ionic compounds are soluble in water. We can use the solubility rules to predict if a compound will be soluble in water. Chapter 8 © 2011 Pearson Education, Inc. 45

Example 8. 14 Applying Solubility Rules State whether each of the following compounds is soluble or insoluble in water: (a) sodium sulfate, Na 2 SO 4 (b) aluminum nitrate, Al(NO 3)3 (c) barium sulfate, Ba. SO 4 (d) lead(II) chromate, Pb. Cr. O 4 (e) ammonium sulfide, (NH 4)2 S Solution We refer to the solubility rules in Table 8. 2. (a) Sodium sulfate contains the alkali metal ion Na+. According to Rule 1, Na 2 SO 4 is soluble. (b) Aluminum nitrate contains the nitrate ion NO 3–. According to Rule 3, Al(NO 3)3 is soluble. (c) Barium sulfate contains the sulfate ion SO 42–. According to the Rule 5 exception, Ba. SO 4 is insoluble. (d) Lead(II) chromate contains the chromate ion Cr. O 42–. According to Rule 7, Pb. Cr. O 4 is insoluble. (e) Ammonium sulfide contains the ammonium ion NH 4+. According to Rule 1, (NH 4)2 S is soluble.

Exercise 8. 14 Applying Solubility Rules Practice Exercise State whether each of the following compounds is soluble or insoluble in water: (a) calcium hydroxide, Ca(OH)2 (b) magnesium carbonate, Mg. CO 3 (c) mercury(II) bromide, Hg. Br 2 (d) lead(II) acetate, Pb(C 2 H 3 O 2)2 (e) zinc phosphate, Zn 3(PO 4)2

Double-Replacement Reactions In a double-replacement reaction, two ionic compounds in aqueous solution switch anions and produce two new compounds. AX + BZ → AZ + BX If either AZ or BX is an insoluble compound, a precipitate will appear and there is a chemical reaction. If no precipitate is formed, there is no reaction. Chapter 8 © 2011 Pearson Education, Inc. 48

Double-Replacement Reactions, Continued Aqueous barium chloride reacts with aqueous potassium chromate as follows: 2 Ba. Cl 2(aq) + K 2 Cr. O 4(aq) → Ba. Cr. O 4(s) + 2 KCl(aq) From the solubility rules, Ba. Cr. O 4 is insoluble, so there is a double-replacement reaction. Aqueous sodium chloride reacts with aqueous lithium nitrate as follows: Na. Cl(aq) + Li. NO 3(aq) → Na. NO 3(aq) + Li. Cl(aq) Both Na. NO 3 and Li. Cl are soluble, so there is no reaction. Chapter 8 © 2011 Pearson Education, Inc. 49

Example 8. 15 Double-Replacement Reactions Write a balanced chemical equation for each of the following double-replacement reactions. (a) Aqueous barium chloride is added to a potassium chromate solution. (b) Aqueous strontium acetate is added to a lithium hydroxide solution. Solution For double-replacement reactions, we switch anions for the two compounds and check the solubility rules for an insoluble compound (a) Barium chloride and potassium chromate give barium chromate and potassium chloride. According to solubility Rule 7, we find that barium chromate is insoluble. The balanced equation is: Ba. Cl 2(aq) + K 2 Cr. O 2(aq) → Ba. Cr. O 4(s) + 2 KCl(aq) (b) Strontium acetate and lithium hydroxide react as follows: Sr(C 2 H 3 O 2)2(aq) + 2 Li. OH(aq) → Sr(OH)2(aq) + 2 Li. C 2 H 3 O 2(aq) However, the solubility rules indicate that Sr(OH)2 and Li. C 2 H 3 O 2 are soluble. Therefore, the equation is written Sr(C 2 H 3 O 2)2(aq) + Li. OH(aq) → NR

Exercise 8. 15 Double-Replacement Reactions Practice Exercise Write a balanced chemical equation for each of the following doublereplacement reactions: (a)Aqueous zinc sulfate is added to a sodium carbonate solution. (b) Aqueous manganese(II) nitrate is added to a potassium hydroxide solution. (c) Aqueous silver nitrate is added to aqueous sodium carbonate. (d) Aqueous sodium chloride is added to aqueous silver nitrate.

