CHEMISTRY Atoms First Chapter 2 ATOMS MOLECULES AND






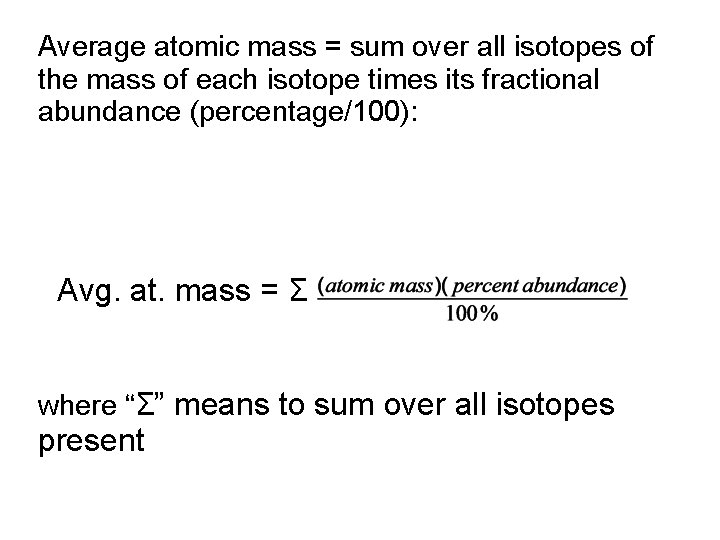
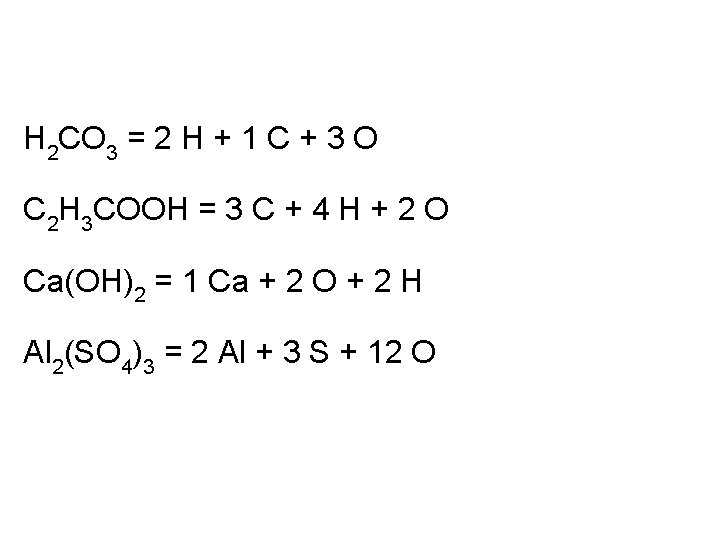





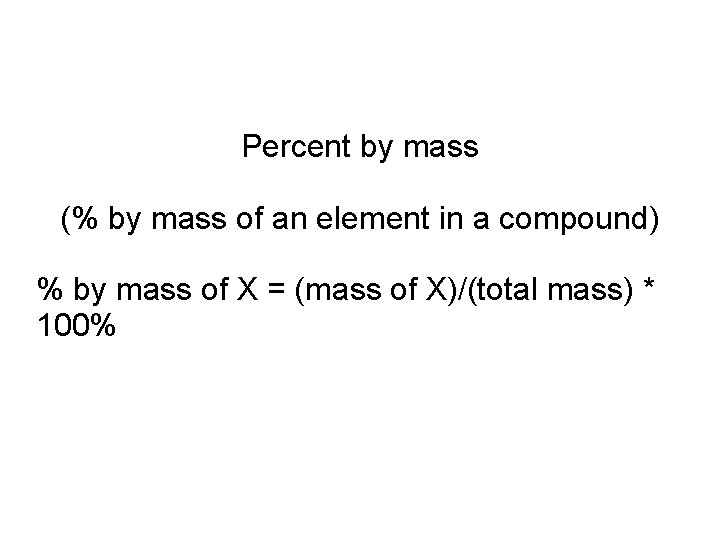


- Slides: 63

CHEMISTRY: Atoms First Chapter 2 ATOMS, MOLECULES, AND IONS Power. Point Image Slideshow

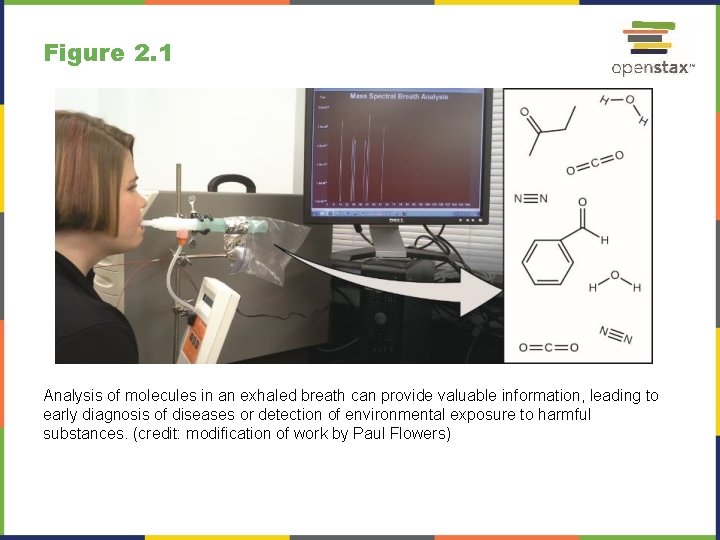
Figure 2. 1 Analysis of molecules in an exhaled breath can provide valuable information, leading to early diagnosis of diseases or detection of environmental exposure to harmful substances. (credit: modification of work by Paul Flowers)

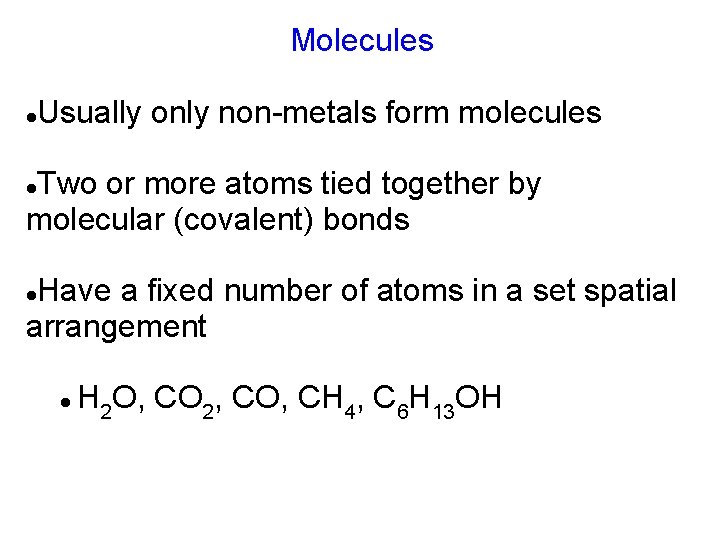
Molecules Usually only non-metals form molecules Two or more atoms tied together by molecular (covalent) bonds Have a fixed number of atoms in a set spatial arrangement H 2 O, CO 2, CO, CH 4, C 6 H 13 OH

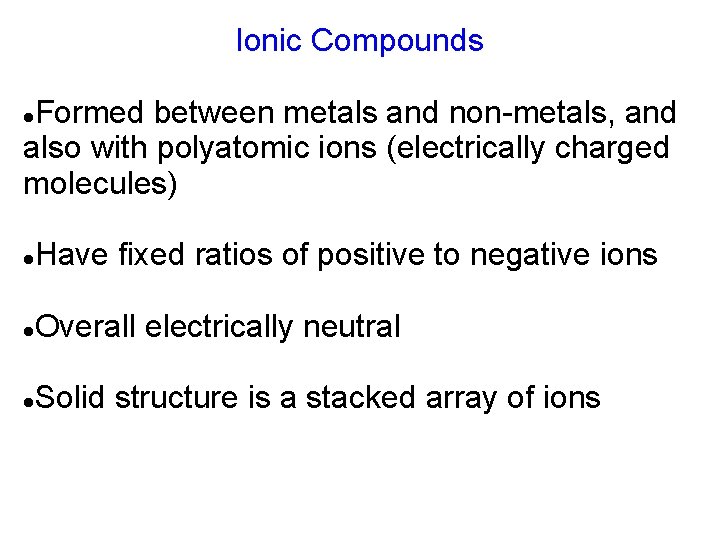
Ionic Compounds Formed between metals and non-metals, and also with polyatomic ions (electrically charged molecules) Have fixed ratios of positive to negative ions Overall electrically neutral Solid structure is a stacked array of ions


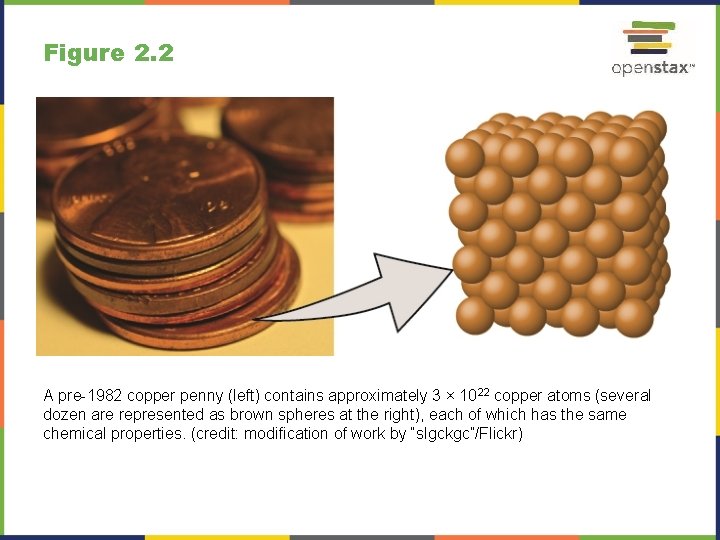
Figure 2. 2 A pre-1982 copper penny (left) contains approximately 3 × 1022 copper atoms (several dozen are represented as brown spheres at the right), each of which has the same chemical properties. (credit: modification of work by “slgckgc”/Flickr)

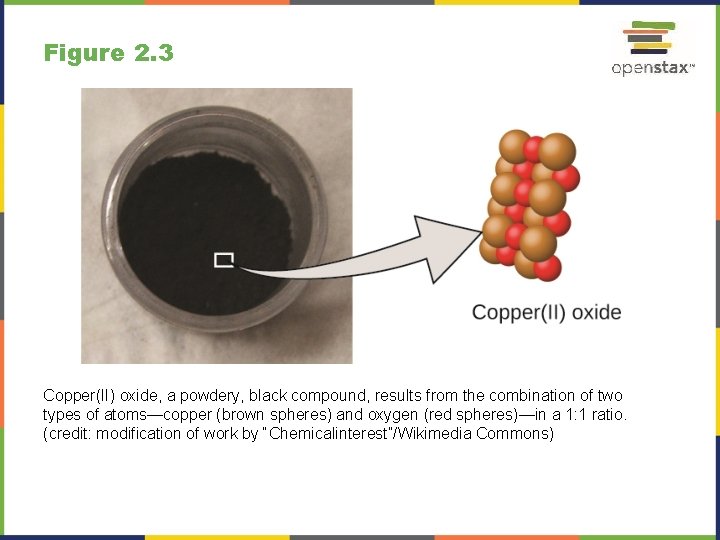
Figure 2. 3 Copper(II) oxide, a powdery, black compound, results from the combination of two types of atoms—copper (brown spheres) and oxygen (red spheres)—in a 1: 1 ratio. (credit: modification of work by “Chemicalinterest”/Wikimedia Commons)

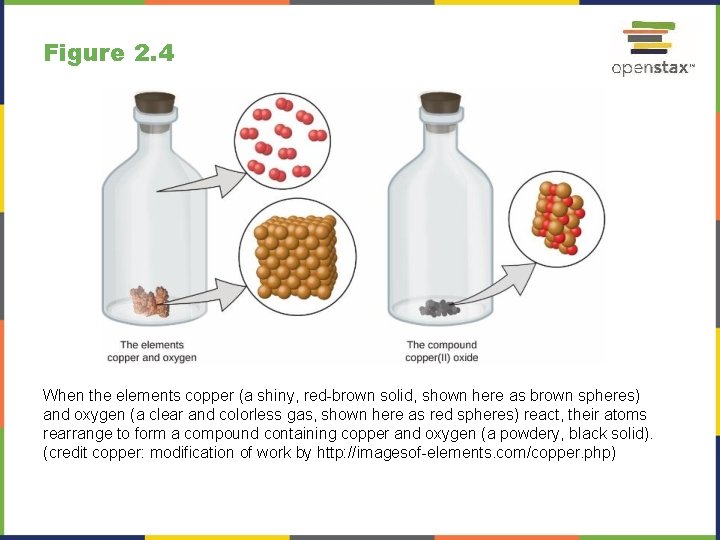
Figure 2. 4 When the elements copper (a shiny, red-brown solid, shown here as brown spheres) and oxygen (a clear and colorless gas, shown here as red spheres) react, their atoms rearrange to form a compound containing copper and oxygen (a powdery, black solid). (credit copper: modification of work by http: //imagesof-elements. com/copper. php)

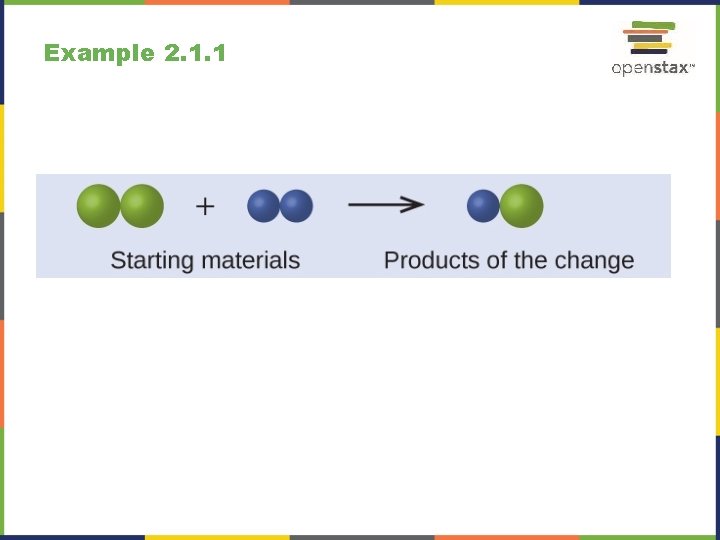
Example 2. 1. 1

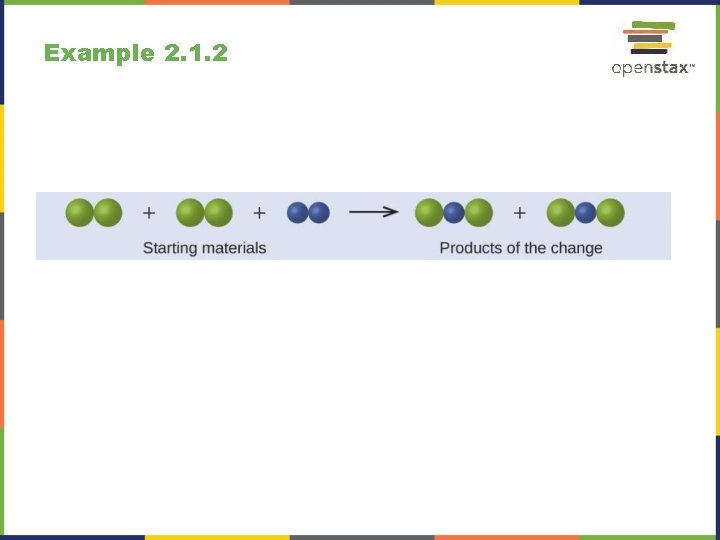
Example 2. 1. 2

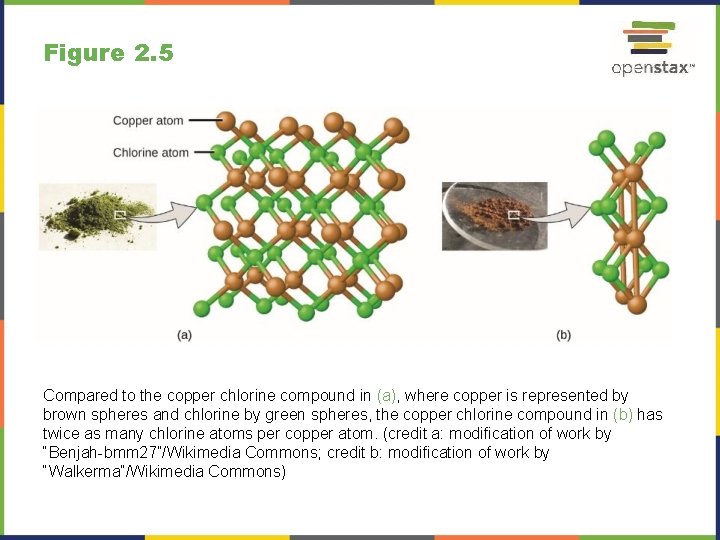
Figure 2. 5 Compared to the copper chlorine compound in (a), where copper is represented by brown spheres and chlorine by green spheres, the copper chlorine compound in (b) has twice as many chlorine atoms per copper atom. (credit a: modification of work by “Benjah-bmm 27”/Wikimedia Commons; credit b: modification of work by “Walkerma”/Wikimedia Commons)

Figure 2. 6 (a) J. J. Thomson produced a visible beam in a cathode ray tube. (b) This is an early cathode ray tube, invented in 1897 by Ferdinand Braun. (c) In the cathode ray, the beam (shown in yellow) comes from the cathode and is accelerated past the anode toward a fluorescent scale at the end of the tube. Simultaneous deflections by applied electric and magnetic fields permitted Thomson to calculate the mass-to-charge ratio of the particles composing the cathode ray. (credit a: modification of work by Nobel Foundation; credit b: modification of work by Eugen Nesper; credit c: modification of work by “Kurzon”/Wikimedia Commons)

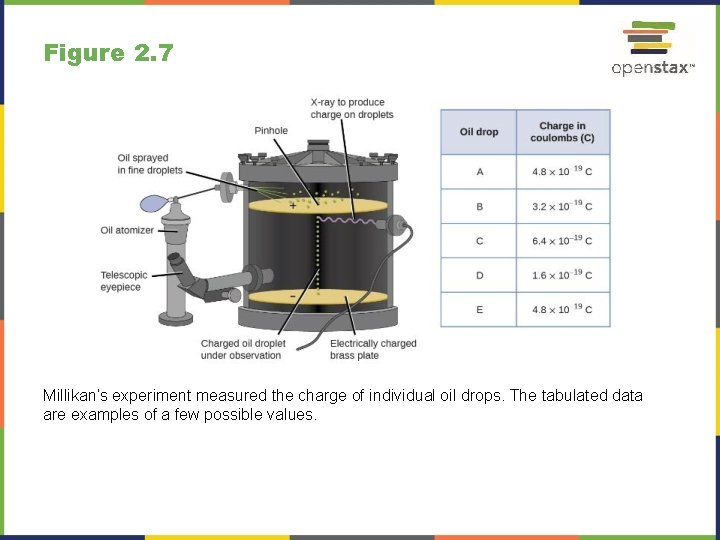
Figure 2. 7 Millikan’s experiment measured the charge of individual oil drops. The tabulated data are examples of a few possible values.

