Asthma Update Suneel Kumar MD Definition of Asthma






























































- Slides: 78

Asthma Update Suneel Kumar MD

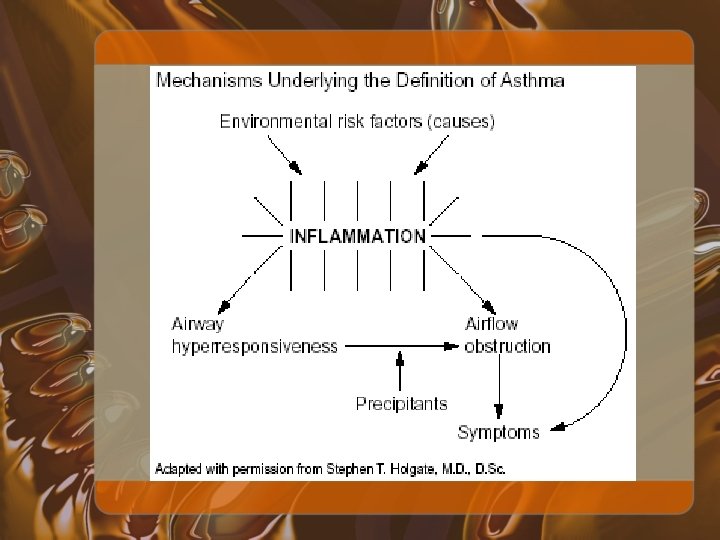
Definition of Asthma • National Asthma Education and Prevention Program (NAEPP) defined as: – Airway obstruction that is reversible (but not completely so in some subjects), either spontaneously or with treatment – Airway inflammation – Increased airway responsiveness to a variety of stimuli

WHO Definition of Asthma • "A chronic inflammatory disorder of the airways in which many cells play a role, in particular mast cells, eosinophils, and T lymphocytes. In susceptible individuals this inflammation causes recurrent episodes of wheezing, breathlessness, chest tightness, and cough particularly at night and/or in the early morning. These symptoms are usually associated with widespread but variable airflow limitation that is at least partly reversible either spontaneously or with treatment. The inflammation also causes an associated increase in airway responsiveness to a variety of stimuli. "


Symptoms • • Cough Wheezing Chest tightness Shortness of breath





Update in Asthma • Current guidelines for treatment of asthma • Beta agonist use • Inhaled corticosteroids • Leukotriene modifiers • Chromones • Anti-Ig. E therapy • Use of exhaled nitric oxide in monitoring asthma • Asthma during pregnancy

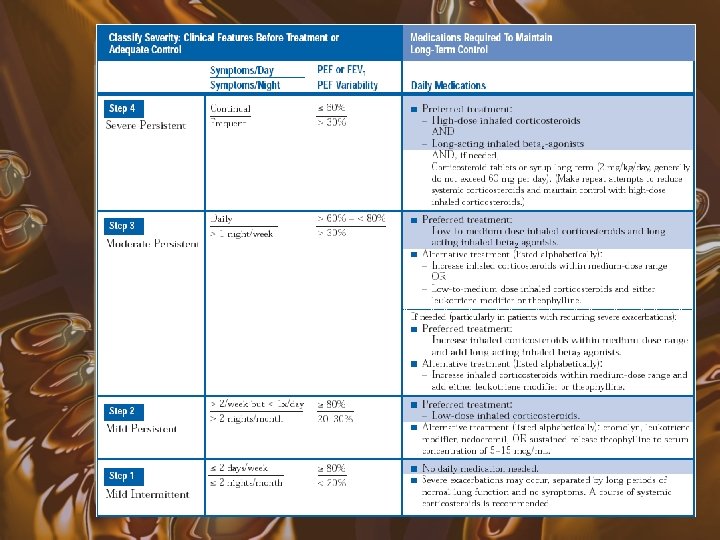
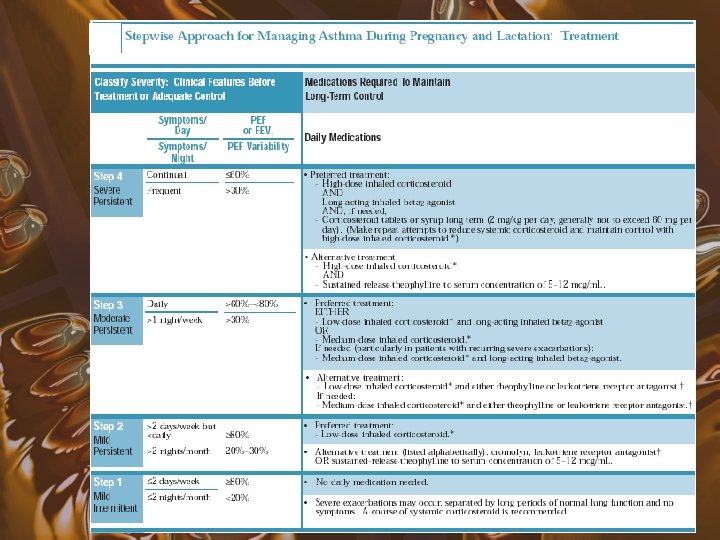
Current Guidelines for Treatment


