CHAPTER 5 THE GASEOUS STATE Vanessa PrasadPermaul Valencia



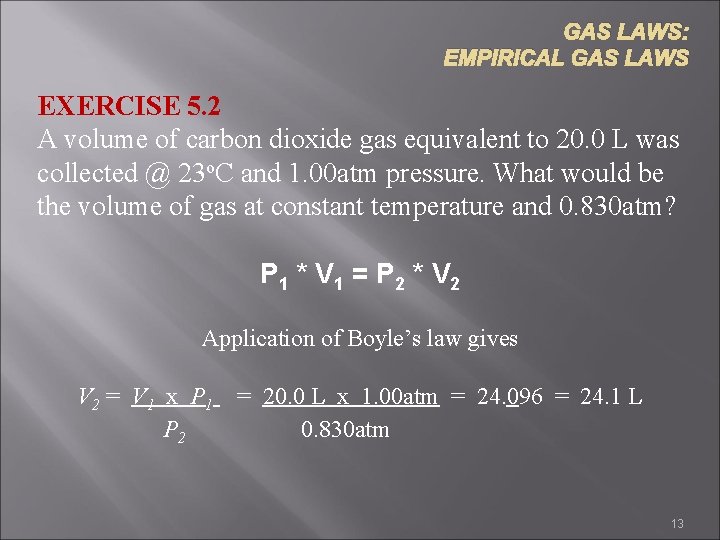








































- Slides: 73

CHAPTER 5: THE GASEOUS STATE Vanessa Prasad-Permaul Valencia Community College CHM 1045 1

GAS LAWS: PRESSURE AND MEASUREMENT Elements that exist as gases at 250 C and 1 atmosphere 2

GAS LAWS: PRESSURE AND MEASUREMENT Physical Characteristics of Gases • • Gases assume the volume and shape of their containers. Gases are the most compressible state of matter. Gases will mix evenly and completely when confined to the same container. Gases have much lower densities than liquids and solids. 3

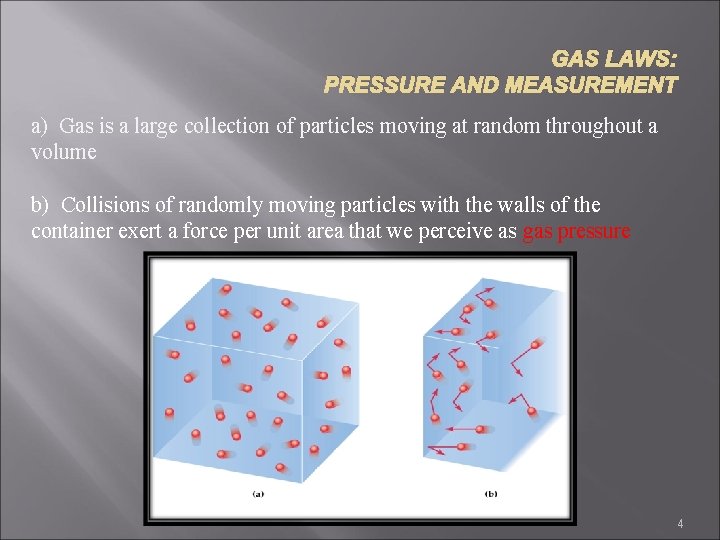
GAS LAWS: PRESSURE AND MEASUREMENT a) Gas is a large collection of particles moving at random throughout a volume b) Collisions of randomly moving particles with the walls of the container exert a force per unit area that we perceive as gas pressure 4

GAS LAWS: PRESSURE AND MEASUREMENT HOW IS PRESSURE DEFINED? The force the gas exerts on a given area of the container in which it is contained. The SI unit for pressure is the Pascal, Pa. Pressure = Force Area • If you’ve ever inflated a tire, you’ve probably made a pressure measurement in pounds (force) per square inch (area). 5

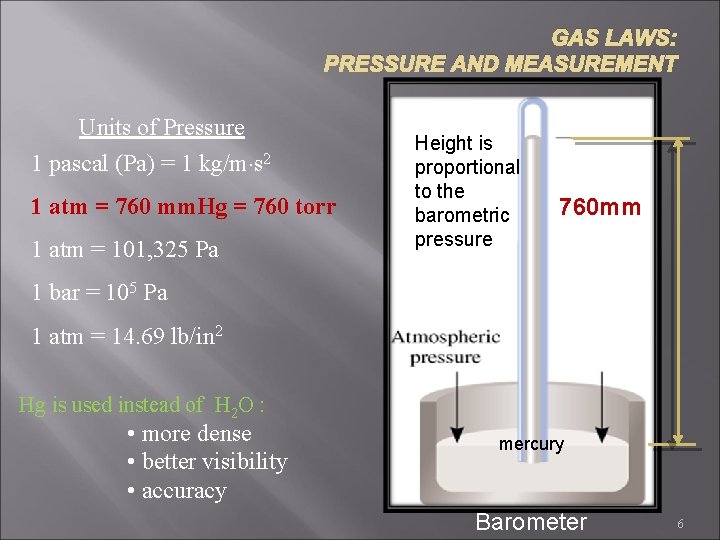
GAS LAWS: PRESSURE AND MEASUREMENT Units of Pressure 1 pascal (Pa) = 1 kg/m·s 2 1 atm = 760 mm. Hg = 760 torr 1 atm = 101, 325 Pa Height is proportional to the barometric pressure 760 mm 1 bar = 105 Pa 1 atm = 14. 69 lb/in 2 Hg is used instead of H 2 O : • more dense • better visibility • accuracy mercury Barometer 6

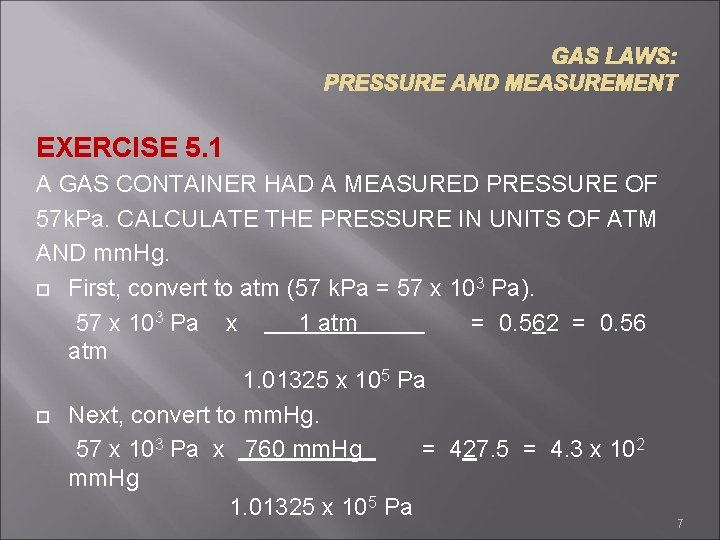
GAS LAWS: PRESSURE AND MEASUREMENT EXERCISE 5. 1 A GAS CONTAINER HAD A MEASURED PRESSURE OF 57 k. Pa. CALCULATE THE PRESSURE IN UNITS OF ATM AND mm. Hg. First, convert to atm (57 k. Pa = 57 x 103 Pa). 57 x 103 Pa x 1 atm = 0. 562 = 0. 56 atm 1. 01325 x 105 Pa Next, convert to mm. Hg. 57 x 103 Pa x 760 mm. Hg = 427. 5 = 4. 3 x 102 mm. Hg 1. 01325 x 105 Pa 7