Figure 2. 8 (a) Thomson suggested that atoms resembled plum pudding, an English dessert consisting of moist cake with embedded raisins (“plums”). (b) Nagaoka proposed that atoms resembled the planet Saturn, with a ring of electrons surrounding a positive “planet. ” (credit a: modification of work by “Man vyi”/Wikimedia Commons; credit b: modification of work by “NASA”/Wikimedia Commons)

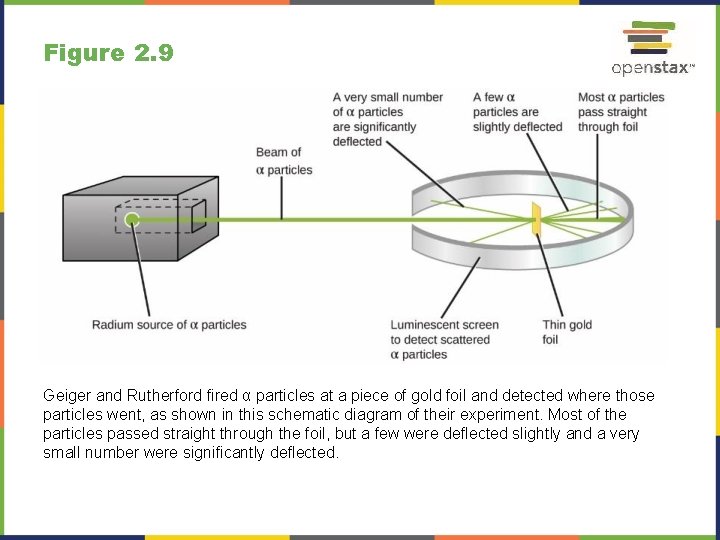
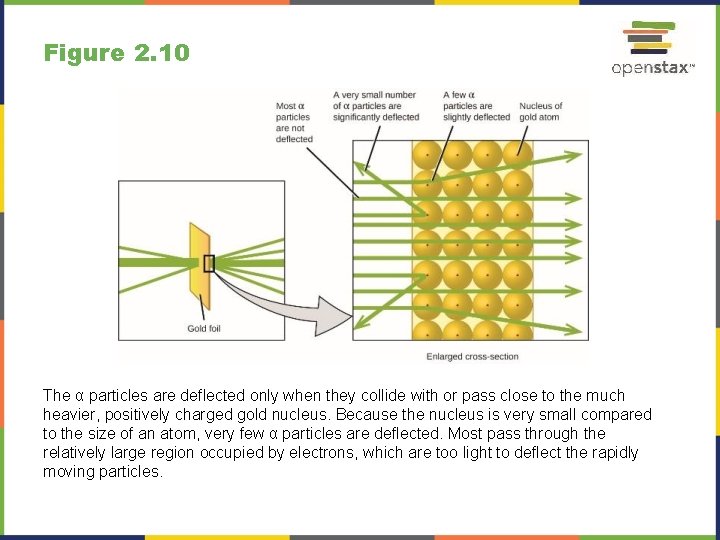
Figure 2. 9 Geiger and Rutherford fired α particles at a piece of gold foil and detected where those particles went, as shown in this schematic diagram of their experiment. Most of the particles passed straight through the foil, but a few were deflected slightly and a very small number were significantly deflected.

Figure 2. 10 The α particles are deflected only when they collide with or pass close to the much heavier, positively charged gold nucleus. Because the nucleus is very small compared to the size of an atom, very few α particles are deflected. Most pass through the relatively large region occupied by electrons, which are too light to deflect the rapidly moving particles.

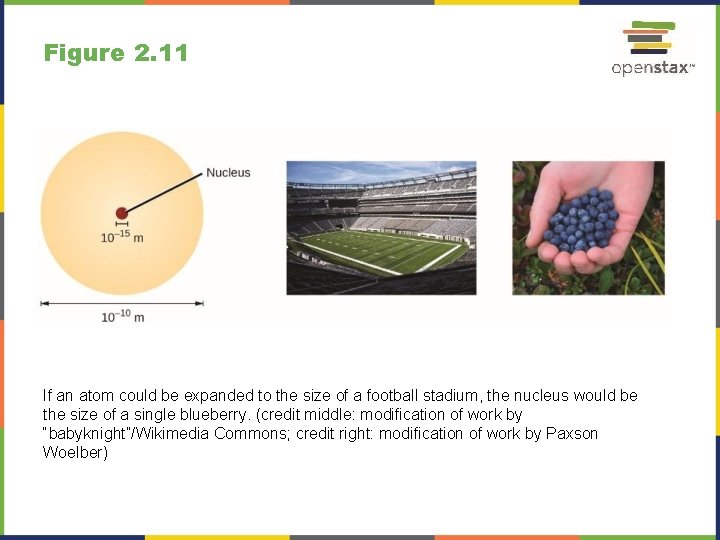
Figure 2. 11 If an atom could be expanded to the size of a football stadium, the nucleus would be the size of a single blueberry. (credit middle: modification of work by “babyknight”/Wikimedia Commons; credit right: modification of work by Paxson Woelber)

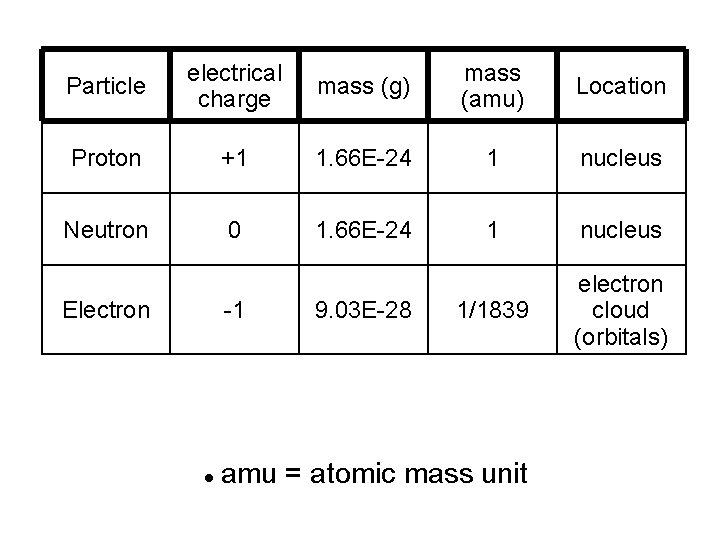
Particle electrical charge mass (g) mass (amu) Location Proton +1 1. 66 E-24 1 nucleus Neutron 0 1. 66 E-24 1 nucleus 1/1839 electron cloud (orbitals) Electron -1 9. 03 E-28 amu = atomic mass unit

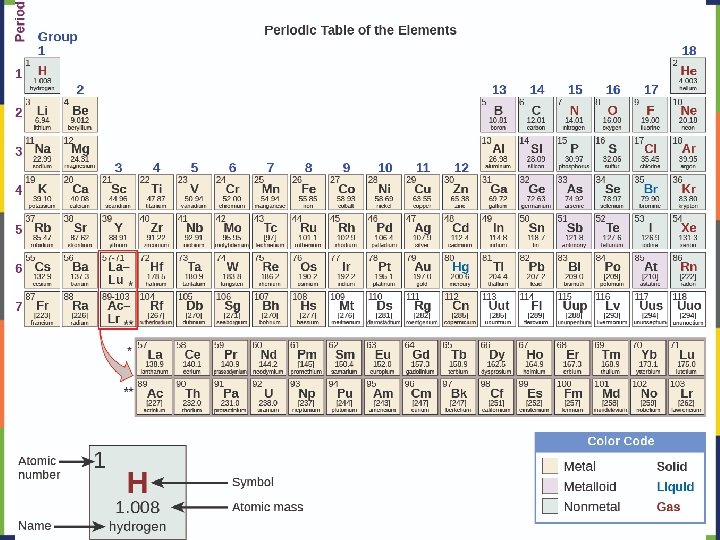
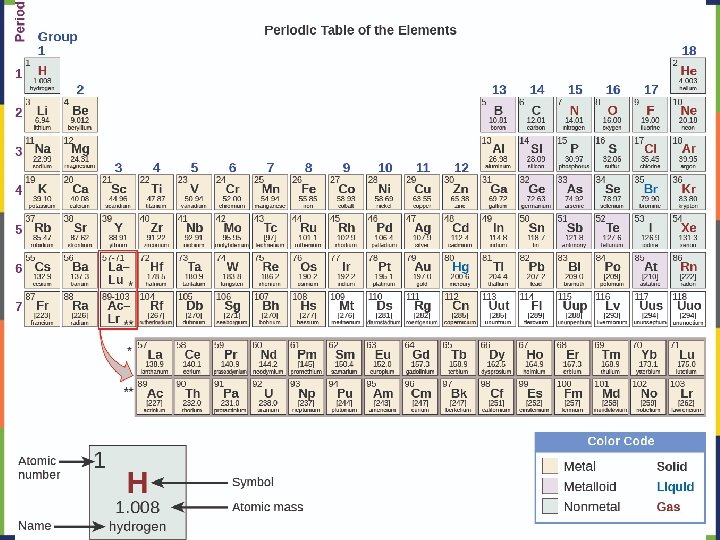
Elements Chemical symbols – used to represent elements - One or two letters, first is capitalized Atom Smallest particle of an element that has the properties of that element Cannot be broken down by chemical means -10 m in diameter ~10 -21 -10 -23 grams mass ~10 21 atoms in a single There about 5 x 10 drop of water

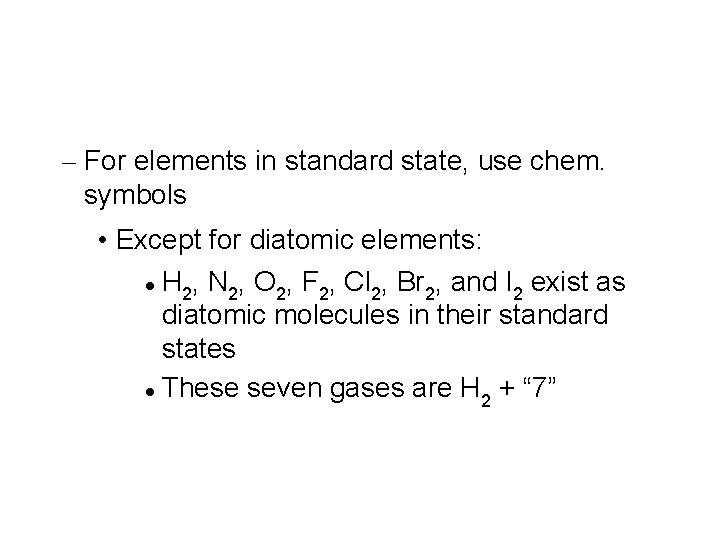
– For elements in standard state, use chem. symbols • Except for diatomic elements: H , N , O , F , Cl , Br , and I exist as 2 2 2 2 diatomic molecules in their standard states These seven gases are H + “ 7” 2


Figure 2. 13 The symbol Hg represents the element mercury regardless of the amount; it could represent one atom of mercury or a large amount of mercury.

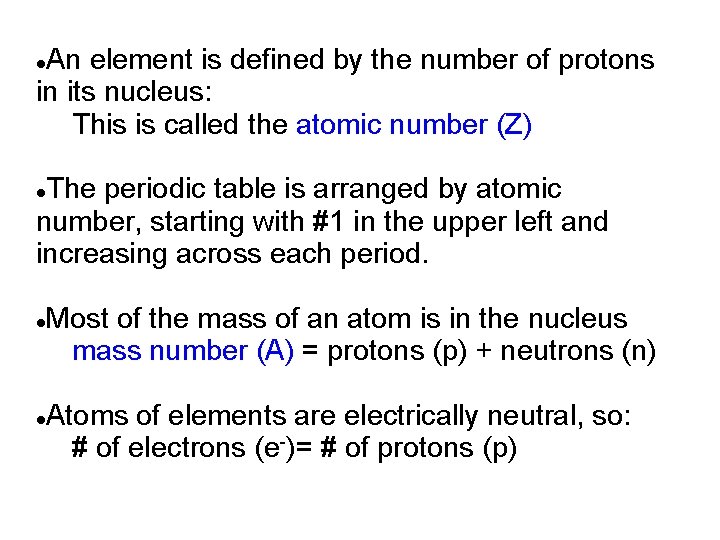
An element is defined by the number of protons in its nucleus: This is called the atomic number (Z) The periodic table is arranged by atomic number, starting with #1 in the upper left and increasing across each period. Most of the mass of an atom is in the nucleus mass number (A) = protons (p) + neutrons (n) Atoms of elements are electrically neutral, so: # of electrons (e-)= # of protons (p)

All atoms of an element have the same atomic number. All atoms of an element will therefore have the same number of electrons. All atoms of an element may not have the same number of neutrons, so may also have different mass numbers. Atoms of an element with different numbers of neutrons are called isotopes.

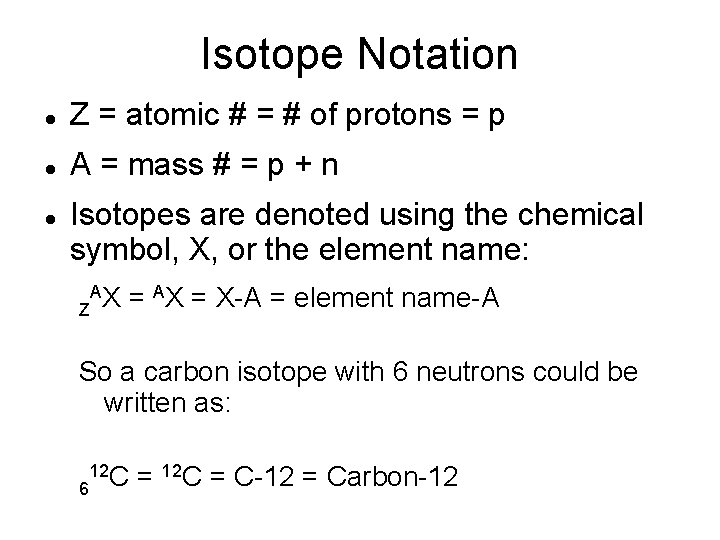
Isotope Notation Z = atomic # = # of protons = p A = mass # = p + n Isotopes are denoted using the chemical symbol, X, or the element name: Z A X = AX = X-A = element name-A So a carbon isotope with 6 neutrons could be written as: 6 12 C = C-12 = Carbon-12

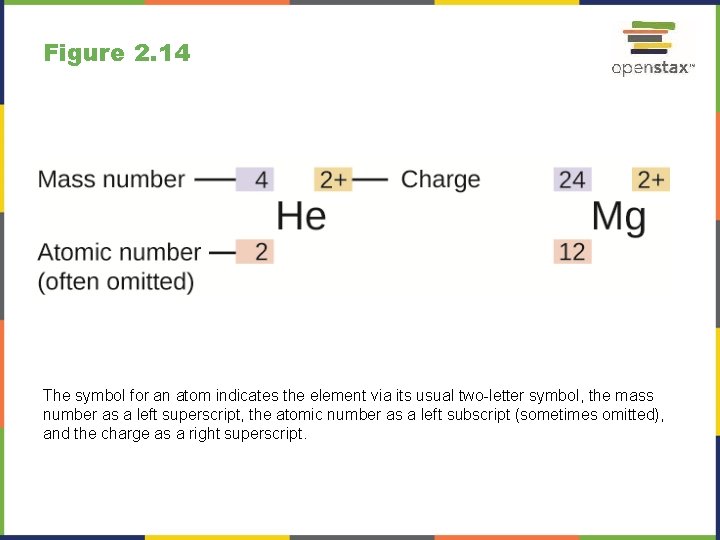
Figure 2. 14 The symbol for an atom indicates the element via its usual two-letter symbol, the mass number as a left superscript, the atomic number as a left subscript (sometimes omitted), and the charge as a right superscript.

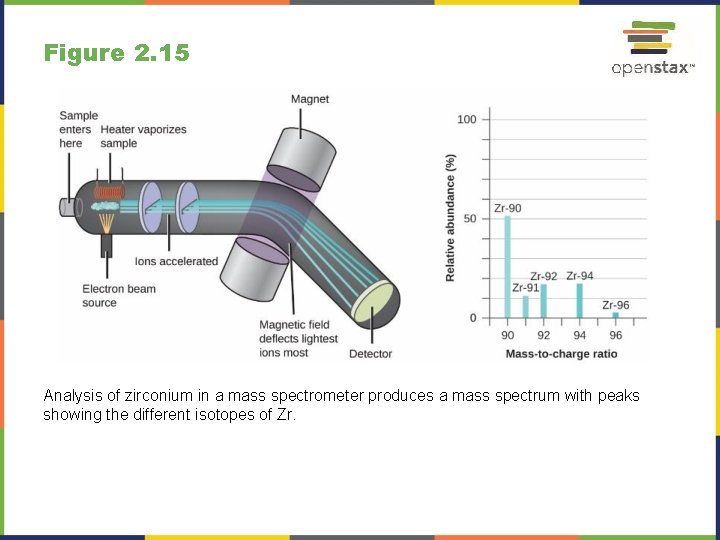
Figure 2. 15 Analysis of zirconium in a mass spectrometer produces a mass spectrum with peaks showing the different isotopes of Zr.

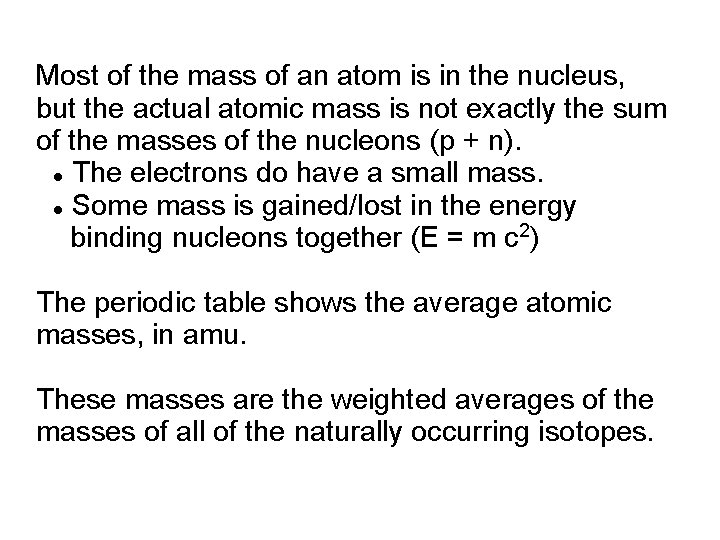
Most of the mass of an atom is in the nucleus, but the actual atomic mass is not exactly the sum of the masses of the nucleons (p + n). The electrons do have a small mass. Some mass is gained/lost in the energy binding nucleons together (E = m c 2) The periodic table shows the average atomic masses, in amu. These masses are the weighted averages of the masses of all of the naturally occurring isotopes.

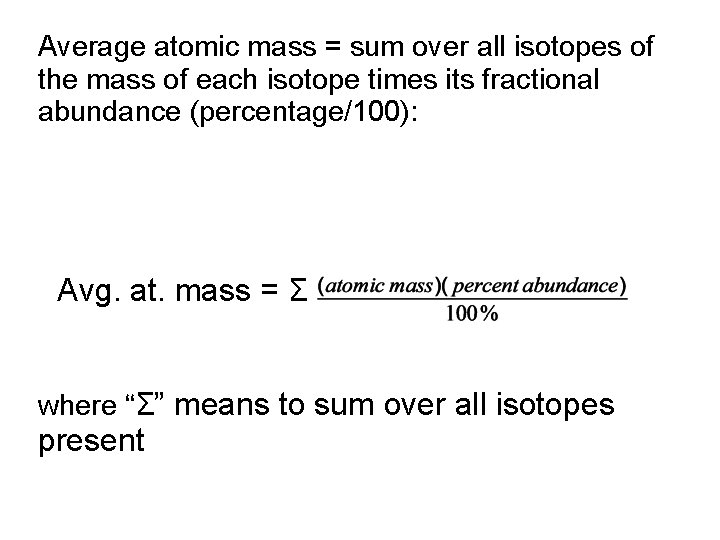
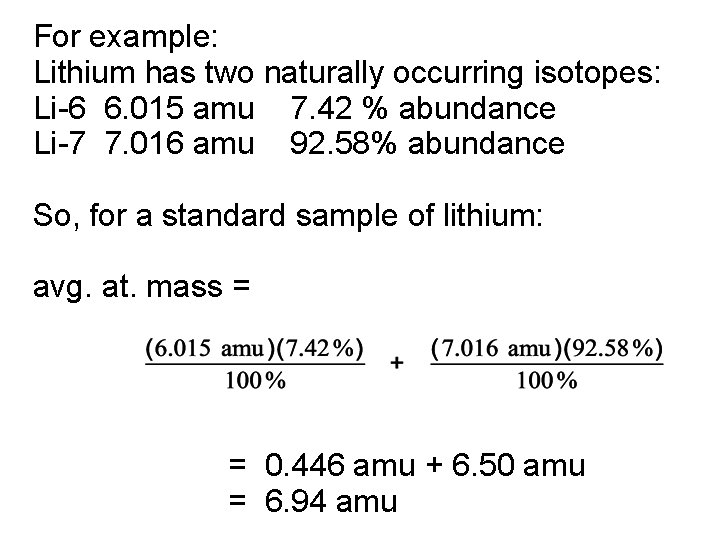
Average atomic mass = sum over all isotopes of the mass of each isotope times its fractional abundance (percentage/100): Avg. at. mass = Σ where “Σ” means to sum over all isotopes present

For example: Lithium has two naturally occurring isotopes: Li-6 6. 015 amu 7. 42 % abundance Li-7 7. 016 amu 92. 58% abundance So, for a standard sample of lithium: avg. at. mass = = 0. 446 amu + 6. 50 amu = 6. 94 amu

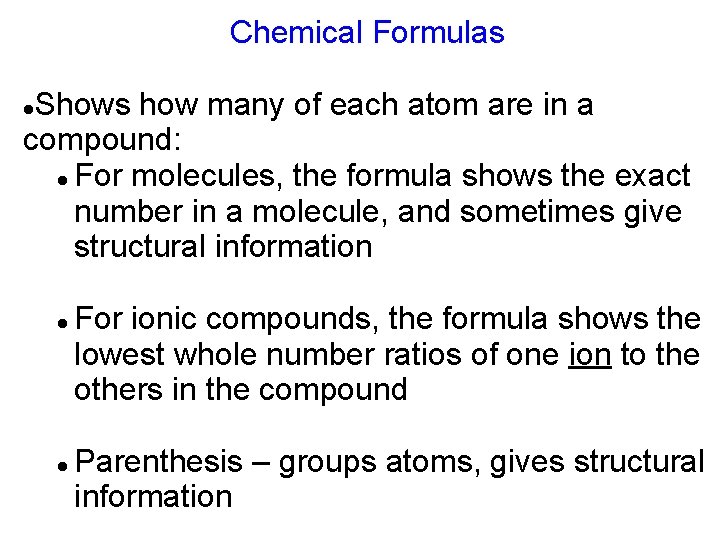
Chemical Formulas Shows how many of each atom are in a compound: For molecules, the formula shows the exact number in a molecule, and sometimes give structural information For ionic compounds, the formula shows the lowest whole number ratios of one ion to the others in the compound Parenthesis – groups atoms, gives structural information

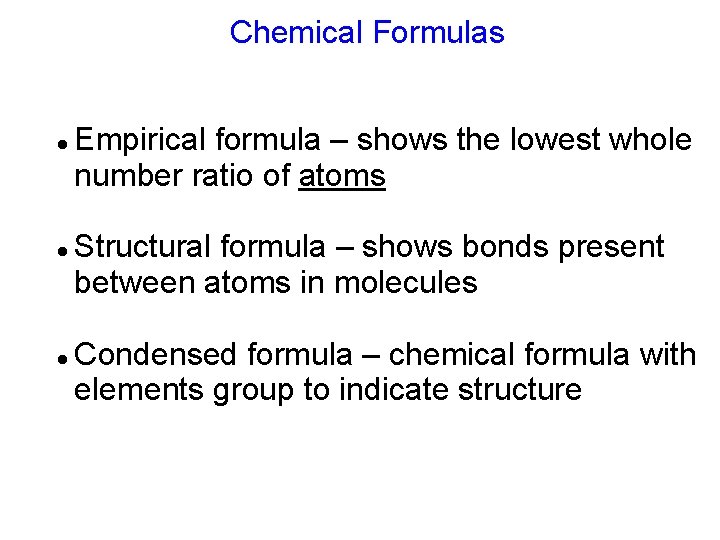
Chemical Formulas Empirical formula – shows the lowest whole number ratio of atoms Structural formula – shows bonds present between atoms in molecules Condensed formula – chemical formula with elements group to indicate structure
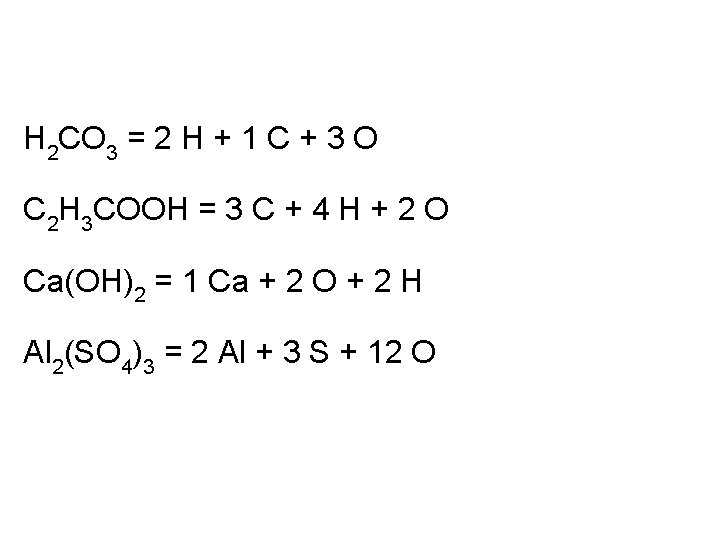
H 2 CO 3 = 2 H + 1 C + 3 O C 2 H 3 COOH = 3 C + 4 H + 2 O Ca(OH)2 = 1 Ca + 2 O + 2 H Al 2(SO 4)3 = 2 Al + 3 S + 12 O

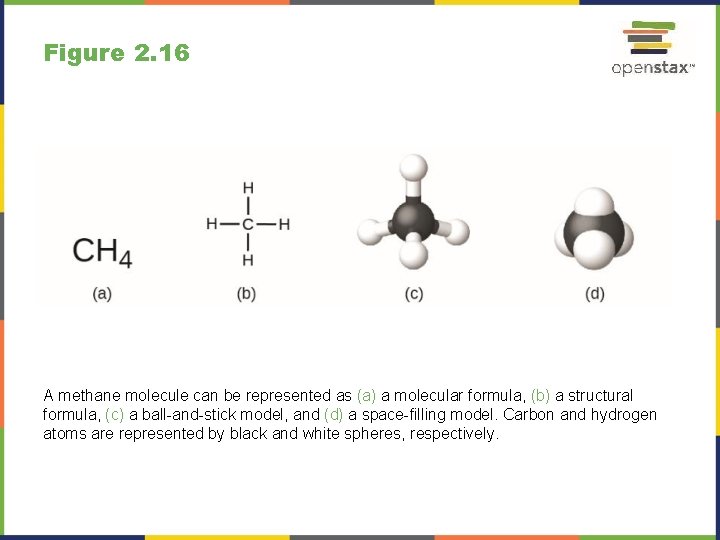
Figure 2. 16 A methane molecule can be represented as (a) a molecular formula, (b) a structural formula, (c) a ball-and-stick model, and (d) a space-filling model. Carbon and hydrogen atoms are represented by black and white spheres, respectively.

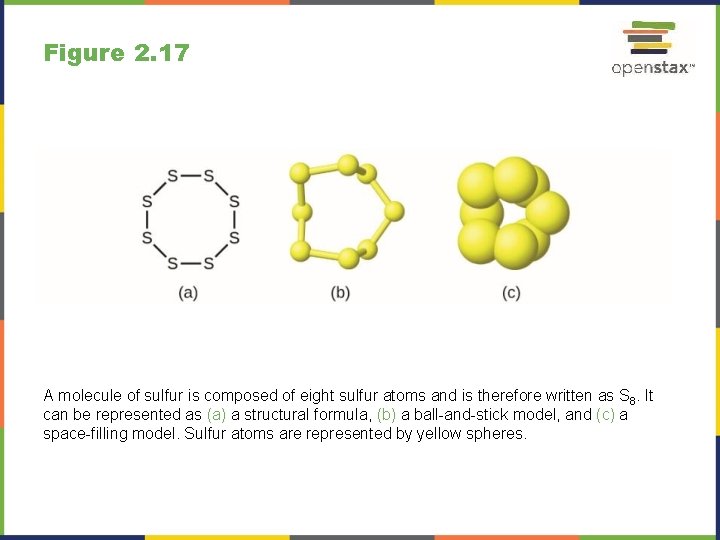
Figure 2. 17 A molecule of sulfur is composed of eight sulfur atoms and is therefore written as S 8. It can be represented as (a) a structural formula, (b) a ball-and-stick model, and (c) a space-filling model. Sulfur atoms are represented by yellow spheres.

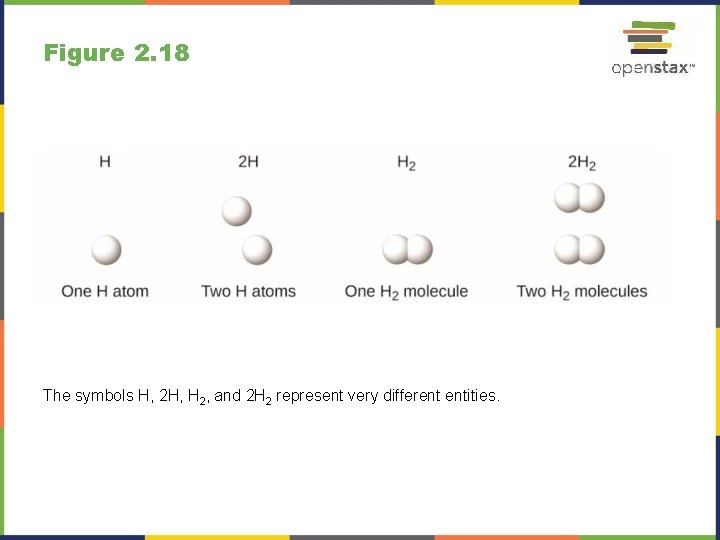
Figure 2. 18 The symbols H, 2 H, H 2, and 2 H 2 represent very different entities.

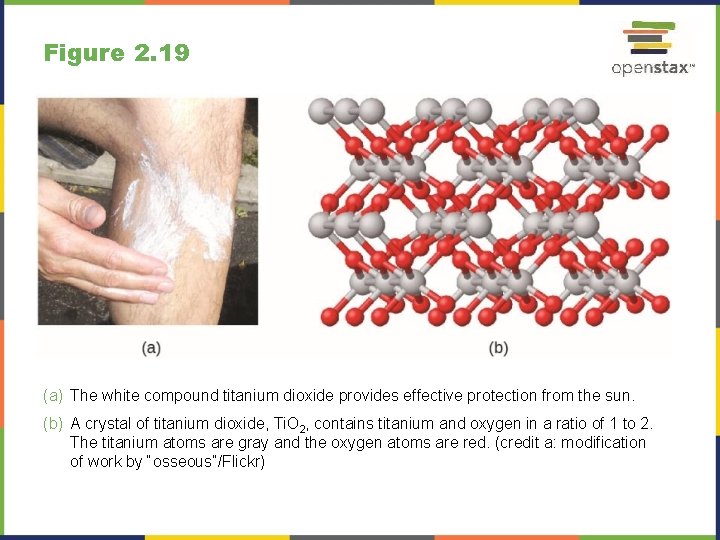
Figure 2. 19 (a) The white compound titanium dioxide provides effective protection from the sun. (b) A crystal of titanium dioxide, Ti. O 2, contains titanium and oxygen in a ratio of 1 to 2. The titanium atoms are gray and the oxygen atoms are red. (credit a: modification of work by “osseous”/Flickr)

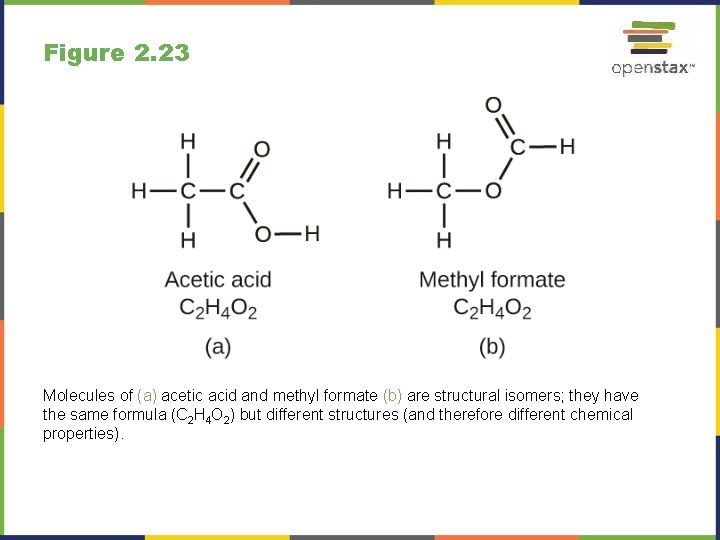
Figure 2. 23 Molecules of (a) acetic acid and methyl formate (b) are structural isomers; they have the same formula (C 2 H 4 O 2) but different structures (and therefore different chemical properties).

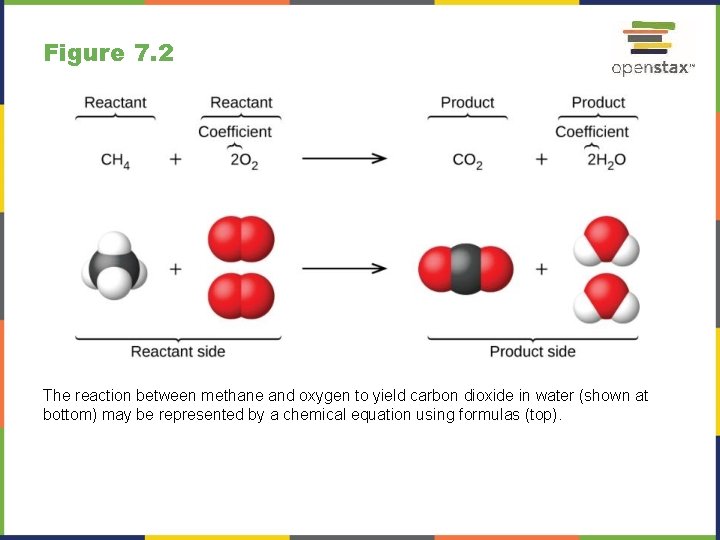
Figure 7. 2 The reaction between methane and oxygen to yield carbon dioxide in water (shown at bottom) may be represented by a chemical equation using formulas (top).

Figure 2. 27 The number of molecules in a single droplet of water is roughly 100 billion times greater than the number of people on earth. (credit: “tanakawho”/Wikimedia commons)

Figure 2. 25 Each sample contains 6. 022 × 1023 atoms — 1. 00 mol of atoms. From left to right (top row): 65. 4 g zinc, 12. 0 g carbon, 24. 3 g magnesium, and 63. 5 g copper. From left to right (bottom row): 32. 1 g sulfur, 28. 1 g silicon, 207 g lead, and 118. 7 g tin. (credit: modification of work by Mark Ott)

Figure 2. 26 Each sample contains 6. 02 × 1023 molecules or formula units— 1. 00 mol of the compound or element. Clock-wise from the upper left: 130. 2 g of C 8 H 17 OH (1 -octanol, formula mass 130. 2 amu), 454. 9 g of Hg. I 2 (mercury(II) iodide, formula mass 459. 9 amu), 32. 0 g of CH 3 OH (methanol, formula mass 32. 0 amu) and 256. 5 g of S 8 (sulfur, formula mass 256. 6 amu). (credit: Sahar Atwa)

Atomic mass – Actual mass of an atom slightly different than mass # due to electrons and nuclear binding energy Average atomic mass Most elements in nature are composed of multiple isotopes The decimal value on the periodic table gives the weighted average of these isotopes in amu (atomic mass units)

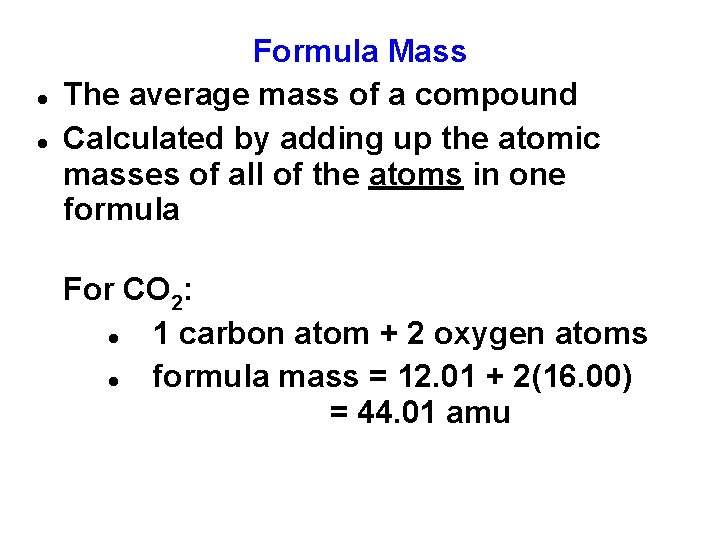
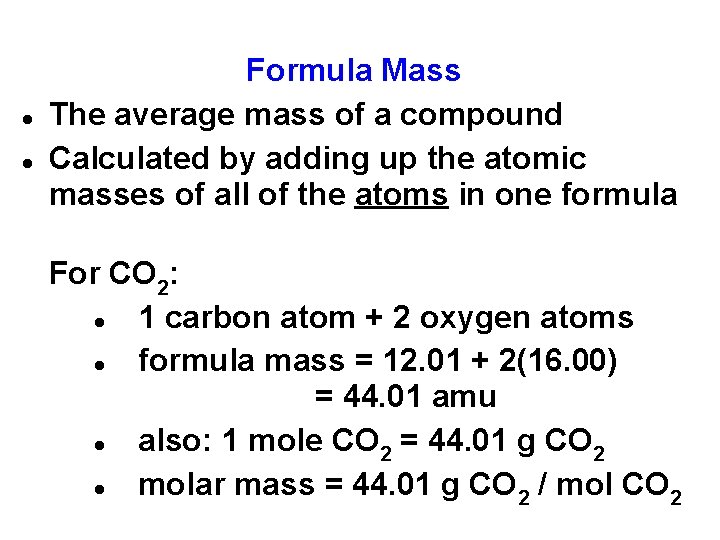
Formula Mass The average mass of a compound Calculated by adding up the atomic masses of all of the atoms in one formula For CO 2: 1 carbon atom + 2 oxygen atoms formula mass = 12. 01 + 2(16. 00) = 44. 01 amu

