Atoms and Molecules What is an atom Atoms





- Slides: 23

Atoms and Molecules

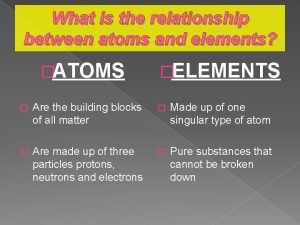
What is an atom? • Atoms are the basic building blocks of matter that make up everyday objects (smallest unit of matter) • A desk, the air, even you are made up of atoms!

Can we see it? • We cannot see atoms with just our eyes or even with glasses • We need a very high powered microscope to see an atom

What is an atom? • The word “atom” means indivisible, meaning it cannot be broken down any further

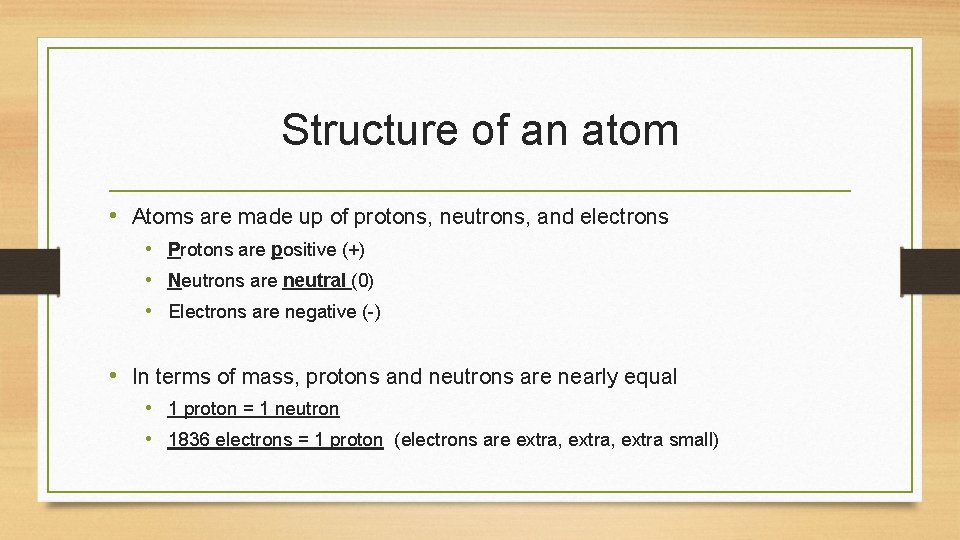
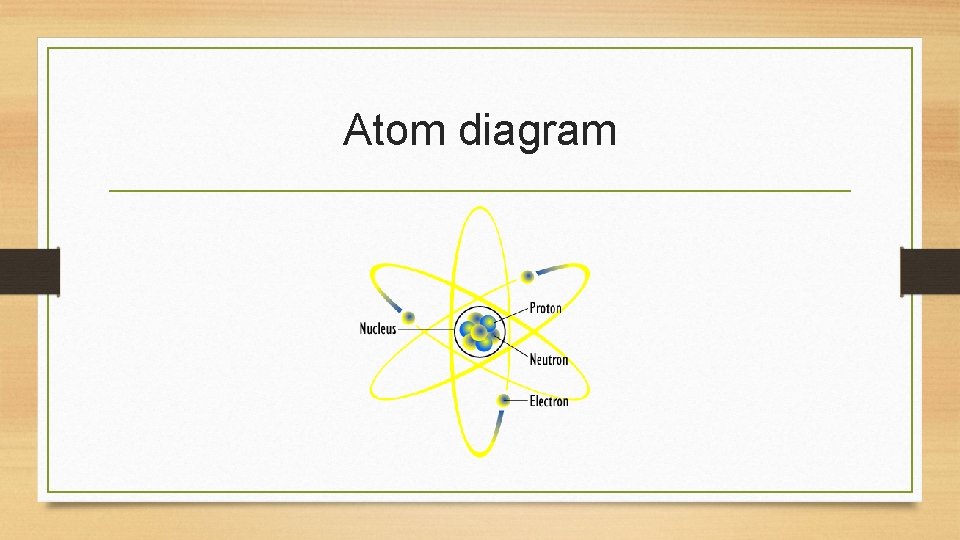
Structure of an atom • Atoms are made up of protons, neutrons, and electrons • Protons are positive (+) • Neutrons are neutral (0) • Electrons are negative (-) • In terms of mass, protons and neutrons are nearly equal • 1 proton = 1 neutron • 1836 electrons = 1 proton (electrons are extra, extra small)