Neutralization Reactions A neutralization reaction is the reaction of an acid and a base. HX + BOH → BX + HOH A neutralization reaction produces a salt and water. H 2 SO 4(aq) + 2 KOH(aq) → K 2 SO 4(aq) + 2 H 2 O(l) Chapter 8 © 2011 Pearson Education, Inc. 52

Example 8. 16 Neutralization Reactions Write a balanced chemical equation for each of the following neutralization reactions: (a) Nitric acid neutralizes an ammonium hydroxide solution. (b) Sulfuric acid neutralizes a potassium hydroxide solution. Solution A neutralization reaction produces a salt and water. (a) Nitric acid and ammonium hydroxide produce ammonium nitrate and water. HNO 3(aq) + NH 4 OH(aq) → NH 4 NO 3(aq) + HOH(l) (b) Sulfuric acid and potassium hydroxide produce potassium sulfate and water. H 2 SO 4(aq) + 2 KOH(aq) → K 2 SO 4(aq) + 2 HOH(l) Practice Exercise Write a balanced chemical equation for each of the following neutralization reactions: (a) Chloric acid neutralizes a strontium hydroxide solution. (b) Phosphoric acid neutralizes a sodium hydroxide solution.

Critical Thinking: Household Chemicals Many common household items contain familiar chemicals Vinegar is a solution of acetic acid. Drain and oven cleaners contain sodium hydroxide. Car batteries contain sulfuric acid. Chapter 8 © 2011 Pearson Education, Inc. 54

Chapter Summary There are four ways to tell if a chemical reaction has occurred: 1. A gas is detected. 2. A precipitate is formed. 3. A permanent color change is seen. 4. Heat or light is given off. An exothermic reaction gives off heat and an endothermic reaction absorbs heat. Chapter 8 © 2011 Pearson Education, Inc. 55

Chapter Summary, Continued There are seven elements that exist as diatomic molecules: 1. H 2 2. N 2 3. O 2 4. F 2 5. Cl 2 6. Br 2 7. I 2 Chapter 8 © 2011 Pearson Education, Inc. 56

Chapter Summary, Continued When we balance a chemical equation, the number of each type of atom must be the same on both the product and reactant sides of the equation. We use coefficients in front of compounds to balance chemical reactions. Chapter 8 © 2011 Pearson Education, Inc. 57

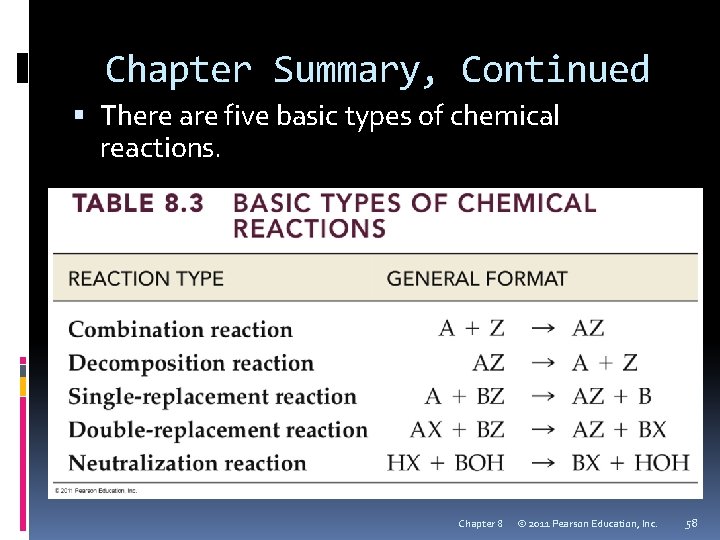
Chapter Summary, Continued There are five basic types of chemical reactions. Chapter 8 © 2011 Pearson Education, Inc. 58

Chapter Summary, Continued In combination reactions, two or more smaller molecules are combined into a more complex molecule. In a decomposition reaction, a molecule breaks apart into two or more simpler molecules. In a single-replacement reaction, a more active metal displaces a less active metal according to the activity series. Chapter 8 © 2011 Pearson Education, Inc. 59

Chapter Summary, Continued In a double-replacement reaction, two aqueous solutions produce a precipitate of an insoluble compound. The insoluble compound can be predicted based on the solubility rules. In a neutralization reaction, an acid and a base react to produce a salt and water. Chapter 8 © 2011 Pearson Education, Inc. 60

When the chemical equation below is properly balanced, what are the coefficients a, b, and c? a N 2(g) + b H 2(g) c NH 3(g) a. b. c. d. 1, 2, 3 1, 3, 2 2, 1, 3 3, 2, 1 © 2011 Pearson Education, Inc.