Mild Intermittent Asthma • • Symptoms < 2 days/week Symptoms < 2 nights/month PEF or FEV 1 > 80% PEF variability < 20% No daily medication needed PRN beta agonists Course of systemic steroids for exacerbations

Mild Persistent Asthma • • • Symptoms > 2 days/wk but < 1 x/day > 2 nights/month PEF or FEV 1 > 80% PEF variability 20 -30% Preferred treatment low dose inhaled corticosteroids • Alternatives include cromolyn, leukotriene modifiers, necromodil, or sustained release theophylline

Moderate Persistent Asthma • • • Symptoms daily > 1 night/week PEF or FEV 1 > 60% and < 80% PEF variability > 30% Preferred treatment is low to medium dose inhaled corticosteroid and a long acting inhaled beta 2 agonist • Alternative includes increasing ICS within moderate dose range, or low to medium dose ICS with either leukotriene modifier or theophylline

Severe Persistent Asthma • • • Continual symptoms Frequent nocturnal attacks PEF or FEV 1 < 60% PEF variability > 30% Preferred treatment is high dose inhaled corticosteroid and long acting beta 2 agonists • If needed, can add systemic corticosteroids

Goals of Therapy • Minimal or no chronic symptoms day or night • Minimal or no exacerbations • No limitations on activities; no school/work missed • Maintain (near) normal pulmonary function • Minimal use of short-acting inhaled beta 2 agonist • Minimal or no adverse effects from medications

Stepwise Approach • Review treatment every 1 to 6 months, and gradually step down treatment • If asthma controlled not maintained, then a step up in treatment may be warranted

Reasons for Poor Asthma Control • • • Inhaler Technique Compliance Environment Also assess for an alternative diagnosis “All that wheezes is not asthma, and not all asthma wheezes”

Factors Affecting Compliance • Support of health care professional and family • Route of drug administration (inhaled vs. oral) • Complexity of drug regimens • Side effects of medications • $$ Cost $$

Beta 2 Agonists

Beta 2 Agonists • • Most potent and rapidly acting bronchodilators currently available for clinical use Given in different forms: – short acting = isoproterenol – intermediate acting = albuterol, metaproterenol, pirbuterol, levalbuterol, terbutaline (IV only), fenoterol [not available in the US] – long acting = salmeterol, formoterol

Mechanism of Action • Beta 2 agonists interact with beta 2 receptors on the surface of a variety of cells that may play a role in asthma pathogenesis • Beta agonists have the potential to relax bronchial smooth muscle, decrease mast cell mediator release, inhibit neutrophil, eosinophil, and lymphocyte functional responses, increase mucociliary transport, and affect vascular tone and edema formation

MDI with Spacer vs. Nebulizer • Equivalent bronchodilation can be achieved by giving beta 2 agonist with a spacer/holding chamber or by nebulizer therapy • Continuous administration with a nebulizer may be more effective in severely obstructed adults and in those who have difficulty with an MDI plus spacer

Chronic Use of Beta Agonists • Arguments against chronic use: – Mortality may be increased – Control of asthma may worsen – Equal or superior efficacy can be achieved with inhaled corticosteroids

Increased Mortality with Chronic Use? • A case-control study using linked health insurance databases in Saskatchewan, Canada* • Increased risk of death or near-death from asthma was associated with the regular use of inhaled beta agonists, especially fenoterol (adjusted OR 6. 1; 4. 1 for albuterol) • Did not appear to be confounded by asthma severity, and there was no relation to non–asthma mortality *Spitzer et al, NEJM Feb 1992; 326(8): 501 -6

Increased Mortality with Chronic Use? • However, increased odds ratios were also noted for theophylline (OR 2. 4) and oral corticosteroids (OR 2. 5) • The case-control design precludes the establishment of causality *Spitzer et al, NEJM Feb 1992; 326(8): 501 -6

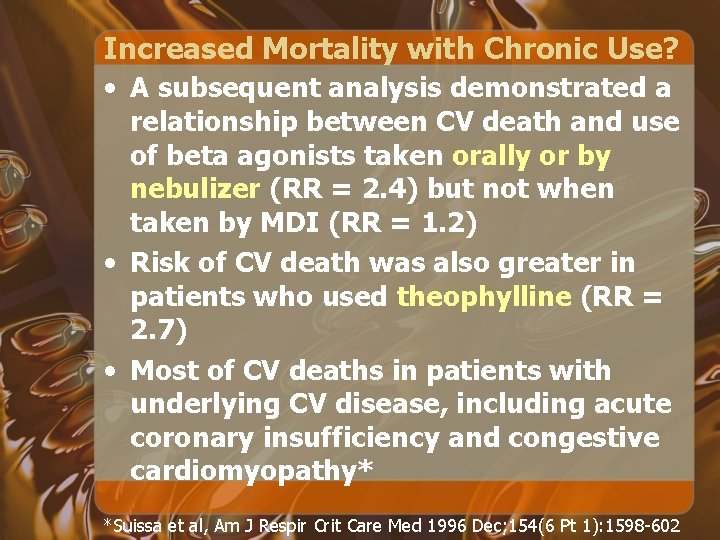
Increased Mortality with Chronic Use? • A subsequent analysis demonstrated a relationship between CV death and use of beta agonists taken orally or by nebulizer (RR = 2. 4) but not when taken by MDI (RR = 1. 2) • Risk of CV death was also greater in patients who used theophylline (RR = 2. 7) • Most of CV deaths in patients with underlying CV disease, including acute coronary insufficiency and congestive cardiomyopathy* *Suissa et al, Am J Respir Crit Care Med 1996 Dec; 154(6 Pt 1): 1598 -602

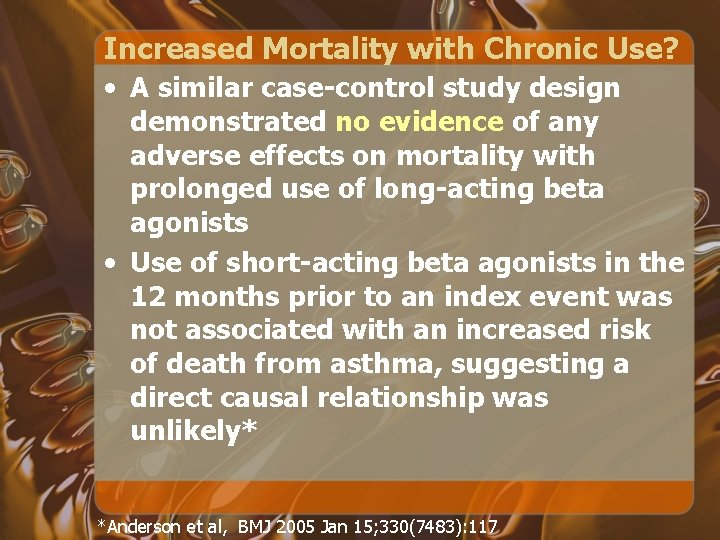
Increased Mortality with Chronic Use? • A similar case-control study design demonstrated no evidence of any adverse effects on mortality with prolonged use of long-acting beta agonists • Use of short-acting beta agonists in the 12 months prior to an index event was not associated with an increased risk of death from asthma, suggesting a direct causal relationship was unlikely* *Anderson et al, BMJ 2005 Jan 15; 330(7483): 117