Structure of an atom • The nucleus is the central or middle part of atoms • The nucleus is made up of protons and neutrons • Question: If the nucleus is always made up of protons and neutrons, what charge does the nucleus always hold?

Structures of an atom • Electrons are outside of the nucleus, orbiting in their electron shells or orbitals • Electrons move faster than the speed of light!!! • In 8 th Grade Science, you need to know that there always the same number of electrons as protons • So…. An atom has a neutral charge (protons and electrons cancel each other)

Atom diagram

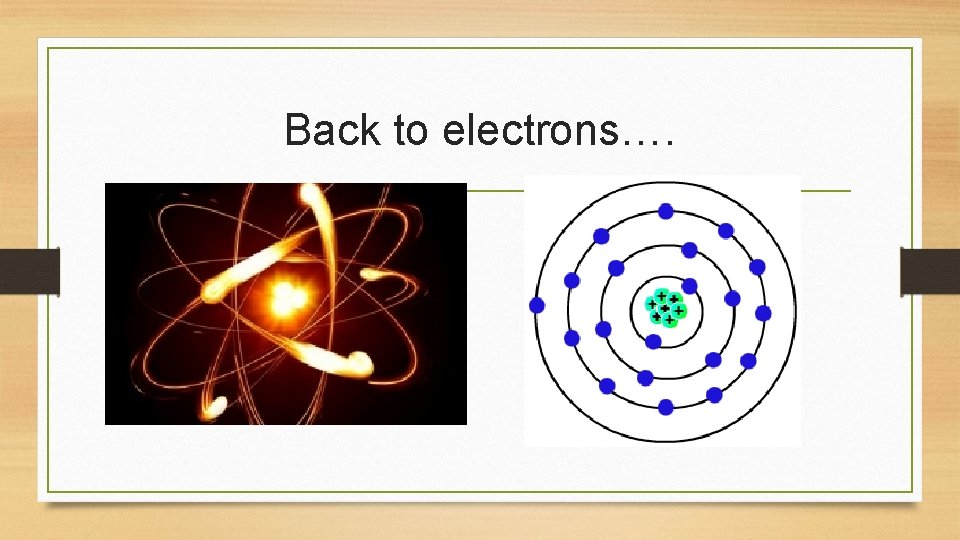
Back to electrons….

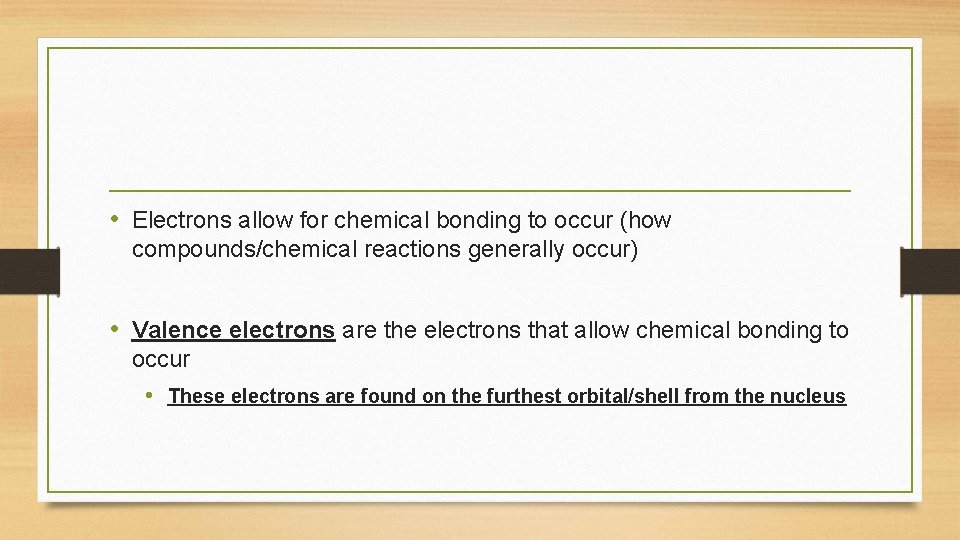
• Electrons allow for chemical bonding to occur (how compounds/chemical reactions generally occur) • Valence electrons are the electrons that allow chemical bonding to occur • These electrons are found on the furthest orbital/shell from the nucleus