If the atomic mass of an isotope is known, then any mass of that isotope can be converted to a number of atoms If the average mass of an element is known, then the number of atoms can also be calculated If the mass of a molecule is known, the we can also calculate the number of molecules in a sample from the mass

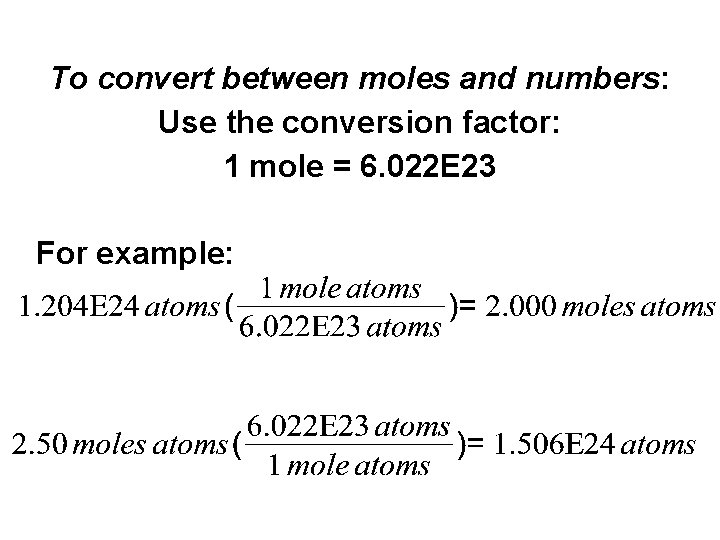
The Mole The key to converting between mass and numbers is the mole 'Mole' is just a number 1 mole (mol) = 6. 022 E 23 (Avogadro's #) We can have a mole of anything: atoms molecules grains of sand. .

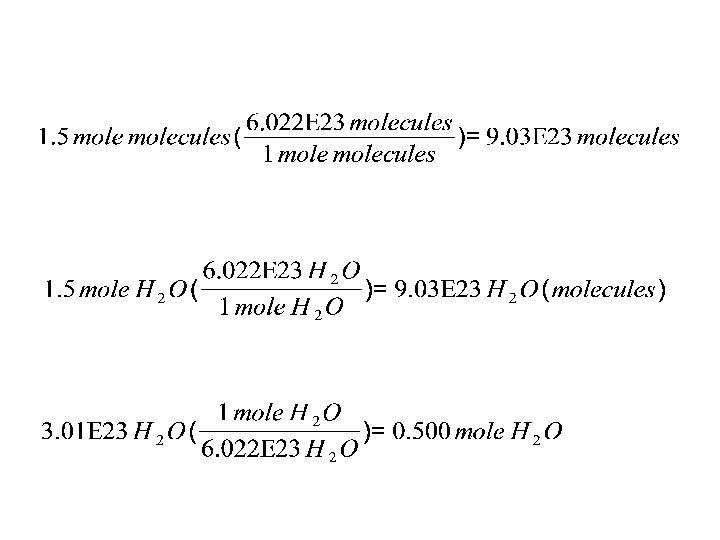
To convert between moles and numbers: Use the conversion factor: 1 mole = 6. 022 E 23 For example:


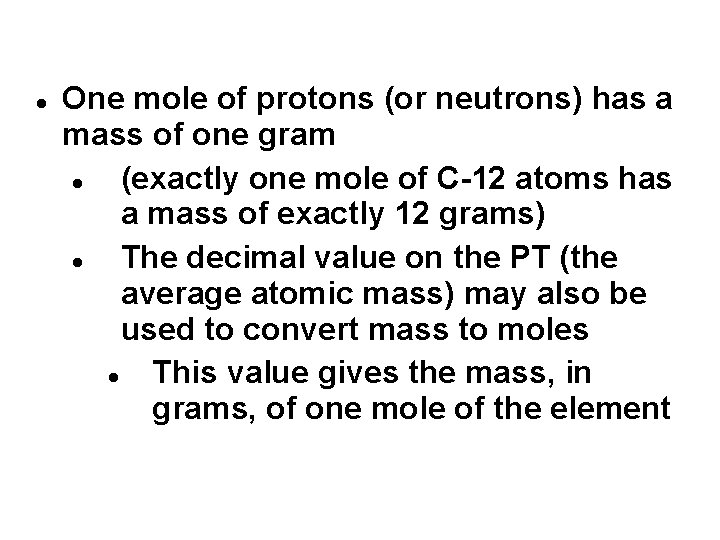
One mole of protons (or neutrons) has a mass of one gram (exactly one mole of C-12 atoms has a mass of exactly 12 grams) The decimal value on the PT (the average atomic mass) may also be used to convert mass to moles This value gives the mass, in grams, of one mole of the element


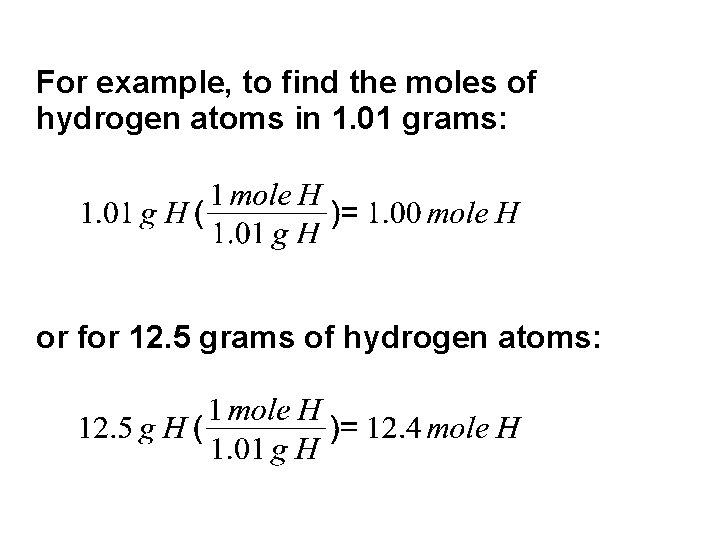
For example, to find the moles of hydrogen atoms in 1. 01 grams: or for 12. 5 grams of hydrogen atoms:

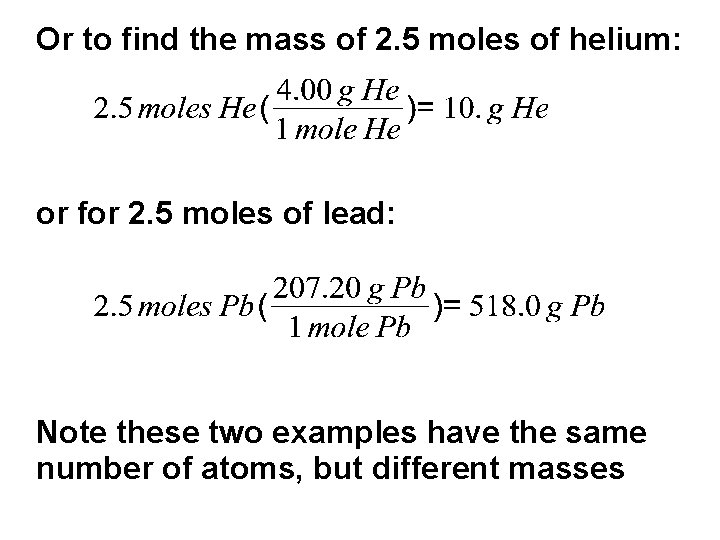
Or to find the mass of 2. 5 moles of helium: or for 2. 5 moles of lead: Note these two examples have the same number of atoms, but different masses

Formula Mass The average mass of a compound Calculated by adding up the atomic masses of all of the atoms in one formula For CO 2: 1 carbon atom + 2 oxygen atoms formula mass = 12. 01 + 2(16. 00) = 44. 01 amu also: 1 mole CO 2 = 44. 01 g CO 2 molar mass = 44. 01 g CO 2 / mol CO 2

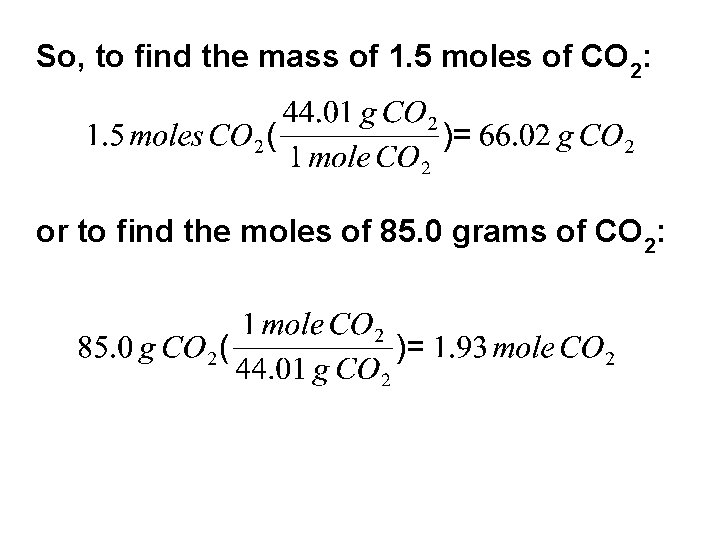
So, to find the mass of 1. 5 moles of CO 2: or to find the moles of 85. 0 grams of CO 2:

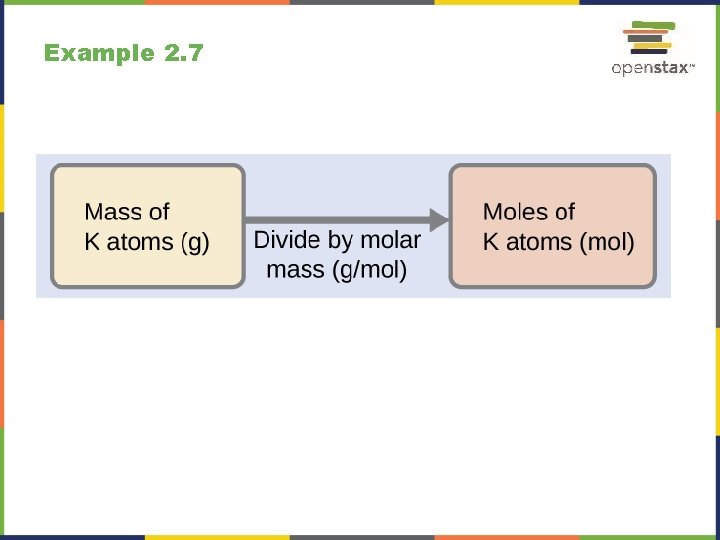
Example 2. 7

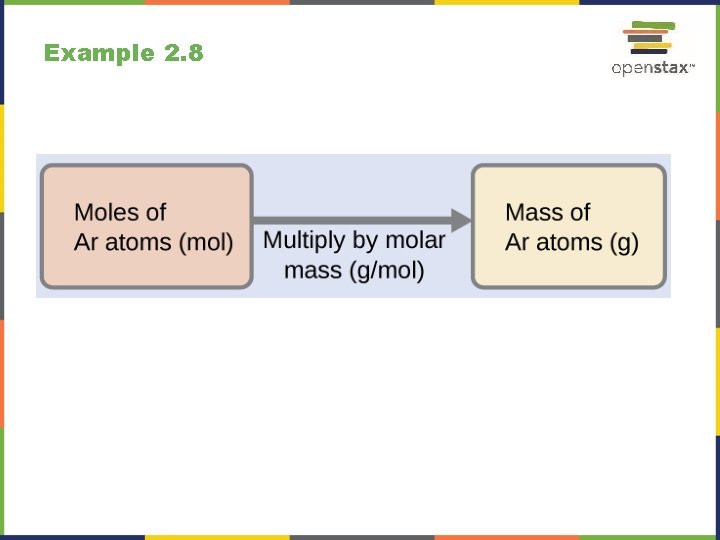
Example 2. 8

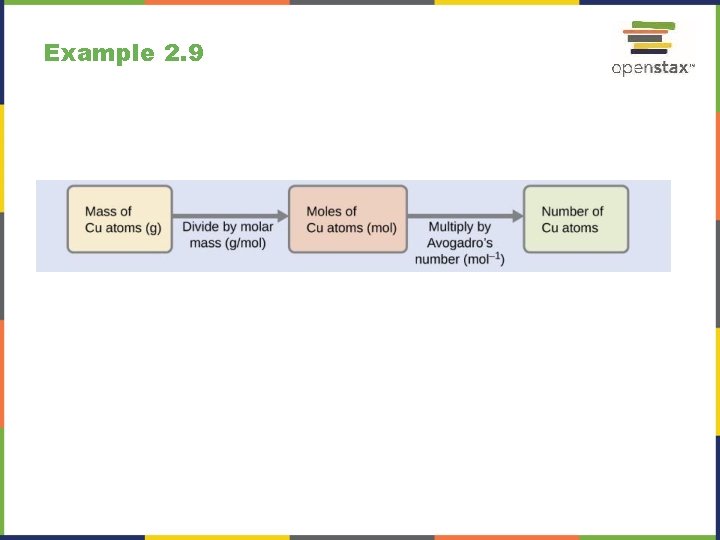
Example 2. 9
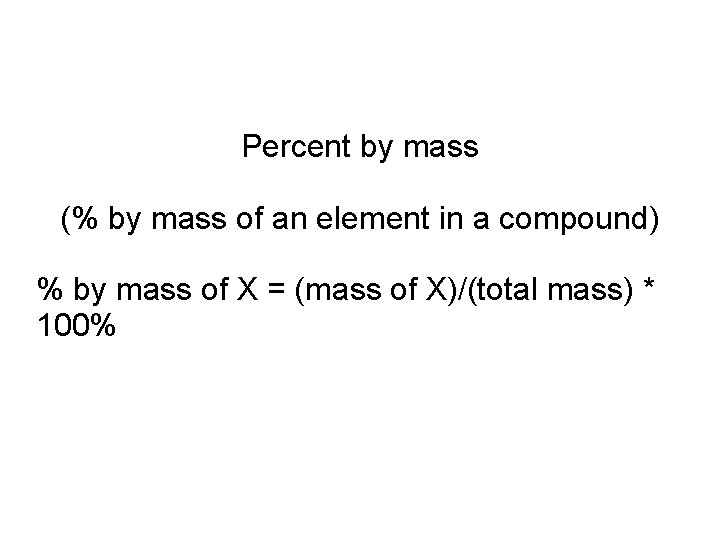
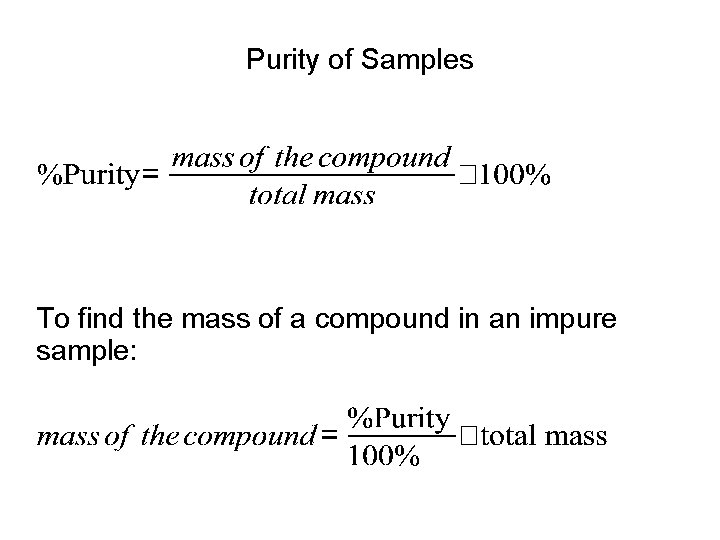
Percent by mass (% by mass of an element in a compound) % by mass of X = (mass of X)/(total mass) * 100%

Purity of Samples To find the mass of a compound in an impure sample:

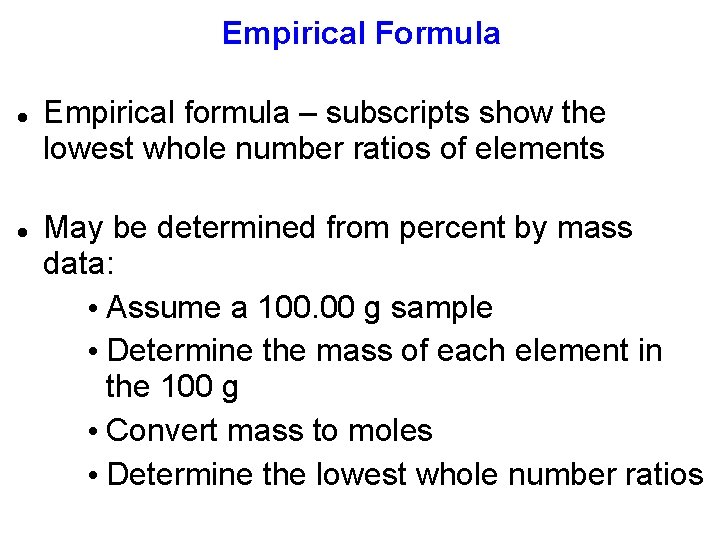
Empirical Formula Empirical formula – subscripts show the lowest whole number ratios of elements May be determined from percent by mass data: • Assume a 100. 00 g sample • Determine the mass of each element in the 100 g • Convert mass to moles • Determine the lowest whole number ratios

For binary ionic compounds, the empirical formula is the chemical formula. For molecular compounds, more data is needed to determine the molecular formula – The molar mass usually needs to be determined – This molar mass will be a multiple of the molar mass of the empirical formula – Divide the molecules molar mass by the empirical formulas molar mass – Multiply the subscripts by this integer to get the molecular formula

This Open. Stax ancillary resource is © Rice University under a CC-BY 4. 0 International license; it may be reproduced or modified but must be attributed to Open. Stax, Rice University and any changes must be noted.