GAS LAWS: EMPIRICAL GAS LAWS You can predict the behavior of gases based on the following properties: • Volume • Amount (moles) • Pressure • Temperature * If two of these physical properties are held constant, it is possible to show a simple relationship between the other two… 8

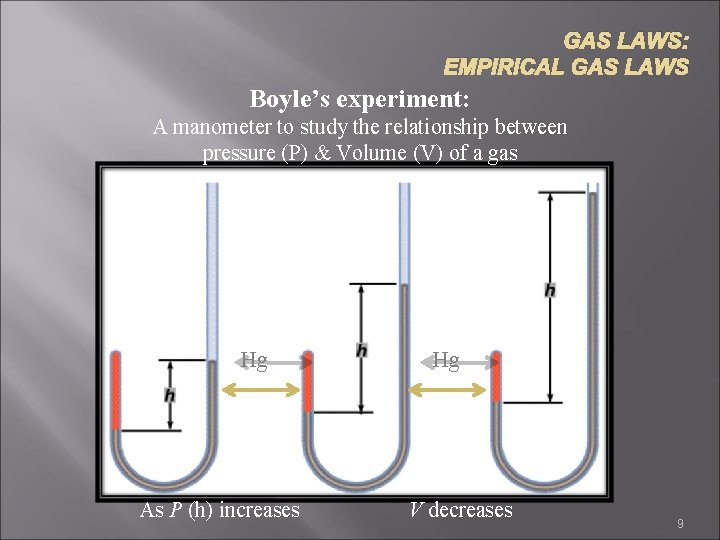
GAS LAWS: EMPIRICAL GAS LAWS Boyle’s experiment: A manometer to study the relationship between pressure (P) & Volume (V) of a gas Hg As P (h) increases Hg V decreases 9

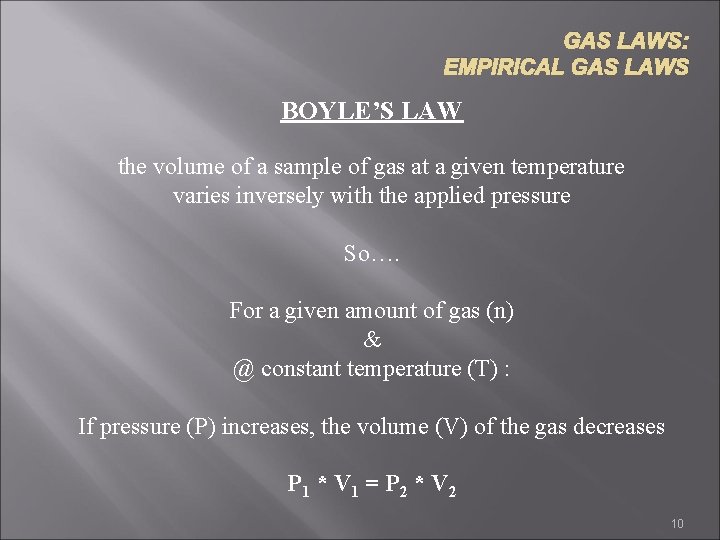
GAS LAWS: EMPIRICAL GAS LAWS BOYLE’S LAW the volume of a sample of gas at a given temperature varies inversely with the applied pressure So…. For a given amount of gas (n) & @ constant temperature (T) : If pressure (P) increases, the volume (V) of the gas decreases P 1 * V 1 = P 2 * V 2 10

Boyle’s Law • Pressure–Volume Law (Boyle’s Law): 11

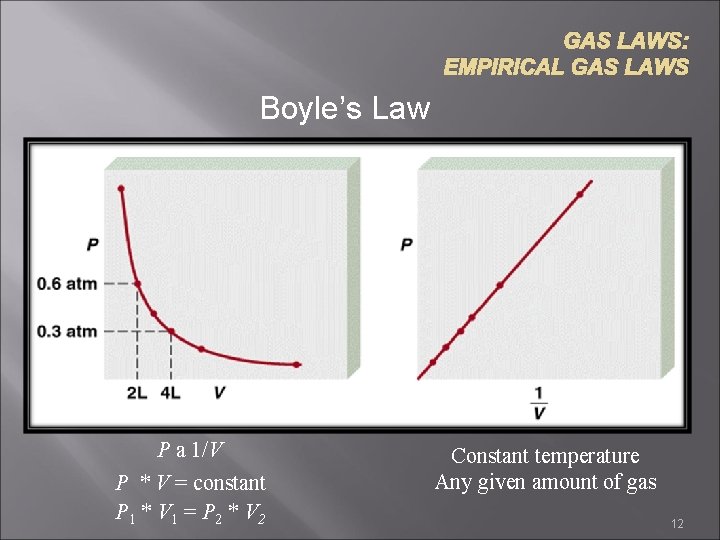
GAS LAWS: EMPIRICAL GAS LAWS Boyle’s Law P a 1/V P * V = constant P 1 * V 1 = P 2 * V 2 Constant temperature Any given amount of gas 12
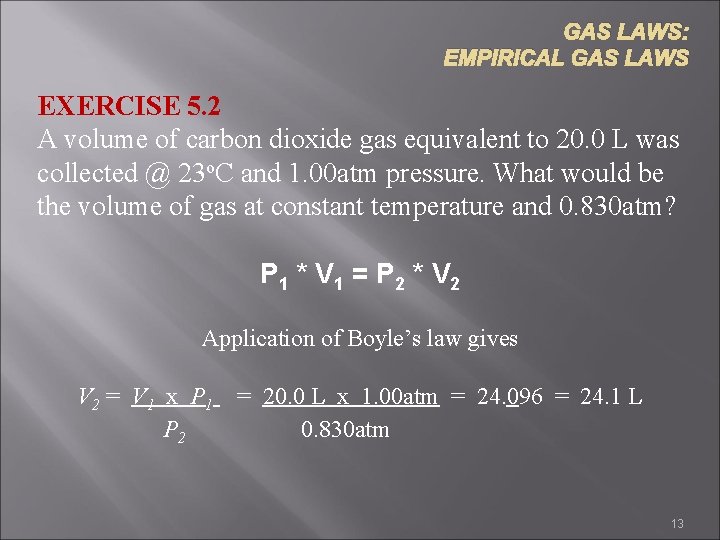
GAS LAWS: EMPIRICAL GAS LAWS EXERCISE 5. 2 A volume of carbon dioxide gas equivalent to 20. 0 L was collected @ 23 o. C and 1. 00 atm pressure. What would be the volume of gas at constant temperature and 0. 830 atm? P 1 * V 1 = P 2 * V 2 Application of Boyle’s law gives V 2 = V 1 x P 1 = 20. 0 L x 1. 00 atm = 24. 096 = 24. 1 L P 2 0. 830 atm 13

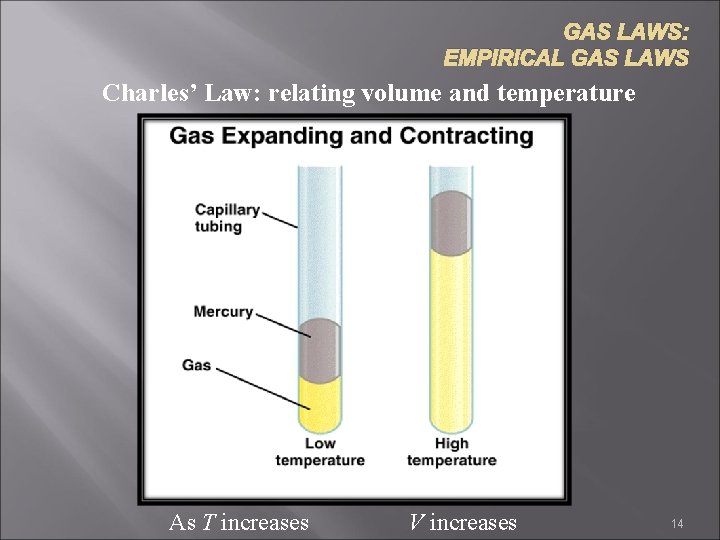
GAS LAWS: EMPIRICAL GAS LAWS Charles’ Law: relating volume and temperature As T increases V increases 14

Charles’ Law • Temperature–Volume Law (Charles’ Law): 15

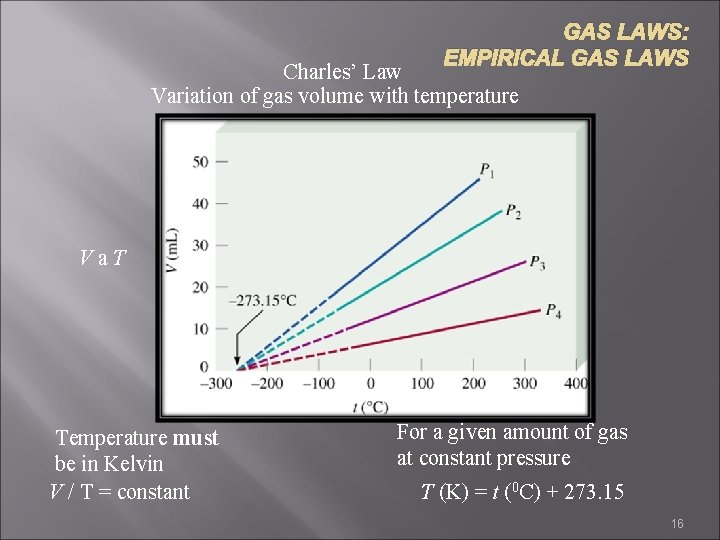
GAS LAWS: EMPIRICAL GAS LAWS Charles’ Law Variation of gas volume with temperature Va. T Temperature must be in Kelvin V / T = constant For a given amount of gas at constant pressure T (K) = t (0 C) + 273. 15 16

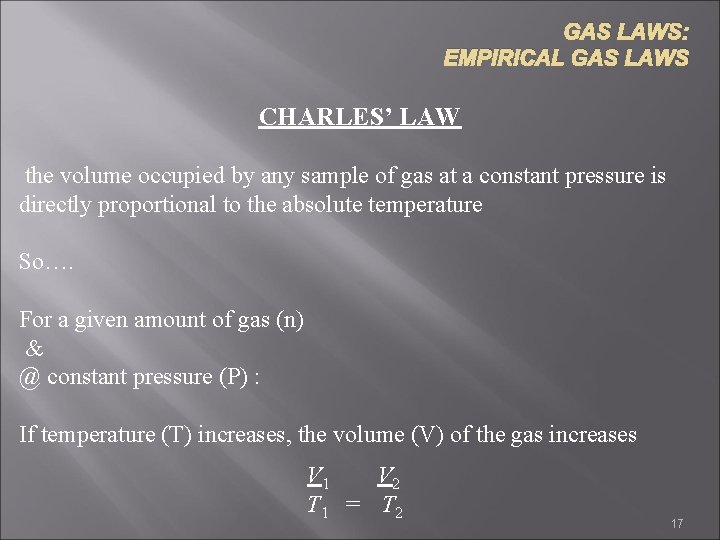
GAS LAWS: EMPIRICAL GAS LAWS CHARLES’ LAW the volume occupied by any sample of gas at a constant pressure is directly proportional to the absolute temperature So…. For a given amount of gas (n) & @ constant pressure (P) : If temperature (T) increases, the volume (V) of the gas increases V 1 V 2 T 1 = T 2 17

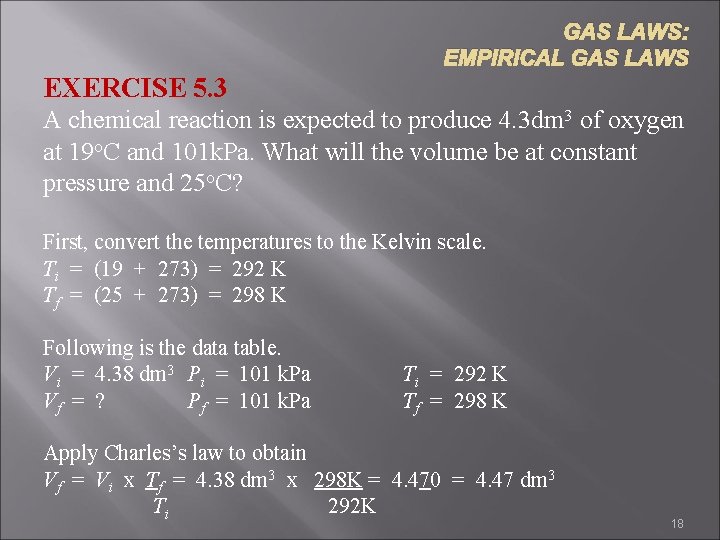
GAS LAWS: EMPIRICAL GAS LAWS EXERCISE 5. 3 A chemical reaction is expected to produce 4. 3 dm 3 of oxygen at 19 o. C and 101 k. Pa. What will the volume be at constant pressure and 25 o. C? First, convert the temperatures to the Kelvin scale. Ti = (19 + 273) = 292 K Tf = (25 + 273) = 298 K Following is the data table. Vi = 4. 38 dm 3 Pi = 101 k. Pa Vf = ? Pf = 101 k. Pa Ti = 292 K Tf = 298 K Apply Charles’s law to obtain Vf = Vi x Tf = 4. 38 dm 3 x 298 K = 4. 470 = 4. 47 dm 3 Ti 292 K 18

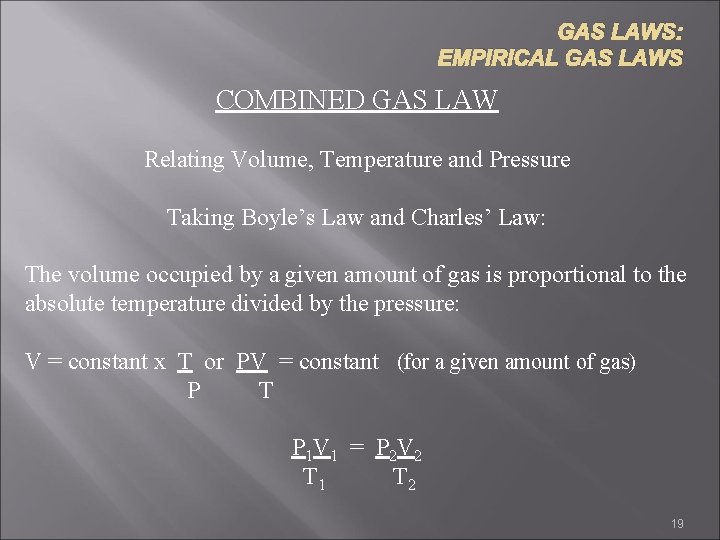
GAS LAWS: EMPIRICAL GAS LAWS COMBINED GAS LAW Relating Volume, Temperature and Pressure Taking Boyle’s Law and Charles’ Law: The volume occupied by a given amount of gas is proportional to the absolute temperature divided by the pressure: V = constant x T or PV = constant (for a given amount of gas) P T P 1 V 1 = P 2 V 2 T 1 T 2 19

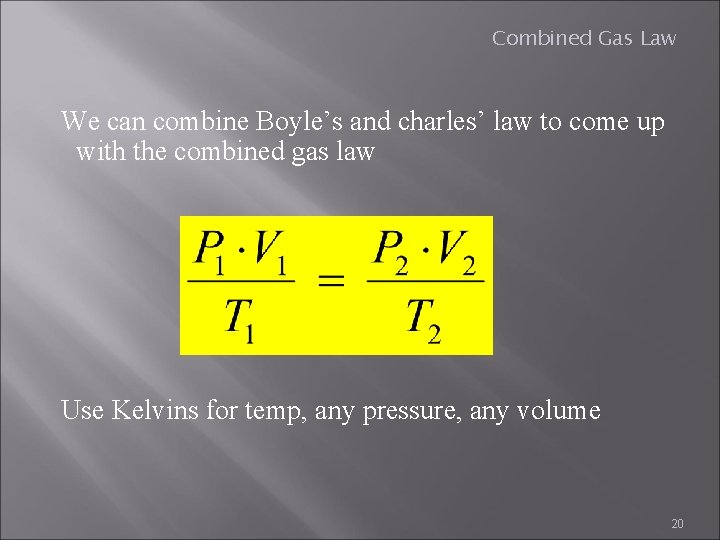
Combined Gas Law We can combine Boyle’s and charles’ law to come up with the combined gas law Use Kelvins for temp, any pressure, any volume 20

GAS LAWS: EMPIRICAL GAS LAWS EXERCISE 5. 4 A balloon contains 5. 41 dm 3 of helium at 24 o. C and 10. 5 k. Pa. Suppose the gas in the balloon is heated to 35 o. C and the pressure is now 102. 8 k. Pa, what is the volume of the gas? First, convert the temperatures to kelvins. Ti = (24 + 273) = 297 K Tf = (35 + 273) = 308 K Following is the data table. Vi = 5. 41 dm 3 Pi = 101. 5 k. Pa Vf = ? Pf = 102. 8 k. Pa Ti = 297 K Tf = 308 K Apply both Boyle’s law and Charles’s law combined to get Vf = Vi x Pi x Tf =5. 41 dm 3 x 101. 5 k. Pa x 308 K = 5. 539 = 5. 54 dm 3 P f Ti 102. 8 k. Pa 297 K 21

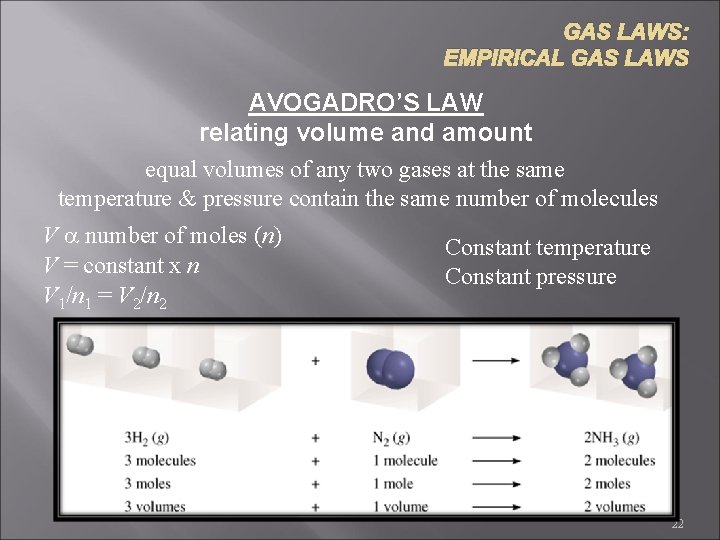
GAS LAWS: EMPIRICAL GAS LAWS AVOGADRO’S LAW relating volume and amount equal volumes of any two gases at the same temperature & pressure contain the same number of molecules V a number of moles (n) Constant temperature V = constant x n Constant pressure V 1/n 1 = V 2/n 2 22

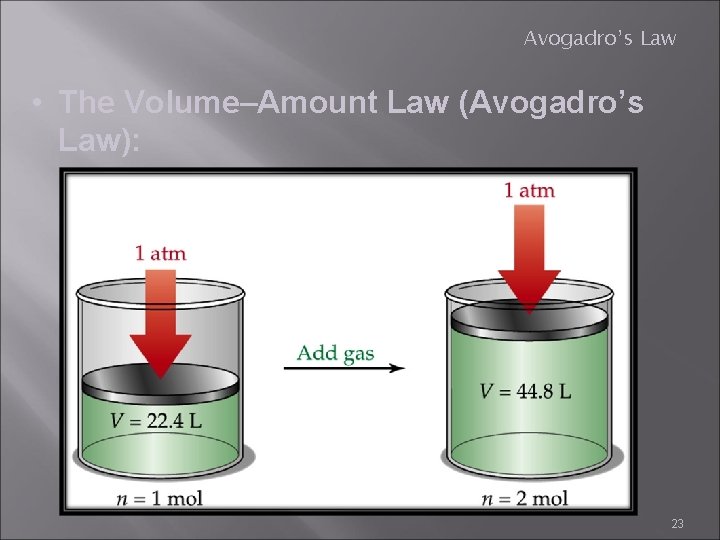
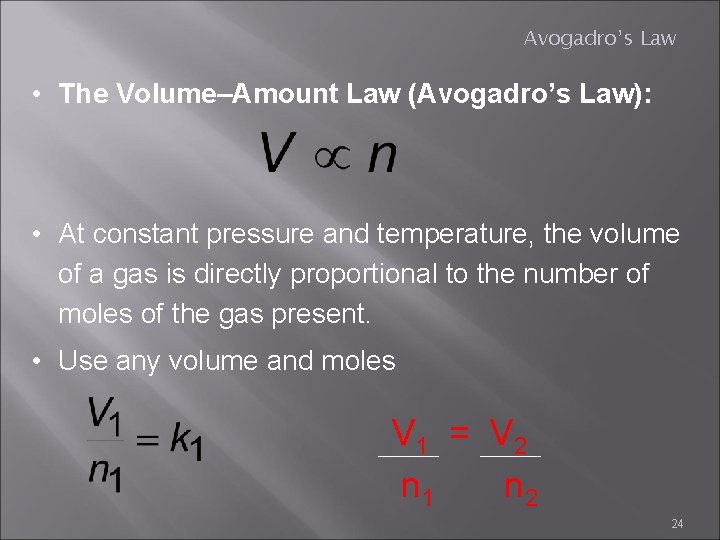
Avogadro’s Law • The Volume–Amount Law (Avogadro’s Law): 23