How many valence electrons do we have here (in the element Sodium, or salt)?

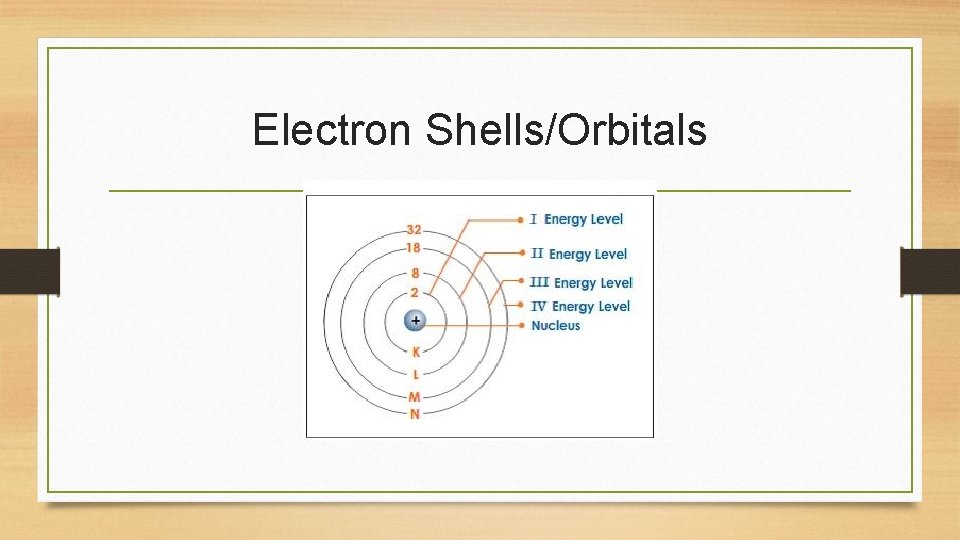
Electron Shells/Orbitals • Electron shells can only hold so many electrons • • Shell 1 - 2 electrons Shell 2 - 8 electrons Shell 3 - 18 electrons Shell 4 - 32 electrons • Atoms always keep trying to fill their shells!!!

Electron Shells/Orbitals

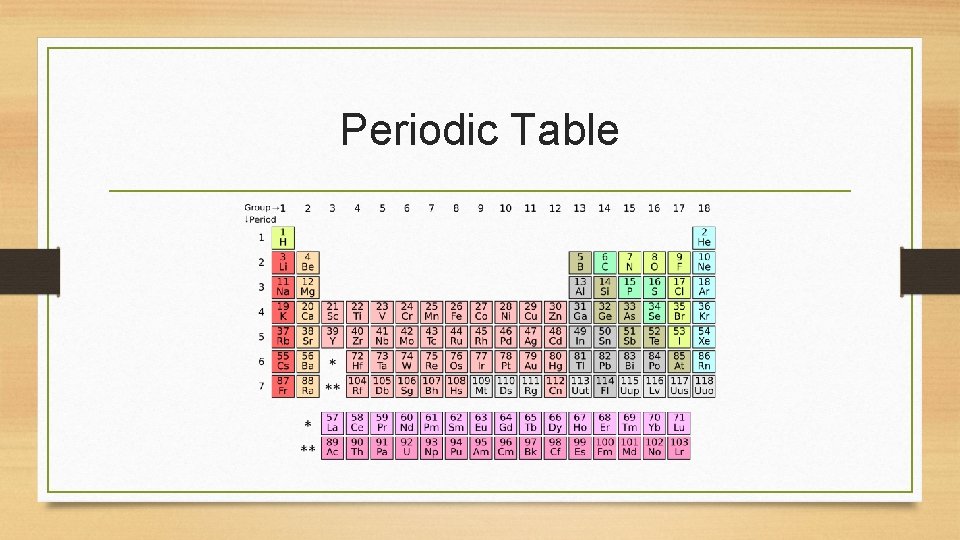
Periodic Table

How is the periodic table arranged? • • We read the periodic table like a book, or a magazine We read left to right (horizontally) Thus, moving from left to right, atomic numbers always increase by 1 Elements that are found in the same period (row/horizontal/left to right) have the same number of electron shells/orbitals • Elements that are found in the same group (vertical/column/up and down) have the same number of valence electrons • The periodic table is very useful for learning about chemistry and elements!!

What is an element? • An element is a substance that is made entirely from one type of atom. • Hydrogen, helium, carbon, and nitrogen are a few to name • There are 118 elements known! Most of these are natural occurring

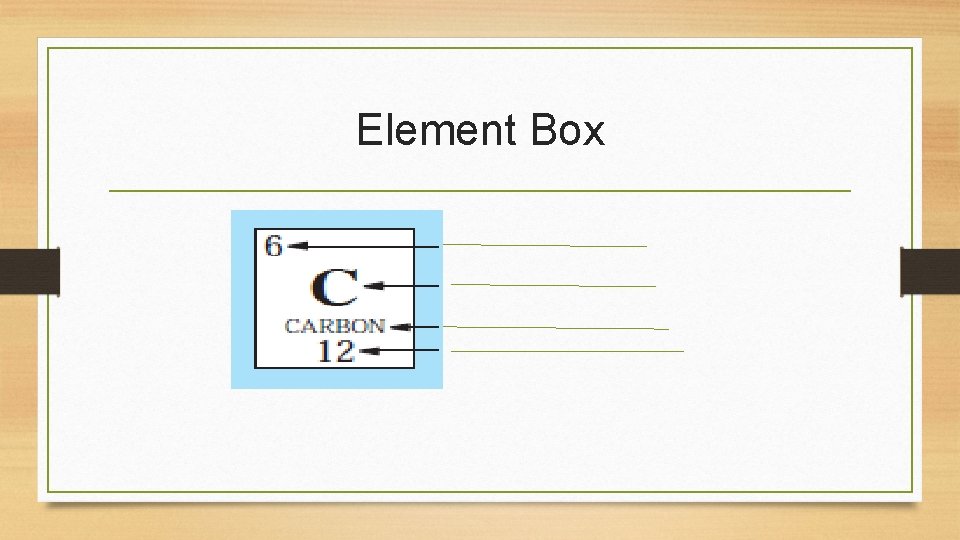
Element Box

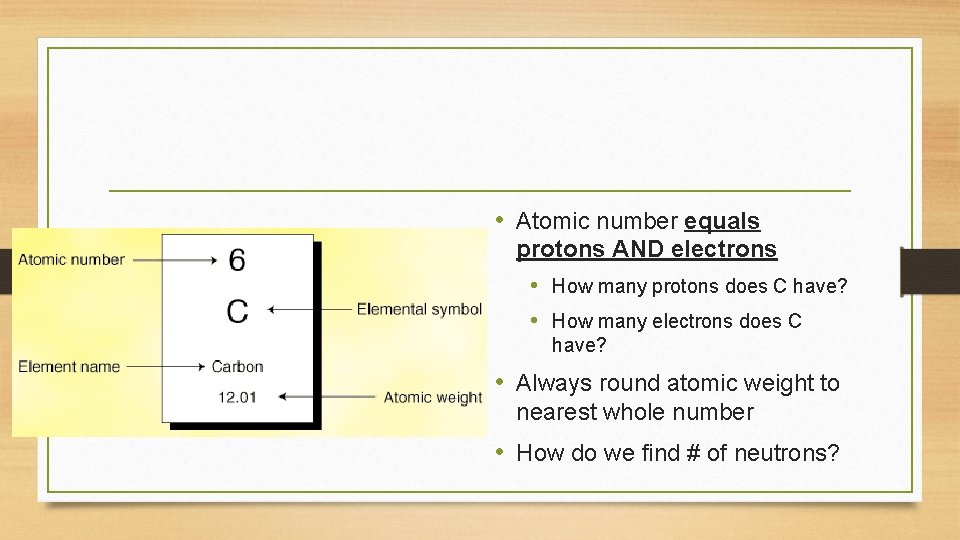
• Atomic number equals protons AND electrons • How many protons does C have? • How many electrons does C have? • Always round atomic weight to nearest whole number • How do we find # of neutrons?