Which is classified as a combination reaction? a. b. c. d. Mg(s) + Cu(NO 3)2(aq) Mg(NO 3)2(aq) + Cu(s) Mg(OH)2(s) + H 2 SO 4(aq) 2 H 2 O(l) + Mg. SO 4(aq) 2 Mg. O(s) 2 Mg(s) + O 2(g) 3 Mg(s) + N 2(g) Mg 3 N 2(g) © 2011 Pearson Education, Inc.

A short version of the activity series is Ba > Zn > Pb > Cu. Use the activity series to determine which one of the following will result in a single-replacement reaction. a. b. c. d. Cu metal is added to a barium chloride solution. Lead metal is added to a zinc nitrate solution. Barium metal is added to a lead(II) acetate solution. Zinc metal is added to a barium nitrate solution. © 2011 Pearson Education, Inc.

Which is insoluble in water? a. b. c. d. Ag. Cl Na. Cl Ca. Cl 2 Cu. Cl 2 © 2011 Pearson Education, Inc.

Which is the balanced chemical equation for the neutralization reaction in which hydrobromic acid neutralizes a calcium hydroxide solution? a. b. c. d. HBr(aq) + Ca. OH(aq) H 2 O(l) + Ca. Br(aq) HBr(aq) + Ca(OH)2(aq) H 2 O(l) + Ca. Br 2(aq) 2 HBr(aq) + Ca(OH)2(aq) 2 H 2 O(l) + Ca. Br 2(aq) 2 HBr. O 3(aq) + Ca(OH)2(aq) 2 H 2 O(l) + Ca(Br. O 3)2(aq) © 2011 Pearson Education, Inc.
 Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Are kc and kp equal
Are kc and kp equal Types of reactions
Types of reactions Chemical blanking process
Chemical blanking process Section 1 chemical changes
Section 1 chemical changes Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chm 130 chapter 12 practice problems answer key
Chm 130 chapter 12 practice problems answer key Chapter 9 chemical reactions
Chapter 9 chemical reactions Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 11 chemical reactions answer key
Chapter 11 chemical reactions answer key Predict the products of the following reactions.
Predict the products of the following reactions. Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key Examples of double replacement reactions
Examples of double replacement reactions What is an active metal
What is an active metal Redox examples
Redox examples Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Chemistry 151 final exam
Chemistry 151 final exam Chemistry
Chemistry Chm 241
Chm 241 Chm 201
Chm 201 Chm 1045
Chm 1045 Chm 111
Chm 111 Contoh mesin non konvensional
Contoh mesin non konvensional Chmportal
Chmportal Chm logo
Chm logo Empirical formula pogil
Empirical formula pogil 7-3 practice problems chemistry answers
7-3 practice problems chemistry answers Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Different types of redox reactions
Different types of redox reactions How to identify types of chemical reactions
How to identify types of chemical reactions Types of reactions chemistry
Types of reactions chemistry What are the 5 types of chemical reactions
What are the 5 types of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Principles of immunochemical reaction
Principles of immunochemical reaction Predicting products of chemical reactions
Predicting products of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions Activity series of metals
Activity series of metals Unit 11 chemical reactions
Unit 11 chemical reactions Unit 4: toxins lesson 73 worksheet answers
Unit 4: toxins lesson 73 worksheet answers Four types of chemical reactions
Four types of chemical reactions What is the role of enzymes in chemical reactions
What is the role of enzymes in chemical reactions Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Chemical reactions in everyday life
Chemical reactions in everyday life What are five chemical changes
What are five chemical changes Chemical reactions reactants and products
Chemical reactions reactants and products 5 general types of chemical reactions
5 general types of chemical reactions Chemical reactions study guide
Chemical reactions study guide Rate constant and equilibrium constant
Rate constant and equilibrium constant What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions 5 chemical reactions
5 chemical reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Site:slidetodoc.com
Site:slidetodoc.com Chemical reactions summary
Chemical reactions summary Chemical reaction of bread
Chemical reaction of bread Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry