Atoms Molecules Name Period Antioch Upper Grade School













- Slides: 38

Atoms & Molecules Name__________ Period_____

Antioch Upper Grade School Science Safety Contract 2015 - 2016 PURPOSE Science is a hands-on laboratory class. However, science activities may have potential hazards. We will use some equipment and animals that may be dangerous if not handled properly. Safety in the science classroom is an important part of the scientific process. To ensure a safe classroom, a list of rules had been developed and is called the Science Safety Contract. These rules must be followed at all times. Additional safety instructions will be given for each activity. No science student will be allowed to participate in science activities until this contract has been signed. SAFETY RULES q Conduct yourself in a responsible manner at all times in the science room. Horseplay, practical jokes, and pranks will not be tolerated. q Follow all written and verbal instruction carefully. Ask your teacher questions if you do not understand the instructions. q Do not touch any equipment, supplies, animals, or other materials in the science room without permission from the teacher. q Perform only authorized and approved experiments. Do not conduct any experiments when the teacher is out of the room. q Never eat, drink, chew gum, or taste anything in the science room. q Keep hands away from face, eyes, and mouth while using science materials or when working with either chemicals or animals. Wash your hands with soap and water before leaving the science room. q Wear safety glasses or goggles when instructed. Never remove safety glasses or goggles during an experiment. There will be no exceptions to this rule. q Keep your work area and the science room neat and clean. Bring only your laboratory instruction, worksheets, and writing instruments to the work area. q Clean all work areas and equipment at the end of the experiment. Return all equipment clean and in working order to the proper storage area. q Follow your teacher’s instruction to dispose of any waste materials generated in an experiment. q Report any accident (fire, spill, breakage, etc. ) injury (cut, burn, etc. ), or hazardous condition (broken equipment, etc. ) to the teacher immediately. q Consider all chemicals used in the science room to be dangerous. Do not touch or smell any chemicals unless specifically instructed to do so. q Handle all animals with care and respect. q Always carry a microscope with both hands. Hold the arm with one hand; place the other hand under the base. q Never open storage cabinets or enter the prep/storage room without permission from the teacher. q Do not remove chemicals, equipment, supplies, or animals form the science room without permission from the teacher. q Handle all glassware with care. Never pick up hot or broken glassware with your bare hands. q Use extreme caution when using matches, a burner, or hot plate. Only light burners when instructed and do not put anything into a flame unless specifically instructed to do so. Do not leave a lit burner unattended. q Dress properly – long hair must be tied back, no dangling jewelry, and not loose or baggy clothing. Wear aprons when instructed. q Learn where the safety equipment is located and how to use it. Know here the exits are located and what to do in case of an emergency or fire drill. I, _______________, have read and understand each of the above safety rules set forth in this contract. I agree to follow them to ensure not only my own safety but also the safety of others in the classroom or laboratory. I also agree to follow the general rules of appropriate behavior for a classroom at all times to avoid accidents and to provide a safe learning environment for everyone. I understand that if I do not follow all the rules and safety precautions, I will not be allowed to participate in science activities. __________________________ ____________ Student Signature Date 2

Directions: Complete the following chart with information about atoms. You may use words, draw pictures or diagrams to convey the information. What do you THINK you KNOW about…. What do you WANT to know about…. What do you LEARN about…. 3

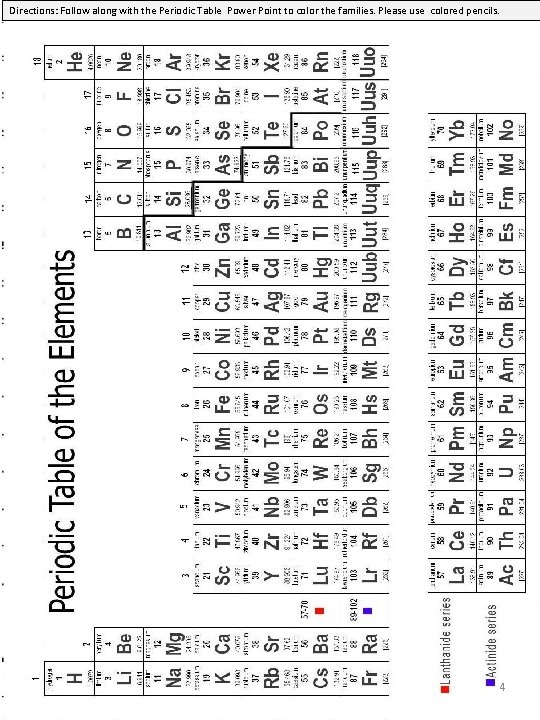
Directions: Follow along with the Periodic Table Power Point to color the families. Please use colored pencils. 4

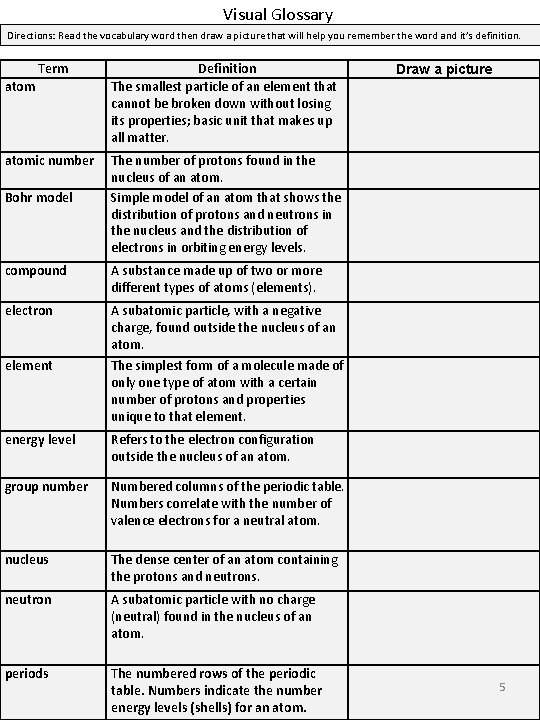
Visual Glossary Directions: Read the vocabulary word then draw a picture that will help you remember the word and it’s definition. Term Definition The smallest particle of an element that cannot be broken down without losing its properties; basic unit that makes up all matter. atomic number Bohr model The number of protons found in the nucleus of an atom. Simple model of an atom that shows the distribution of protons and neutrons in the nucleus and the distribution of electrons in orbiting energy levels. compound A substance made up of two or more different types of atoms (elements). electron A subatomic particle, with a negative charge, found outside the nucleus of an atom. element The simplest form of a molecule made of only one type of atom with a certain number of protons and properties unique to that element. energy level Refers to the electron configuration outside the nucleus of an atom. group number Numbered columns of the periodic table. Numbers correlate with the number of valence electrons for a neutral atom. nucleus The dense center of an atom containing the protons and neutrons. neutron A subatomic particle with no charge (neutral) found in the nucleus of an atom. periods The numbered rows of the periodic table. Numbers indicate the number energy levels (shells) for an atom Draw a picture 5

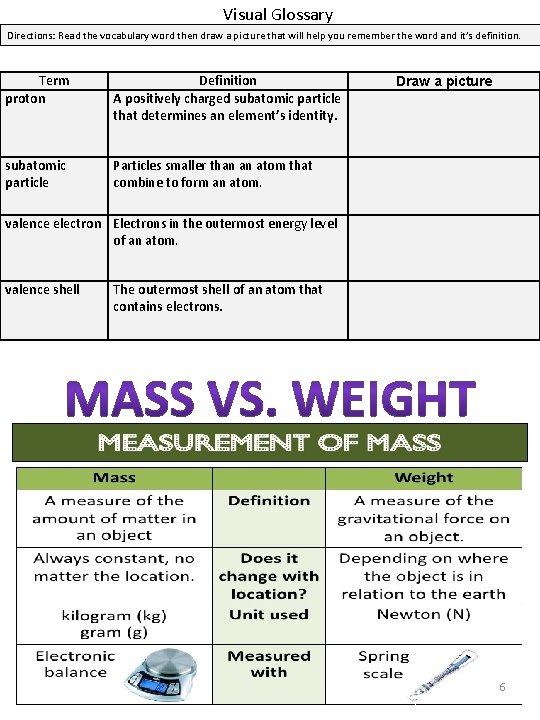
Visual Glossary Directions: Read the vocabulary word then draw a picture that will help you remember the word and it’s definition. Term proton Definition A positively charged subatomic particle that determines an element’s identity. subatomic particle Particles smaller than an atom that combine to form an atom. Draw a picture valence electron Electrons in the outermost energy level of an atom. valence shell The outermost shell of an atom that contains electrons. 6

Directions: Look at the Periodic Table of Elements and list the elements you encounter on a daily basis. Element name Use

WHAT IS MATTER ? ? ? Matter is the Stuff Around You Matter is everything around you. Atoms and molecules are the building blocks of matter. Matter is anything that has mass and volume (takes up space). Matter has properties that observed through our senses. What’s in the Bag Activity: In this activity, you will test your skills in determining an object’s Properties without looking directly at the object. Procedure: 1. 2. ? DO NOT OPEN THE BAG until the end ! The bag contains a mystery object that you are being asked to identify using all your senses. 3. For 5 minutes use make observations about the object in the bag. 4. You may touch, smell, shake and listen through the bag. 5. List your observations: 6. List what you cannot identify: 7. Make a conclusion about the object’s identity: 8. Share your observations and your conclusion with your shoulder partner and get their opinion. List their ideas: 9. When the teacher calls on you share your observations and your conclusion with the class. 10. Wait for the teacher to cue you to open the bag and reveal the true identity of the object. 11. Did you correctly identify the object? YES or NO 12. If NO state why: ___________________________ 13. If YES state why: __________________________ 14. What do you think is the most important sense needed to identify an unknown object? 8

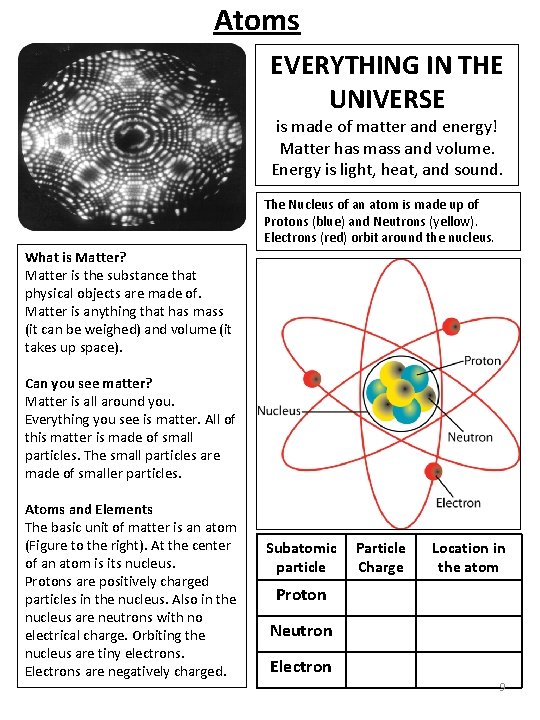
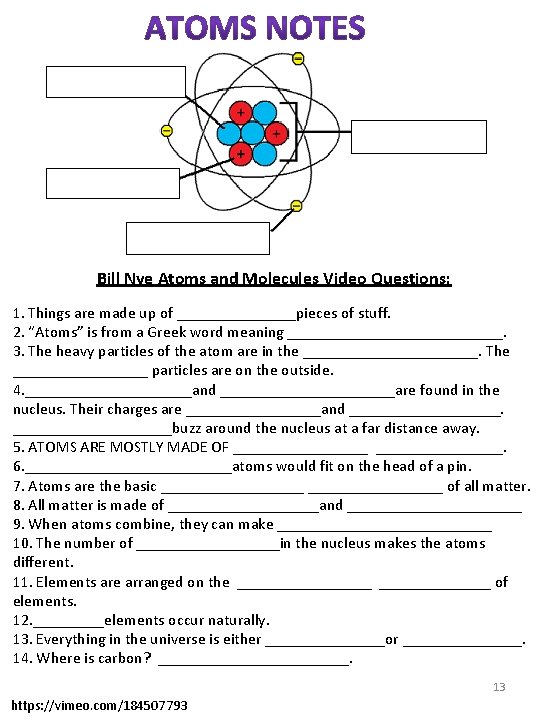
Atoms EVERYTHING IN THE UNIVERSE is made of matter and energy! Matter has mass and volume. Energy is light, heat, and sound. The Nucleus of an atom is made up of Protons (blue) and Neutrons (yellow). Electrons (red) orbit around the nucleus. What is Matter? Matter is the substance that physical objects are made of. Matter is anything that has mass (it can be weighed) and volume (it takes up space). Can you see matter? Matter is all around you. Everything you see is matter. All of this matter is made of small particles. The small particles are made of smaller particles. Atoms and Elements The basic unit of matter is an atom (Figure to the right). At the center of an atom is its nucleus. Protons are positively charged particles in the nucleus. Also in the nucleus are neutrons with no electrical charge. Orbiting the nucleus are tiny electrons. Electrons are negatively charged. Subatomic Particle particle Charge Location in the atom Proton Neutron Electron 9

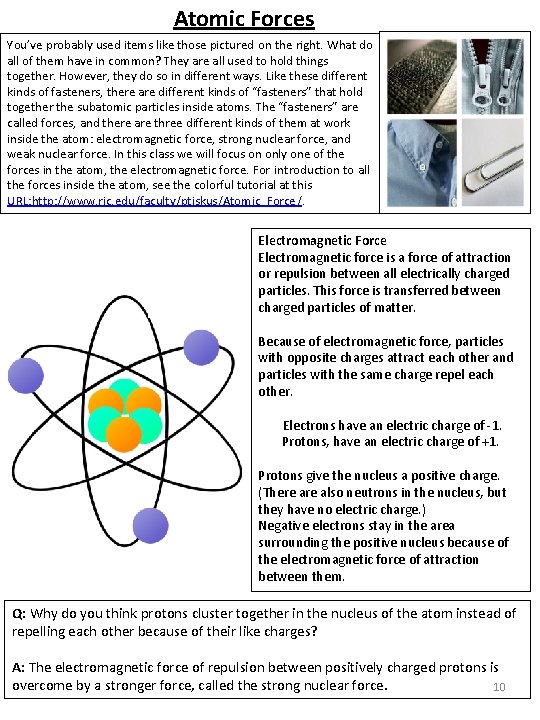
Atomic Forces You’ve probably used items like those pictured on the right. What do all of them have in common? They are all used to hold things together. However, they do so in different ways. Like these different kinds of fasteners, there are different kinds of “fasteners” that hold together the subatomic particles inside atoms. The “fasteners” are called forces, and there are three different kinds of them at work inside the atom: electromagnetic force, strong nuclear force, and weak nuclear force. In this class we will focus on only one of the forces in the atom, the electromagnetic force. For introduction to all the forces inside the atom, see the colorful tutorial at this URL: http: //www. ric. edu/faculty/ptiskus/Atomic_Force/. Electromagnetic Force Electromagnetic force is a force of attraction or repulsion between all electrically charged particles. This force is transferred between charged particles of matter. Because of electromagnetic force, particles with opposite charges attract each other and particles with the same charge repel each other. Electrons have an electric charge of -1. Protons, have an electric charge of +1. Protons give the nucleus a positive charge. (There also neutrons in the nucleus, but they have no electric charge. ) Negative electrons stay in the area surrounding the positive nucleus because of the electromagnetic force of attraction between them. Q: Why do you think protons cluster together in the nucleus of the atom instead of repelling each other because of their like charges? A: The electromagnetic force of repulsion between positively charged protons is overcome by a stronger force, called the strong nuclear force. 10

https: //www. youtube. com/watch? v=34 t. Kk. ET_TFE 5. . The total of an atom’s protons and neutrons is it’s __________________ 1. A ________ of ________ at man’s command was found in the atom. 3: 50 0: 57 6. These different members of the same atom family science calls _________ 2. The atom’s binding force is like a __________ that holds the nucleus together 2: 43 3: 11 4: 00 7. Other are busy being what science calls _________ 3. The atom’ are identified by numbers, that is the number of __________ in their nucleus. 4: 26 8. This spontaneous changing of elements is called natural __________ 4. The heaviest of all natural elements is __________ with 92 protons in the nucleus of the atom. 3: 30 4: 54

9. Describe what happened when scientists were experimenting with the transmutation of Uranium. Describe the double miracle of science. ____________________________________________ Starting at 6: 00 ____________________________________________ 10. How much dynamite is equal the energy released in the complete fission of a baseball size amount of Uranium 235? ______________________ Starting at 8: 15 Directions: Name the many giants of the atomic age. 13: 57 13: 40 13: 55 14: 03

Bill Nye Atoms and Molecules Video Questions: 1. Things are made up of ________pieces of stuff. 2. “Atoms” is from a Greek word meaning ______________. 3. The heavy particles of the atom are in the ___________. The _________ particles are on the outside. 4. ___________and ___________are found in the nucleus. Their charges are _________and __________buzz around the nucleus at a far distance away. 5. ATOMS ARE MOSTLY MADE OF _________. 6. _____________atoms would fit on the head of a pin. 7. Atoms are the basic _________ of all matter. 8. All matter is made of __________and ___________ 9. When atoms combine, they can make ______________ 10. The number of _________in the nucleus makes the atoms different. 11. Elements are arranged on the _________ of elements. 12. _____elements occur naturally. 13. Everything in the universe is either ________or ________. 14. Where is carbon? ____________. 13 https: //vimeo. com/184507793

Atomic Structure Power notes Prentice Hall pages 92 -101 1. What is meant by the word subatomic particles? ________________________________ 2. What are the 3 main subatomic particles in an atom? _________________ 3. The nucleus is considered to be the _________ of the atom. 4. What 2 subatomic particles can be found in the nucleus? ____________________ 5. Protons have a _____ charge and weigh ______ AMU 6. Neutrons have a _____ charge and weigh ______AMU 7. What does the atomic number represent? _______________ 8. How many protons are in the nucleus of a carbon atom_____? Nitrogen____? 9. What is an isotope? ________________________ How many neutrons do the following isotopes of Hydrogen have? Protium_____ Deuterium_____ Tritium_______ 10. What is the mass number? ________________________ 11. How many neutrons does Uranium 235 have? _______Uranium 238? _____ 12. The atomic mass of an element is __________________ 13. Electrons have a __________charge and weigh______AMU. 14. Electrons occupy the space around the nucleus called the _________ 15. How many electrons can each to the following energy levels hold? The lowest or the first holds_____ the second level holds_____the third level holds_____.

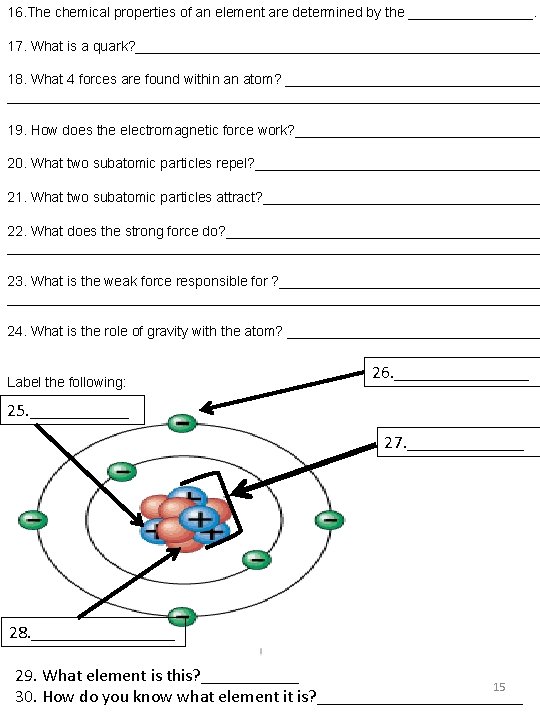
16. The chemical properties of an element are determined by the ________. 17. What is a quark? __________________________ 18. What 4 forces are found within an atom? ___________________________________________________ 19. How does the electromagnetic force work? ________________ 20. What two subatomic particles repel? ___________________ 21. What two subatomic particles attract? __________________ 22. What does the strong force do? _______________________________________________________ 23. What is the weak force responsible for ? ____________________________________________________ 24. What is the role of gravity with the atom? _________________ Label the following: 26. ________ 25. ______ 27. _______ 28. ________ 29. What element is this? ______ 15 30. How do you know what element it is? ____________

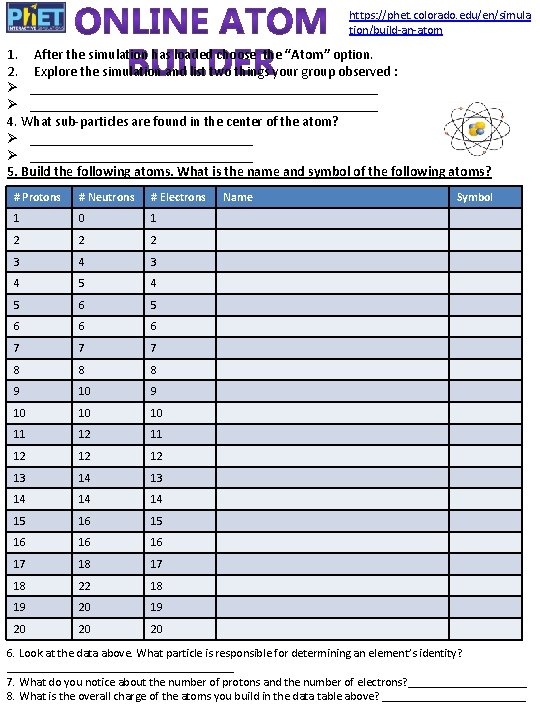
https: //phet. colorado. edu/en/simula tion/build-an-atom 1. After the simulation has loaded choose the “Atom” option. 2. Explore the simulation and list two things your group observed : Ø __________________________________________________ 4. What sub-particles are found in the center of the atom? Ø ________________________________ 5. Build the following atoms. What is the name and symbol of the following atoms? # Protons # Neutrons # Electrons 1 0 1 2 2 2 3 4 5 4 5 6 6 6 7 7 7 8 8 8 9 10 10 10 11 12 12 12 13 14 14 14 15 16 16 16 17 18 22 18 19 20 20 20 Name Symbol 6. Look at the data above. What particle is responsible for determining an element’s identity? ___________________ 7. What do you notice about the number of protons and the number of electrons? __________ 8. What is the overall charge of the atoms you build in the data table above? ____________

17

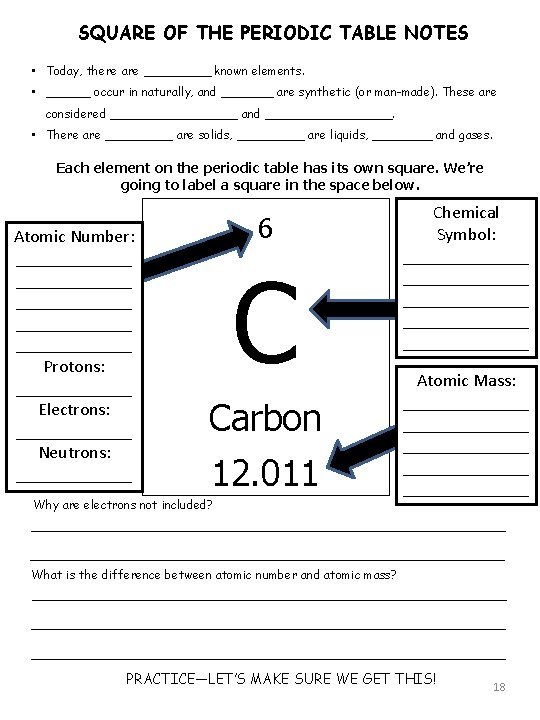
SQUARE OF THE PERIODIC TABLE NOTES • Today, there are _____ known elements. • ______ occur in naturally, and _______ are synthetic (or man-made). These are considered _________ and _________. • There are _____ are solids, _____ are liquids, ____ and gases. Each element on the periodic table has its own square. We’re going to label a square in the space below. Atomic Number: _____________ _______ Protons: _______ Electrons: _______ Neutrons: _______ 6 C Carbon 12. 011 Why are electrons not included? Chemical Symbol: ______________ _______ Atomic Mass: ______________ _______ What is the difference between atomic number and atomic mass? PRACTICE—LET’S MAKE SURE WE GET THIS! 18

We are going to spend some time looking at the periodic table and answering questions about it—we need to make sure that we understand EXACTLY what we’re looking at when we see one square of the periodic table. Sulfer What is the chemical symbol for Sulfur? What is the atomic number of the element Sulfur? What is the atomic mass of the element Sulfur? How many protons does Sulfur have? _____ How many neutrons does Sulfur have? ____ How many electrons does Sulfur have? _______ Lithium What is the chemical symbol for lithium? What is the atomic number of the element lithium? What is the atomic mass of the element lithium? How many protons does lithium have? _____ How many neutrons does lithium have? ____ How many electrons does lithium have? _______ Calcium What is the chemical symbol for calcium? What is the atomic number of the element calcium? What is the atomic mass of the element calcium? How many protons does calcium have? ____ How many neutrons does calcium have? ____ How many electrons does calcium have? _______ Strontium What is the chemical symbol for strontium? What is the atomic number of the element strontium? What is the atomic mass of the element strontium? How many protons does strontium have? ____ How many neutrons does strontium have? _______ How many electrons does strontium have? _______ 19

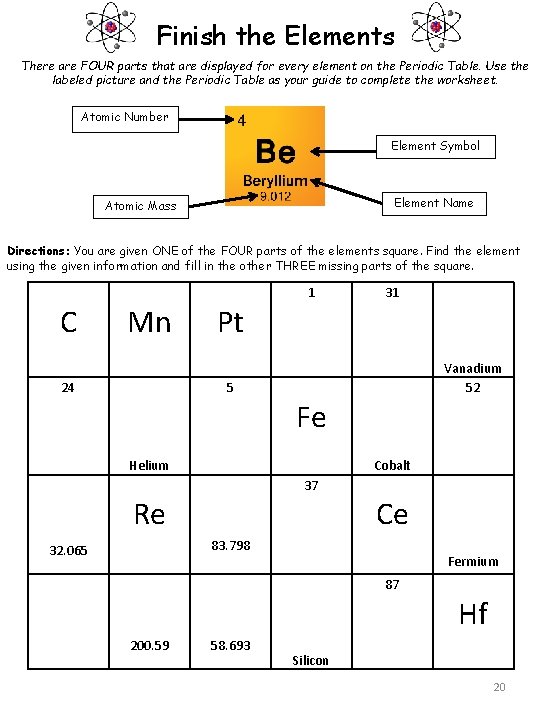
Finish the Elements There are FOUR parts that are displayed for every element on the Periodic Table. Use the labeled picture and the Periodic Table as your guide to complete the worksheet. Atomic Number Element Symbol Element Name Atomic Mass Directions: You are given ONE of the FOUR parts of the elements square. Find the element using the given information and fill in the other THREE missing parts of the square. C Mn Pt 1 31 24 32. 065 Helium 5 Re Fe 37 Vanadium 52 Cobalt Ce 83. 798 Fermium 200. 59 58. 693 87 Hf Silicon 20

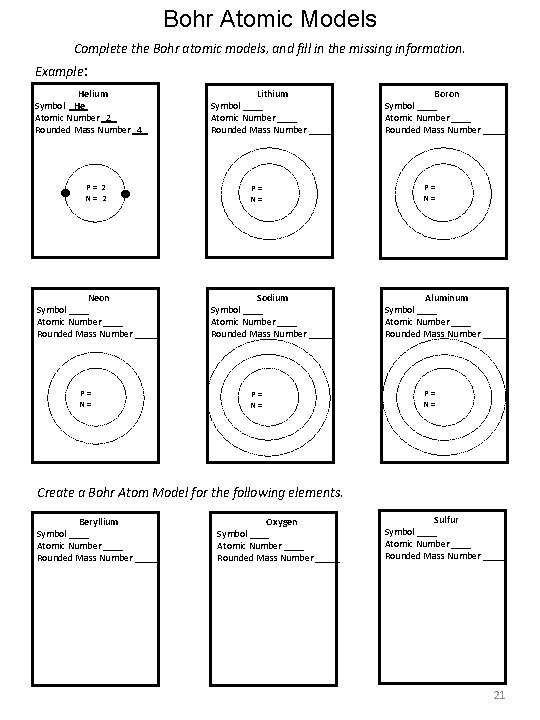
Bohr Atomic Models Complete the Bohr atomic models, and fill in the missing information. Example: Helium Symbol He Atomic Number 2 Rounded Mass Number 4 P = 2 Neon Symbol ____ Atomic Number ____ Rounded Mass Number _____ P = N = Lithium Symbol ____ Atomic Number ____ Rounded Mass Number _____ P = N = Sodium Symbol ____ Atomic Number ____ Rounded Mass Number _____ P = N = Boron Symbol ____ Atomic Number ____ Rounded Mass Number _____ P = N = Aluminum Symbol ____ Atomic Number ____ Rounded Mass Number _____ P = N = Create a Bohr Atom Model for the following elements. Beryllium Symbol ____ Atomic Number ____ Rounded Mass Number _____ Oxygen Symbol ____ Atomic Number ____ Rounded Mass Number _____ Sulfur Symbol ____ Atomic Number ____ Rounded Mass Number _____ 21

In Summary. . . For any element: Number of Protons = Atomic Number of Electrons = Number of Protons = Atomic Number of Neutrons = Mass Number - Atomic Number Step 1 - Gather Information The first thing you will need to do is find some information about your element. Go to the Periodic Table of Elements and find your element. Choose an element from 3 -20 (The models get more difficult as the atomic number gets larger) Atomic Number What element did you choose? _____________ Use the Table of Elements to find your element's atomic number and atomic weight. The atomic number is the number located in the upper right or left corner and the atomic weight is the number located on the bottom, as in this example for krypton: What is your element’s atomic mass? _________ What is your element’s atomic number? ______ Atomic Mass Step 2 - The Number of Protons is. . . The atomic number is the number of protons in an atom of an element. In our example, krypton's atomic number is 36. This tells us that an atom of krypton has 36 protons in its nucleus. The interesting thing here is that every atom of krypton contains 36 protons. If an atom doesn't have 36 protons, it can't be an atom of krypton. Adding or removing protons from the nucleus of an atom creates a different element. For example, removing one proton from an atom of krypton creates an atom of bromine! How many protons does an atom of your element have in it’s nucleus? _____ Step 3 - The Number of Electrons is. . . By definition, atoms have no overall electrical charge. That means that there must be a balance between the positively charged protons and the negatively charged electrons. Atoms must have equal numbers of protons and electrons. In our example, an atom of krypton must contain 36 electrons since it contains 36 protons. How many electrons does an atom of your element have whirling about the nucleus? ___________

With the Bohr Model electrons are arranged around atoms in a special way. Energy Level Electron Capacity 1 2 2 8 3 18 4 32 How many energy levels will your atom have? How many electrons will be in each energy level? Level 1: Level 2: Level 3: Level 4: . Step 4 - The Number of Neutrons is. . . The atomic weight is basically a measurement of the total number of particles in an atom's nucleus. First round the atomic weight to the nearest whole number. Your element’s atomic weight rounded to nearest whole number ______ In our example, krypton's mass is 84 since its atomic weight, 83. 80, rounds up to 84. Mass Number minus Number of Protons (atomic number) = Number of Neutrons For krypton, this equation becomes: 84 minus 36 = 48 (Number of Neutrons) Your element’s mass number ______ minus ______ the number of protons (atomic number) = _____ (neutrons). The interesting thing here is that adding or removing neutrons from an atom does not create a different element. Rather, it creates a heavier or lighter version of that element. These different versions are called isotopes and most elements are actually a mixture of different isotopes. If you could grab atoms of krypton and count the number of neutrons each one had, you would find that most would have 48, others would have 47, some would have 50, some others would have 46, a few would have 44 and a very few would have 42. You would count different numbers of neutrons because krypton is a mixture of six isotopes. Step 5 – Putting it all together Draw a diagram of the atom you chose to make: How many? : Protons _____ Neurons ____ Energy levels _____ Electrons _____ Element name ___________ Atomic number_____ Atomic mass (not rounded) ______ Period number____ Group number_____ Teacher’s initials http: //www. kscience. co. uk/animations/atom. htm Go to this site to build your atom online. 23

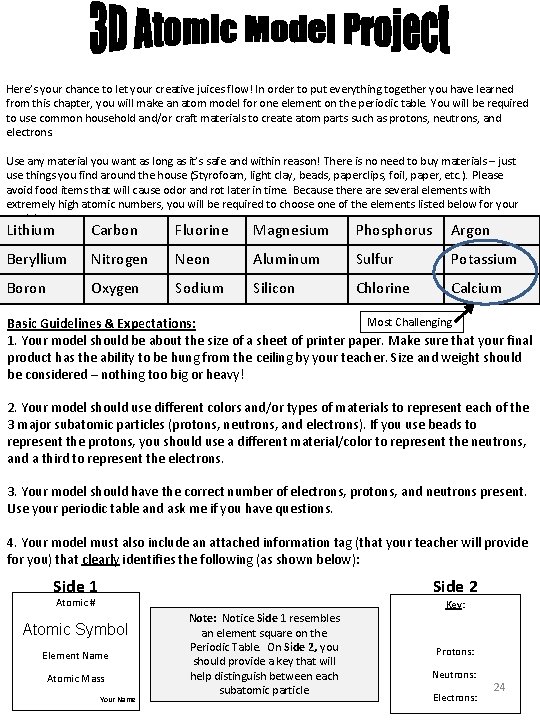
Here’s your chance to let your creative juices flow! In order to put everything together you have learned from this chapter, you will make an atom model for one element on the periodic table. You will be required to use common household and/or craft materials to create atom parts such as protons, neutrons, and electrons. Use any material you want as long as it’s safe and within reason! There is no need to buy materials – just use things you find around the house (Styrofoam, light clay, beads, paperclips, foil, paper, etc. ). Please avoid food items that will cause odor and rot later in time. Because there are several elements with extremely high atomic numbers, you will be required to choose one of the elements listed below for your model: Carbon Fluorine Magnesium Phosphorus Argon Lithium Beryllium Nitrogen Neon Aluminum Sulfur Potassium Boron Oxygen Sodium Silicon Chlorine Calcium Most Challenging Basic Guidelines & Expectations: 1. Your model should be about the size of a sheet of printer paper. Make sure that your final product has the ability to be hung from the ceiling by your teacher. Size and weight should be considered – nothing too big or heavy! 2. Your model should use different colors and/or types of materials to represent each of the 3 major subatomic particles (protons, neutrons, and electrons). If you use beads to represent the protons, you should use a different material/color to represent the neutrons, and a third to represent the electrons. 3. Your model should have the correct number of electrons, protons, and neutrons present. Use your periodic table and ask me if you have questions. 4. Your model must also include an attached information tag (that your teacher will provide for you) that clearly identifies the following (as shown below): Side 1 Side 2 Atomic # Atomic Symbol Element Name Atomic Mass Your Name Note: Notice Side 1 resembles an element square on the Periodic Table. On Side 2, you should provide a key that will help distinguish between each subatomic particle Key: Protons: Neutrons: Electrons: 24

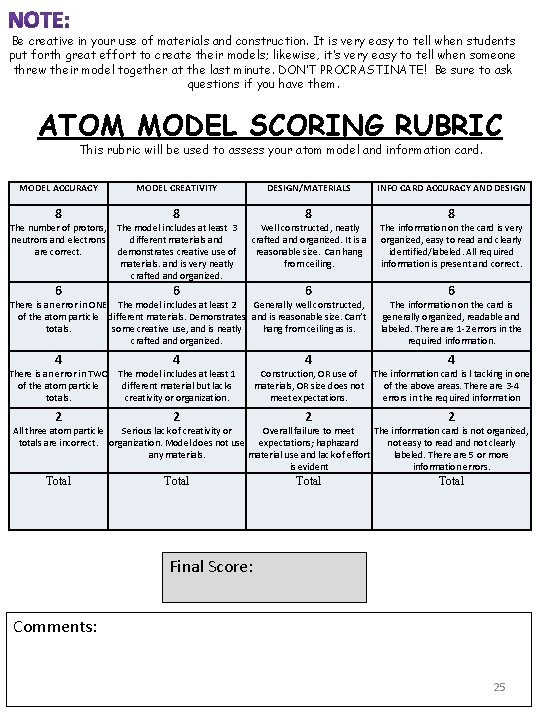
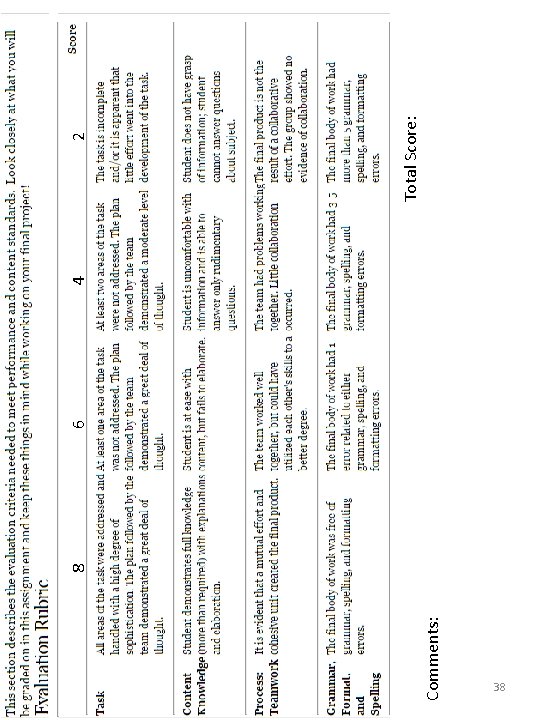
Be creative in your use of materials and construction. It is very easy to tell when students put forth great effort to create their models; likewise, it’s very easy to tell when someone threw their model together at the last minute. DON’T PROCRASTINATE! Be sure to ask questions if you have them. ATOM MODEL SCORING RUBRIC This rubric will be used to assess your atom model and information card. MODEL ACCURACY MODEL CREATIVITY 8 8 6 6 6 4 4 2 2 Total The number of protons, The model includes at least 3 neutrons and electrons different materials and are correct. demonstrates creative use of materials. and is very neatly crafted and organized. DESIGN/MATERIALS 8 Well constructed, neatly crafted and organized. It is a reasonable size. Can hang from ceiling. There is an error in ONE The model includes at least 2 Generally well constructed, of the atom particle different materials. Demonstrates and is reasonable size. Can’t totals. some creative use, and is neatly hang from ceiling as is. crafted and organized. There is an error in TWO The model includes at least 1 of the atom particle different material but lacks totals. creativity or organization. INFO CARD ACCURACY AND DESIGN 8 The information on the card is very organized, easy to read and clearly identified/labeled. All required information is present and correct. 6 The information on the card is generally organized, readable and labeled. There are 1 -2 errors in the required information. Construction, OR use of The information card is l tacking in one materials, OR size does not of the above areas. There are 3 -4 meet expectations. errors in the required information All three atom particle Serious lack of creativity or Overall failure to meet The information card is not organized, totals are incorrect. organization. Model does not use expectations; haphazard not easy to read and not clearly any materials. material use and lack of effort labeled. There are 5 or more is evident information errors. Final Score: Comments: 25

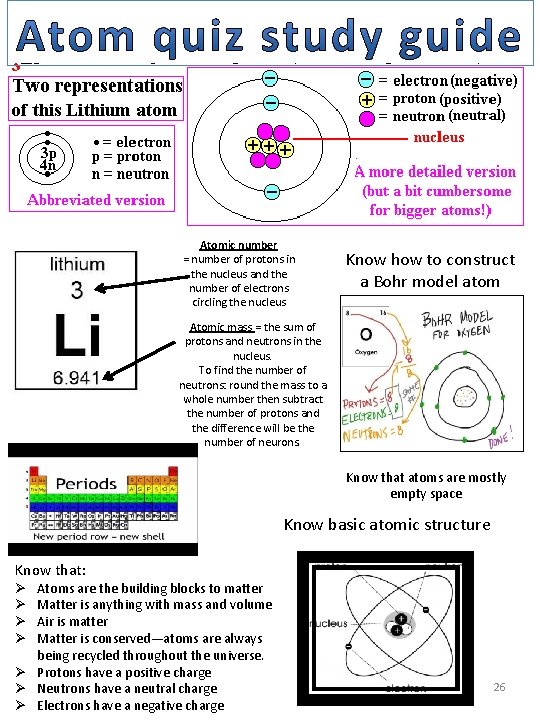
Atomic number = number of protons in the nucleus and the number of electrons circling the nucleus Know how to construct a Bohr model atom Atomic mass = the sum of protons and neutrons in the nucleus. To find the number of neutrons: round the mass to a whole number then subtract the number of protons and the difference will be the number of neurons. Know that atoms are mostly empty space Know basic atomic structure Know that: Atoms are the building blocks to matter Matter is anything with mass and volume Air is matter Matter is conserved—atoms are always being recycled throughout the universe. Ø Protons have a positive charge Ø Neutrons have a neutral charge Ø Electrons have a negative charge Ø Ø 26


What is antimatter? Slow down! "Antimatter? " "Pure energy? " What is this, Star Trek? The idea of antimatter is strange, made all the stranger because the universe appears to be composed entirely of matter. Antimatter seems to go against everything you know about the universe. But you can see evidence for antimatter in this early bubble chamber photo. The magnetic field in this chamber makes negative particles curl left and positive particles curl right. Many electron-positron pairs appear as if from nowhere, but are in fact from photons, which don't leave a trail. Positrons (anti-electrons) behave just like the electrons but curl in the opposite way because they have the opposite charge. (One such electron-positron pair is highlighted. ) If antimatter and matter are exactly equal but opposite, then why is there so much more matter in the universe than antimatter? Well. . . we don't know. It is a question that keeps physicists up at night. (The usual symbol for an antiparticle is a bar over the corresponding particle symbol. For example, the "up quark" u has an "up antiquark" designated by U, pronounced u-bar. The antiparticle of a quark is an antiquark, the antiparticle of a proton is an antiproton, and so on. The antielectron is called a positron and is designated as e+. What is your reaction to these theoretical types of matter? How might we use antimatter or dark matter when (or if) we find it? ____________________________________________________________________________________ __________________________________________ Dark matter Ready for a mind-boggling idea? The majority of the universe may not be made of the same type of matter as the Earth. We infer from gravitational effects the presence of this dark matter, a type of matter that we cannot see. There is strong evidence that it might not be made up of protons, neutrons, and electrons. What is dark matter, then? We don't know. Perhaps it is composed of neutrinos, or even more 28 exotic forms of matter, like neutralinos, one of theoretical supersymmetric

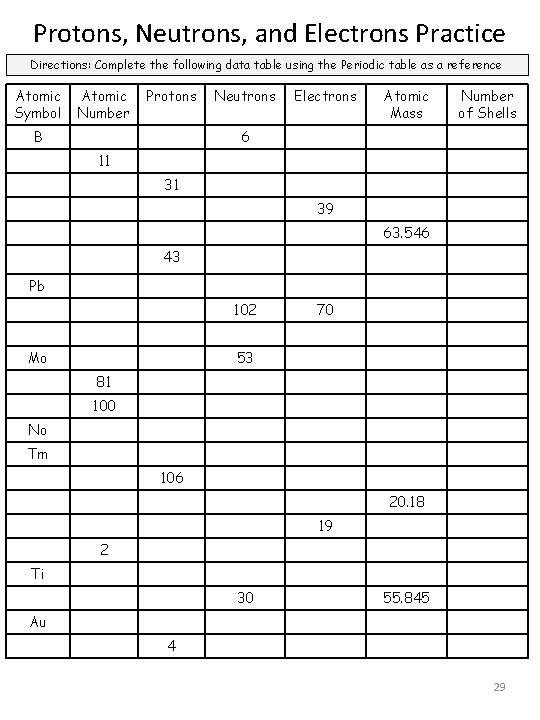
Protons, Neutrons, and Electrons Practice Directions: Complete the following data table using the Periodic table as a reference Atomic Symbol Atomic Number Protons B Neutrons Electrons Atomic Mass Number of Shells 6 11 31 39 63. 546 43 Pb 102 Mo 70 53 81 100 No Tm 106 20. 18 19 2 Ti 30 55. 845 Au 4 29

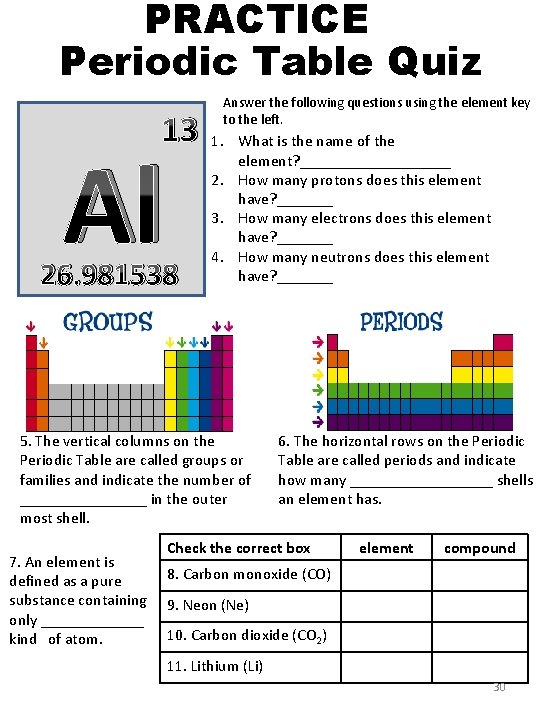
PRACTICE Periodic Table Quiz 13 Al 26. 981538 Answer the following questions using the element key to the left. 1. What is the name of the element? __________ 2. How many protons does this element have? _______ 3. How many electrons does this element have? _______ 4. How many neutrons does this element have? _______ 5. The vertical columns on the Periodic Table are called groups or families and indicate the number of ________ in the outer most shell. 7. An element is defined as a pure substance containing only _______ kind of atom. 6. The horizontal rows on the Periodic Table are called periods and indicate how many _________ shells an element has. Check the correct box element compound 8. Carbon monoxide (CO) 9. Neon (Ne) 10. Carbon dioxide (CO 2) 11. Lithium (Li) 30

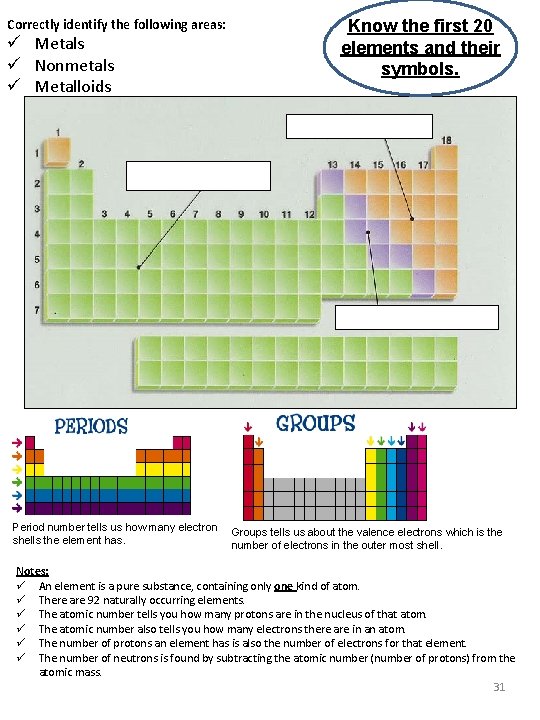
Correctly identify the following areas: ü Metals ü Nonmetals ü Metalloids Know the first 20 elements and their symbols. Period number tells us how many electron Groups tells us about the valence electrons which is the shells the element has. number of electrons in the outer most shell. Notes: ü An element is a pure substance, containing only one kind of atom. ü There are 92 naturally occurring elements. ü The atomic number tells you how many protons are in the nucleus of that atom. ü The atomic number also tells you how many electrons there are in an atom. ü The number of protons an element has is also the number of electrons for that element. ü The number of neutrons is found by subtracting the atomic number (number of protons) from the atomic mass. 31

32

Organic Molecules Card Game 4 to 6 players Playing time: as little as 5 -10 minutes per game, though some games make take 20 or more minutes. Play the game several times. Each game will be different. Background information: § The molecules you will make in this game may or may not be actual molecules found in nature. § There are so many real organic molecules that, chances are, your molecules will be at least very similar to real ones. § Here is what the letters stand for: H= hydrogen, C= carbon, O= oxygen, N= nitrogen, Cl= chlorine, Br= bromine, F= fluorine. (You may have noticed that there a lot of hydrogen cards in the game. 90% of all atoms in the universe are hydrogen!) § The lines on the cards represent electrons that the atom would like to share with another atom. You will need: • copies of the pattern pages printed onto card stock (any color-- you can make them brightly col- ored if you wish!) • Scissors NOTE: If you are making more than one set of cards, I strongly recommend making each set a different color. It will be easy to make sure all the cards get back into the right sets. Otherwise, you will have to count each type of card to make sure one set doesn’t have more or less than another. Life is too short to waste time counting cards! How to play: § Give each player five cards. The rest go face down in a draw pile. Put one of the carbons (with no double bonds) face up to be the starter card. The players take turns laying down cards, trying to get rid of all the cards in their hand. The first player to get rid of all their cards wins the game. HOWEVER: the last card he lays down MUST complete a molecule. If a player lays down his last card on an incomplete mol- ecule, he must draw another card. He may not lay down this new card immediately, but must wait till his next turn to play it. § The lines represent bonds. You must match single bonds to single bonds and double bonds to double bonds. A molecule is complete when no bonds are “left hanging. ” Each bond line must have some- thing attached to it. § Notice that on the double bond O, there are dotted lines. This is so that you can turn the card caddy-corner and match the double bond with two single bonds. § If a player cannot lay down a card, he must take one from the draw pile. He may lay this card down immediately if he can do so. § If a molecule is finished and all players are still holding cards, simple begin another molecule. You must use a carbon with four single bonds as the starter card for a new molecule. § Remember, if order to win the game, the player must lay down his last card as the final atom in a molecule! 33

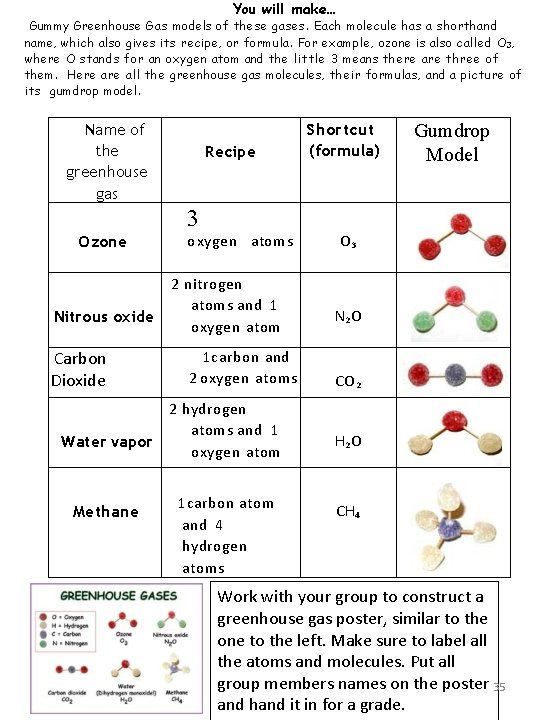
Get your Gummy Greenhouse Gases! Got gumdrops? Then you can build models of molecules. Molecules are tiny structures that make up just about all matter—including you! Molecules themselves are made of atoms, the basic building blocks of matter. Using just four kinds of atoms as building blocks, you can construct many different types of molecules. In this project, you will build models of some gas molecules. These kinds of gas molecules are part of the air. They are called greenhouse gases. We will explain why later. For now, get ready for some gummy fun! Materials: Gumdrops, any size, four different colors. These atoms are usually modeled using red for oxygen, white for hydrogen, gray for carbon, and blue for nitrogen. However, some of these colors are mighty hard to find in gumdrops. So use any colors you like. Here's how many you will need of each (but don't forget to get extras for sneaking into your mouth): o Red: 13 Oxygen o White: 7 Hydrogen o Gray (or black): 3 Carbon o Green: 2 Nitrogen Round wooden toothpicks Construction paper, 1 large sheet (12 x 18, for example) Felt pen or crayons Here are the colors we used for our gumdrop building block atoms: Oxygen Hydrogen Carbon Nitrogen 34

You will make… Gummy Greenhouse Gas models of these gases. Each molecule has a shorthand name, which also gives its recipe, or formula. For example, ozone is also called O 3 , where O stands for an oxygen atom and the little 3 means there are three of them. Here all the greenhouse gas molecules, their formulas, and a picture of its gumdrop model. Name of the greenhouse gas Recipe Shortcut (formula) Gumdrop Model 3 Ozone Nitrous oxide Carbon Dioxide oxygen atoms 2 nitrogen atoms and 1 oxygen atom 1 carbon and 2 oxygen atoms 2 hydrogen atoms and 1 Water vapor oxygen atom Methane 1 carbon atom and 4 hydrogen atoms O 3 N 2 O CO 2 H 2 O CH 4 Work with your group to construct a greenhouse gas poster, similar to the one to the left. Make sure to label all the atoms and molecules. Put all group members names on the poster 35 and hand it in for a grade.

36

http: //education. jlab. org/qa/atom _model. html 37

Comments: 38 8 6 4 Total Score: 2