Avogadro’s Law • The Volume–Amount Law (Avogadro’s Law): • At constant pressure and temperature, the volume of a gas is directly proportional to the number of moles of the gas present. • Use any volume and moles V 1 = V 2 n 1 n 2 24

GAS LAWS: EMPIRICAL GAS LAWS Avogadro’s Number = one mole of any gas contains the same number of molecules 6. 023 x 1023 Must occupy the same volume at a given temperature and pressure The conditions 0 0 C and 1 atm are called standard temperature and pressure (STP). Experiments show that at STP, 1 mole of an ideal gas occupies 22. 4 L. 25

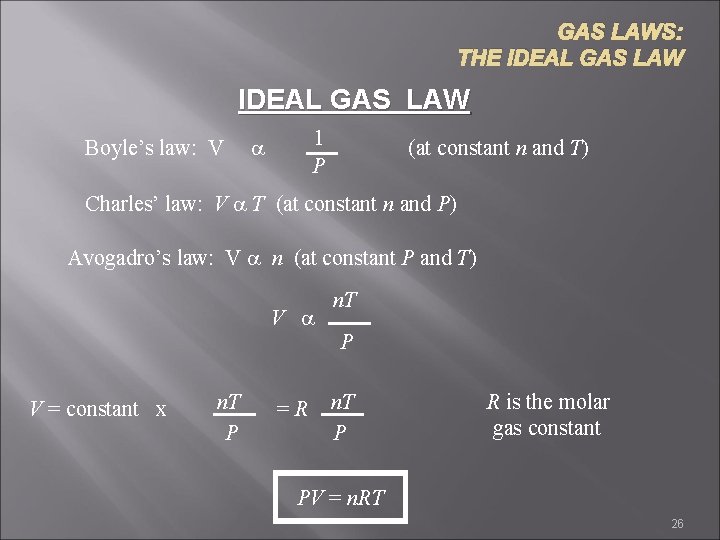
GAS LAWS: THE IDEAL GAS LAW Boyle’s law: V 1 P a (at constant n and T) Charles’ law: V a T (at constant n and P) Avogadro’s law: V a n (at constant P and T) V a V = constant x n. T P =R n. T P R is the molar gas constant PV = n. RT 26

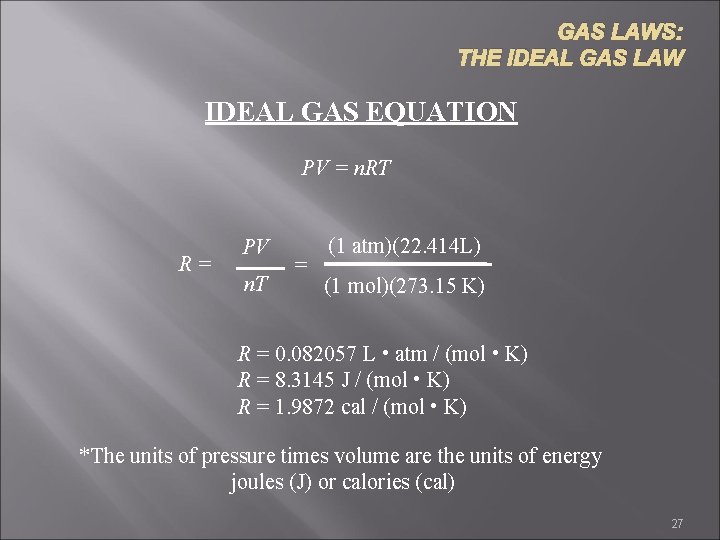
GAS LAWS: THE IDEAL GAS LAW IDEAL GAS EQUATION PV = n. RT R= PV n. T = (1 atm)(22. 414 L) (1 mol)(273. 15 K) R = 0. 082057 L • atm / (mol • K) R = 8. 3145 J / (mol • K) R = 1. 9872 cal / (mol • K) *The units of pressure times volume are the units of energy joules (J) or calories (cal) 27

The Ideal Gas Law Ideal gases obey an equation incorporating the laws of Charles, Boyle, and Avogadro. 1 mole of an ideal gas occupies 22. 414 L at STP conditions are 273. 15 K and 1 atm pressure The gas constant R = 0. 08206 L·atm·K– 1·mol– 1 P has to be in atm V has to be in L T has to be in K 28

GAS LAWS: THE IDEAL GAS LAW EXERCISE 5. 5 Show that the moles of gas are proportional to the pressure for constant volume and temperature Use the ideal gas law, PV = n. RT, and solve for n: n = PV RT n = V x P RT Note that everything in parentheses is constant. Therefore, you can write n = constant x P Or, expressing this as a proportion, n P 29

GAS LAWS: THE IDEAL GAS LAW EXERCISE 5. 6 What is the pressure in a 50. 0 L gas cylinder that contains 3. 03 kg of oxygen at 23 o. C? T = 23 o. C + 273 K = 296 K V = 50. 0 L R = 0. 08206 L. atm/K. mol n = 3. 03 kg x 1000 g x 1 mol = 94. 688 mol O 2 1 kg 31. 998 g P=? PV= n. RT P = n. RT = 0. 0347 mol x 0. 08206 L. atm x 296 K V K. mol = 46. 0 atm 50. 0 L 30

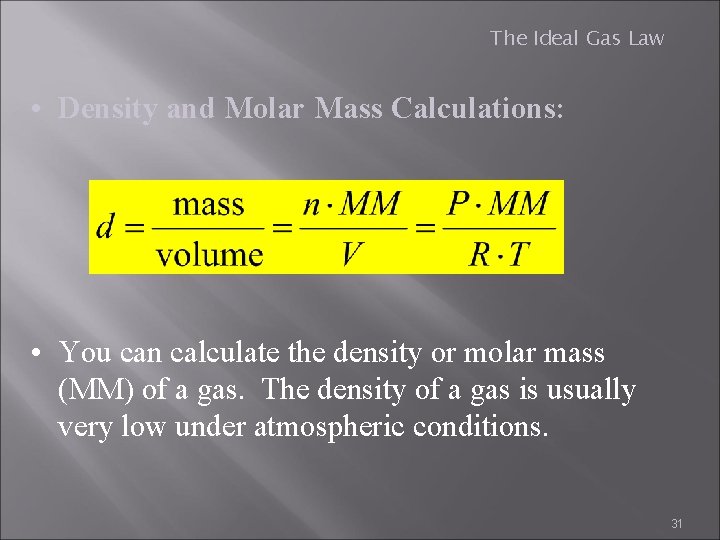
The Ideal Gas Law • Density and Molar Mass Calculations: • You can calculate the density or molar mass (MM) of a gas. The density of a gas is usually very low under atmospheric conditions. 31

GAS LAWS: THE IDEAL GAS LAW EXERCISE 5. 7 Calculate the density of helium (g/L) at 21 o. C and 752 mm. Hg. The density of air under these conditions is 1. 188 g/L. What is the difference in mass between 1 liter of air and 1 liter of helium? Variable P V T n Value 752 mm. Hg x 1 atm = 0. 98947 atm 760 mm. Hg 1 L (exact number) (21 + 273) = 294 K ? 32

GAS LAWS: THE IDEAL GAS LAW Using the ideal gas law, solve for n, the moles of helium. n = PV = 0. 98947 atm x 1 L RT 0. 08206 L. atm/ K. mol x 294 K Now convert mol He to grams. 0. 04101 mol He x 4. 00 g He 1. 00 mol He 1 L = 0. 4101 mol = 0. 16404 g He = 0. 164 g/L Therefore, the density of He at 21°C and 752 mm. Hg is 0. 164 g/L. The difference in mass between one liter of air and one liter of He: mass air − mass He = 1. 188 g − 0. 16404 g = 1. 02396 = 1. 024 g difference 33

GAS LAWS: THE IDEAL GAS LAW EXERCISE 5. 8 A sample of a gaseous substance at 25 o. C and 0. 862 atm has a density of 2. 26 g/L. What is the molecular mass of the substance? Variable P V T n Value 0. 862 atm 1 L (exact number) (25 + 273) = 298 K ? From the ideal gas law, PV = n. RT, you obtain n = PV = 0. 862 atm x 1 L RT 0. 08206 L. atm/K. Mol x 298 K = 0. 03525 mol 34

GAS LAWS: THE IDEAL GAS LAW Dividing the mass of the gas by moles gives you the mass per mole (the molar mass). Molar mass = grams of gas = moles of gas 2. 26 g 0. 03525 mol = 64. 114 g/mol Therefore, the molecular mass is 64. 1 amu. 35

GAS LAWS: GAS MIXTURES Dalton’s Law of Partial Pressures V and T are constant P 1 P 2 Ptotal = P 1 + P 2 36

Dalton’s Law of Partial Pressures • For a two-component system, the moles of components A and B can be represented by the mole fractions (XA and XB). Mole fraction is related to the total pressure by: 37

GAS LAWS: GAS MIXTURES EXERCISE 5. 10 A 10. 0 L flask contains 1. 031 g O 2 and 0. 572 g CO 2 at 18 o. C. What are the partial pressures of each gas? What is the total pressure? What is the mole fraction of oxygen in this mixture? Each gas obeys the ideal gas law. 1. 031 g O 2 x 1 mol = 0. 0322188 mol O 2 32. 00 g P = n. RT = 0. 0322 mol x 0. 0821 L. atm/K. mol x 291 K = 0. 076936 atm V 10. 0 L 0. 572 g CO 2 x 1 mol = 0. 012997 mol CO 2 44. 01 g P = n. RT = 0. 0130 mol x 0. 0821 L. atm/K. mol x 291 K = 0. 031036 atm V 10. 0 L 38

GAS LAWS: GAS MIXTURES The total pressure is equal to the sum of the partial pressures: PT = PO 2 + PCO 2 = 0. 076936 atm + 0. 031036 atm = 0. 10797 = 0. 1080 atm The mole fraction of oxygen in the mixture is Mole fraction O 2 = PO 2 = 0. 076936 atm = 0. 7122 = 0. 712 PT 0. 1080 atm 0. 712 x 100% = 71. 2 mole % of O 2 in this gas mixture 39

Kinetic Molecular Theory This theory presents physical properties of gases in terms of the motion of individual molecules. • Average Kinetic Energy Kelvin Temperature • Gas molecules are points separated by a great distance • Particle volume is negligible compared to gas volume • Gas molecules are in constant random motion • Gas collisions are perfectly elastic • Gas molecules experience no attraction or repulsion 40

Kinetic Molecular Theory 41

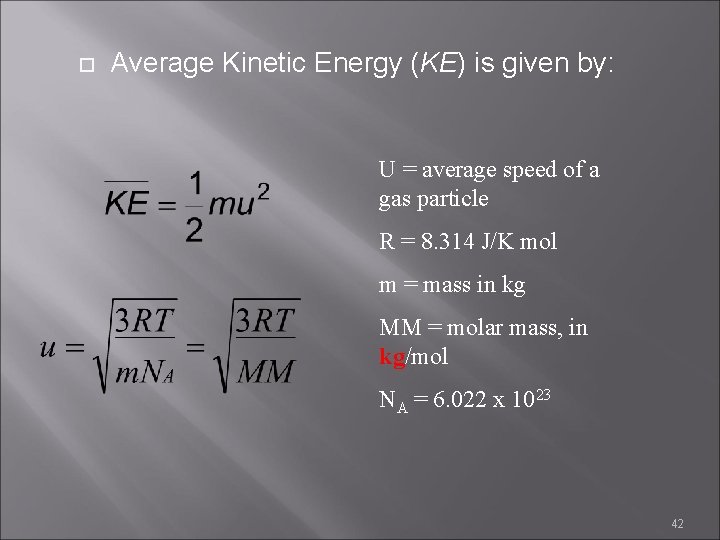
Average Kinetic Energy (KE) is given by: U = average speed of a gas particle R = 8. 314 J/K mol m = mass in kg MM = molar mass, in kg/mol NA = 6. 022 x 1023 42

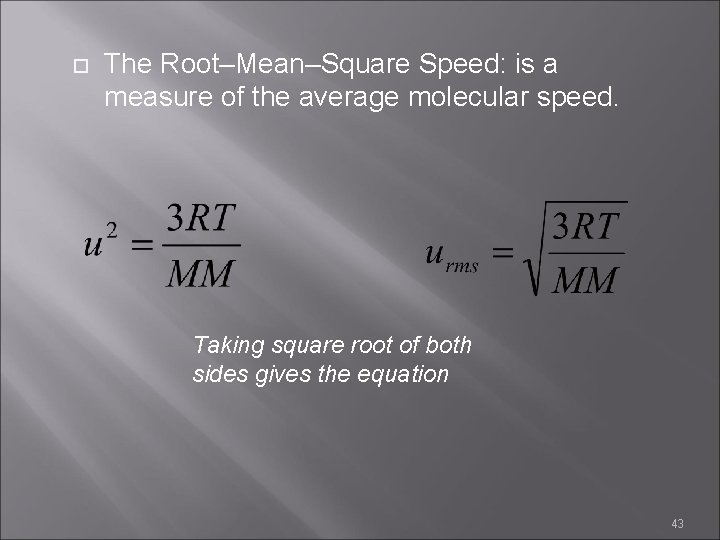
The Root–Mean–Square Speed: is a measure of the average molecular speed. Taking square root of both sides gives the equation 43

Example 17: Calculate the root–mean–square speeds of helium atoms and nitrogen molecules in m/s at 25°C. 44

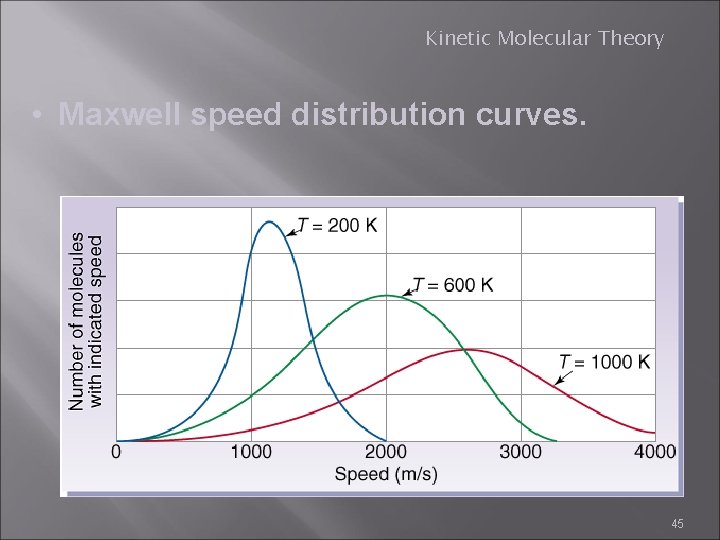
Kinetic Molecular Theory • Maxwell speed distribution curves. 45

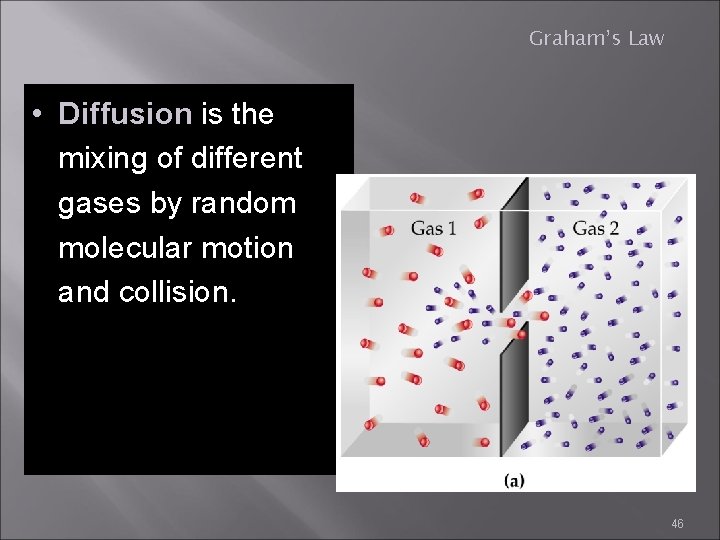
Graham’s Law • Diffusion is the mixing of different gases by random molecular motion and collision. 46

Graham’s Law • Effusion is when gas molecules escape without collision, through a tiny hole into a vacuum. 47

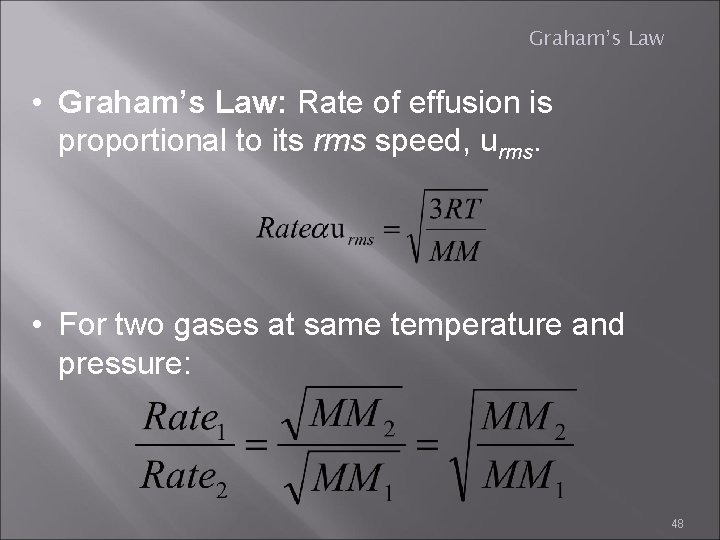
Graham’s Law • Graham’s Law: Rate of effusion is proportional to its rms speed, urms. • For two gases at same temperature and pressure: 48

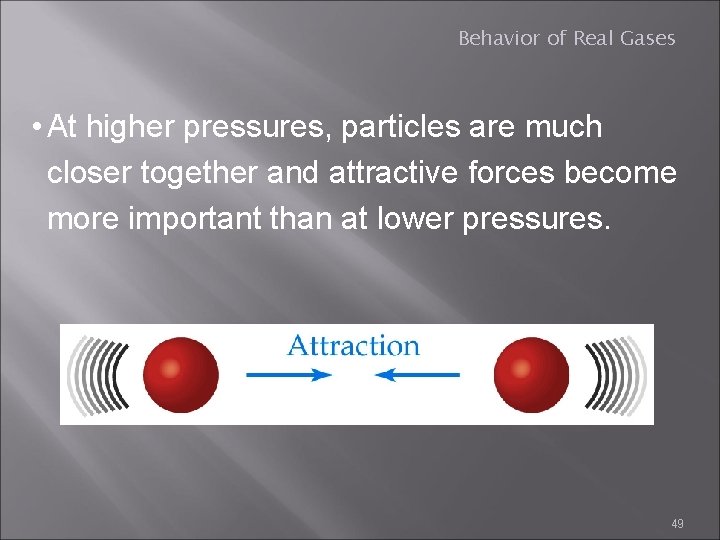
Behavior of Real Gases • At higher pressures, particles are much closer together and attractive forces become more important than at lower pressures. 49

Behavior of Real Gases • The volume taken up by gas particles is actually less important at lower pressures than at higher pressure. As a result, the volume at high pressure will be greater than the ideal value. 50

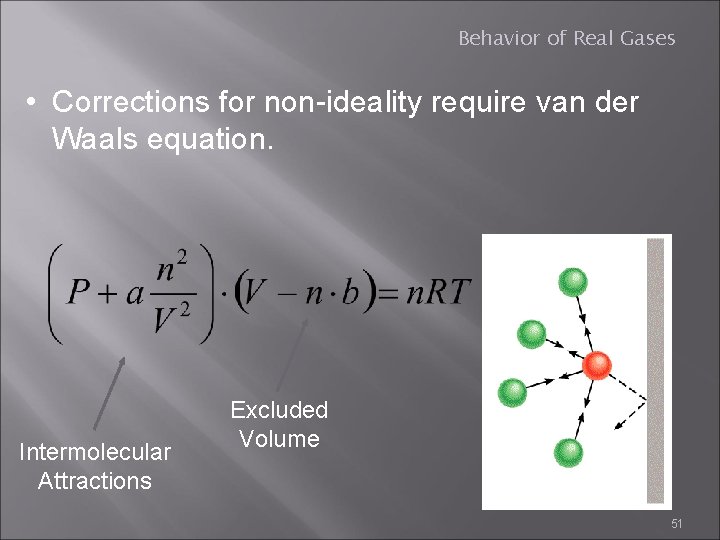
Behavior of Real Gases • Corrections for non-ideality require van der Waals equation. Intermolecular Attractions Excluded Volume 51

Example 1: Boyle’s Law A sample of argon gas has a volume of 14. 5 L at 1. 56 atm of pressure. What would the pressure be if the gas was compressed to 10. 5 L? (at constant temperature and moles of gas) 52

Example 2: Charles’ Law A sample of CO 2(g) at 35 C has a volume of 8. 56 x 10 -4 L. What would the resulting volume be if we increased the temperature to 85 C? (at constant moles and pressure) 53

Example 3: Avogadro’s Law 6. 53 moles of O 2(g) has a volume of 146 L. If we decreased the number of moles of oxygen to 3. 94 moles what would be the resulting volume? (constant pressure and temperature) 54

Example 4: Combined Gas Law Oxygen gas is normally sold in 49. 0 L steel containers at a pressure of 150. 0 atm. What volume would the gas occupy if the pressure was reduced to 1. 02 atm and the temperature raised from 20 o. C to 35 o. C? 55

Example 5: Gas Laws An inflated balloon with a volume of 0. 55 L at sea level, where the pressure is 1. 0 atm, is allowed to rise to a height of 6. 5 km, where the pressure is about 0. 40 atm. Assuming that the temperature remains constant, what is the final volume of the balloon? 56

Example 6: Ideal Gas Law Sulfur hexafluoride (SF 6) is a colorless, odorless, very unreactive gas. Calculate the pressure (in atm) exerted by 1. 82 moles of the gas in a steel vessel of volume 5. 43 L at 69. 5°C. 57

Example 7: Ideal Gas Law What is the volume (in liters) occupied by 7. 40 g of CO 2 at STP? 58

Example 8: Density & MM What is the molar mass of a gas with a density of 1. 342 g/L at STP? 59

Example 9: Density & MM What is the density of uranium hexafluoride, UF 6, (MM = 352 g/mol) under conditions of STP? 60

Example 10: Density & MM The density of a gaseous compound is 3. 38 g/L at 40°C and 1. 97 atm. What is its molar mass? 61

Example 11: Dalton Exactly 2. 0 moles of Ne and 3. 0 moles of Ar were placed in a 40. 0 L container at 25 o. C. What are the partial pressures of each gas and the total pressure? 62

Example 12: Dalton A sample of natural gas contains 6. 25 moles of methane (CH 4), 0. 500 moles of ethane (C 2 H 6), and 0. 100 moles of propane (C 3 H 8). If the total pressure of the gas is 1. 50 atm, what are the partial pressures of the gases? 63

Example 13: Mole Fraction What is the mole fraction of each component in a mixture of 12. 45 g of H 2, 60. 67 g of N 2, and 2. 38 g of NH 3? 64

Example 14: Partial Pressure On a humid day in summer, the mole fraction of gaseous H 2 O (water vapor) in the air at 25°C can be as high as 0. 0287. Assuming a total pressure of 0. 977 atm, what is the partial pressure (in atm) of H 2 O in the air? 65

Gas Stoichiometry & Example In gas stoichiometry, for a constant temperature and pressure, volume is proportional to moles. Example: Assuming no change in temperature and pressure, calculate the volume of O 2 (in liters) required for the complete combustion of 14. 9 L of butane (C 4 H 10): 2 C 4 H 10(g) + 13 O 2(g) 8 CO 2(g) + 10 H 2 O(l) 66

Example 15: All of the mole fractions of elements in a given compound must add up to? 1. 2. 3. 4. 100 1 50 2 67

Example 16: Hydrogen gas, H 2, can be prepared by letting zinc metal react with aqueous HCl. How many liters of H 2 can be prepared at 742 mm Hg and 15 o. C if 25. 5 g of zinc (MM = 65. 4 g/mol) was allowed to react? Zn(s) + 2 HCl(aq) H 2(g) + Zn. Cl 2(aq) 68

Example 18: Under the same conditions, an unknown gas diffuses 0. 644 times as fast as sulfur hexafluoride, SF 6 (MM = 146 g/mol). What is the identity of the unknown gas if it is also a hexafluoride? 69

Example 19: Diffusion What are the relative rates of diffusion of the three naturally occurring isotopes of neon: 20 Ne, 21 Ne, and 22 Ne? 70

Behavior of Real Gases • Deviations result from assumptions about ideal gases. 1. Molecules in gaseous state do not exert any force, either attractive or repulsive, on one another. 2. Volume of the molecules is negligibly small compared with that of the container. 71

Example 20: Ideal Vs. Van Der Waals Given that 3. 50 moles of NH 3 occupy 5. 20 L at 47°C, calculate the pressure of the gas (in atm) using (a) the ideal gas equation (b) the van der Waals equation. (a = 4. 17, b = 0. 0371) 72

Example 21: Ideal Vs. Van Der Waals Assume that you have 0. 500 mol of N 2 in a volume of 0. 600 L at 300 K. Calculate the pressure in atmospheres using both the ideal gas law and the van der Waals equation. For N 2, a = 1. 35 L 2·atm mol– 2, and b = 0. 0387 L/mol. 73
 Gaseous state chapter
Gaseous state chapter Solar system inner and outer planets
Solar system inner and outer planets Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Xeromorphic plants
Xeromorphic plants Inorganic gases
Inorganic gases Gas exchange in worms
Gas exchange in worms Granular porosity denture
Granular porosity denture Gaseous exchange in animals
Gaseous exchange in animals Gaseous dosage form
Gaseous dosage form Gaseous equilibrium
Gaseous equilibrium Gaseous envelope of the sun
Gaseous envelope of the sun Life science grade 11 gaseous exchange practical
Life science grade 11 gaseous exchange practical Pearson
Pearson Are solutions homogeneous
Are solutions homogeneous Khbo2
Khbo2 Collodion dosage form
Collodion dosage form At 500 k one mole of gaseous oncl
At 500 k one mole of gaseous oncl Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Cong thức tính động năng
Cong thức tính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Nguyên nhân của sự mỏi cơ sinh 8
Nguyên nhân của sự mỏi cơ sinh 8 đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thứ tự các dấu thăng giáng ở hóa biểu
Thứ tự các dấu thăng giáng ở hóa biểu Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te Thẻ vin
Thẻ vin đại từ thay thế
đại từ thay thế điện thế nghỉ
điện thế nghỉ Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Các loại đột biến cấu trúc nhiễm sắc thể
Các loại đột biến cấu trúc nhiễm sắc thể Các số nguyên tố
Các số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết Lời thề hippocrates
Lời thề hippocrates Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan ưu thế lai là gì
ưu thế lai là gì Sự nuôi và dạy con của hổ
Sự nuôi và dạy con của hổ Khi nào hổ con có thể sống độc lập
Khi nào hổ con có thể sống độc lập Hệ hô hấp
Hệ hô hấp Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Desguace valencia 2000
Desguace valencia 2000 Valencia dual enrollment courses
Valencia dual enrollment courses Intencie
Intencie Juan luis jaramillo valencia
Juan luis jaramillo valencia Rango de valor del enlace covalente puro
Rango de valor del enlace covalente puro Diodo
Diodo Bird poo
Bird poo Silicio electrones de valencia
Silicio electrones de valencia Octetos expandidos
Octetos expandidos Electrones de valencia de l
Electrones de valencia de l Juliana valencia montes
Juliana valencia montes Inheritance tax valencia region spain
Inheritance tax valencia region spain Capas de valencia de los elementos
Capas de valencia de los elementos Paraules amb diftong i hiat
Paraules amb diftong i hiat Varicolece
Varicolece