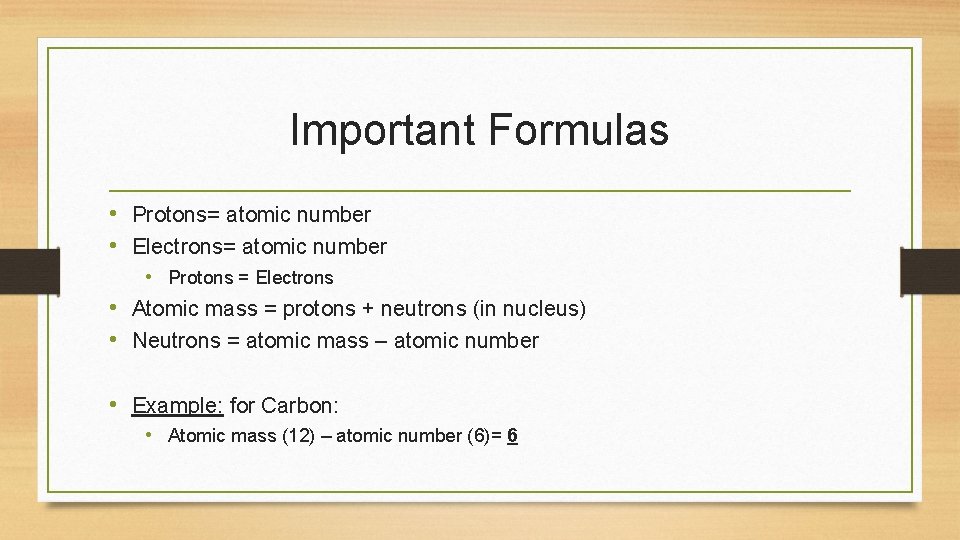
Important Formulas • Protons= atomic number • Electrons= atomic number • Protons = Electrons • Atomic mass = protons + neutrons (in nucleus) • Neutrons = atomic mass – atomic number • Example: for Carbon: • Atomic mass (12) – atomic number (6)= 6

Remember one of ATOM’s rules • Atoms are always neutral because there an equal amount of protons and electrons • If we have an atomic number of 16, then we have 16 protons and 16 electrons, therefore our atom is neutral because protons (+) and electrons (-) cancel each other out

Element Box Practice • Draw & fill in a square for the element Neon. • Also, find the number of neutrons that exist in one atom of neon

Element Box Practice • Draw & fill in a square for the element Nickel. • Also, find the number of neutrons that exist in one atom of nickel

Element and Periodic Table Practice • http: //education. jlab. org/elementmath/
 Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules What is the relationship between atoms and elements
What is the relationship between atoms and elements Solution
Solution How can you count atoms and molecules
How can you count atoms and molecules Ion chapter 11
Ion chapter 11 Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules Collision theory states that
Collision theory states that Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Atoms combine to form
Atoms combine to form The lowest allowable energy state of an atom is called
The lowest allowable energy state of an atom is called Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Regents periodic table
Regents periodic table The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Democritus dalton
Democritus dalton Cecil writes the equation for the reaction of hydrogen
Cecil writes the equation for the reaction of hydrogen Solid liquid gas examples
Solid liquid gas examples Absolute configuration
Absolute configuration Polar and nonpolar molecules similarities
Polar and nonpolar molecules similarities Which sequence of organisms represents a food chain
Which sequence of organisms represents a food chain Shapes and polarity of molecules
Shapes and polarity of molecules