Long Acting Beta Agonist Monotherapy • Prolonged treatment with long-acting beta agonists and without inhaled corticosteroids has been associated with increased mortality • 28 -week placebo-controlled trial assessing the safety of the long-acting beta agonist salmeterol enrolled over 25, 000 patients, but was stopped early when interim analysis revealed a significantly increased risk of death in those not taking concomitant inhaled corticosteroids and, independent of steroid therapy, in African-American patients* *"The Pink Sheet" FDC Reports. Chevy Chase, MD. 2003; 65(4): 10

Tolerance to Beta Agonists • More frequent in chronic use • Induced more with oral rather than inhaled preparations • Tolerance to long acting beta agonists may or may not occur (conflicting data)

Corticosteroids

Glucocorticoids • Most potent antiinflammatory agents available for the treatment of asthma • More effective than beta agonists, theophylline, and cromolyn sodium in reducing airway hyperresponsiveness during maintenance therapy

Glucocorticoid Mechanisms • Alleviating airway inflammation • Reducing collagen and tenascin deposition, two features associated with airway remodeling • Inhibit the synthesis of almost all known cytokines • Alteration of the number and availability of circulating leukocytes • Reduction in vascular permeability • Inhibition of mediator synthesis and release

Steroids and Long Acting Beta Agonists • Results in greater improvements in lung function and symptom control than monotherapy with escalating doses of inhaled glucocorticoid • Act synergistically to activate transcription factors, decrease smooth muscle proliferation, and impair eosinophil adhesion

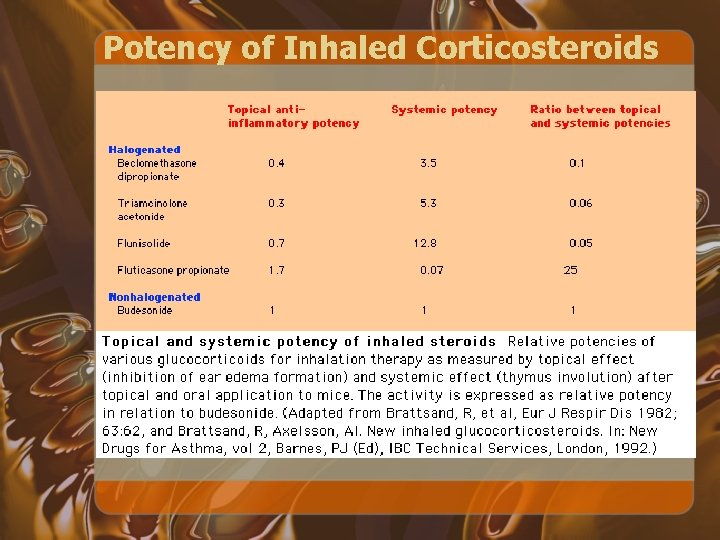
Potency of Inhaled Corticosteroids

Can ICS Cause Osteoporosis? • Three year prospective study looked at dose of inhaled triamcinolone and rate of bone loss in premenopausal women with asthma • There was a dose-related decline in bone density in the total hip and trochanter • No dose-related decline in the femoral neck or spine* *Israel et al, NEJM Sept 2001; 345: 941 -47

Leukotriene Modifiers

Leukotriene Modification • 5 -lipoxygenase pathway is a series of biochemical reactions that result in the transformation of arachidonic acid into leukotrienes • Produced by eosinophils and mast cells when appropriately activated


Leukotriene Modifiers • Zafirlukast (Accolate) and montelukast (Singulair) are inhibitors of the action of LTD 4 at its receptor • Zileuton (Zyflo) is an inhibitor of 5 lipoxygenase • All are oral

Leukotriene Modifiers • Superior effect when compared to placebo in the treatment of patients with mild to moderate asthma • Associated with both a significant decrease in the need for rescue beta agonist therapy and improved asthma symptoms, especially at night • Similar in magnitude to those achieved with inhaled steroids given at recommended doses

Montelukast vs. Beclomethasone • Both agents demonstrated similar efficacy with respect to preventing asthma exacerbations in moderate persistent asthma • However, beclomethasone was significantly superior to montelukast in its capacity to improve objective measures of lung function as measured by average responses

Cause of Churg-Strauss? • Rarely (1 out of 25, 000 to 150, 000 patient-years of treatment) Churg. Strauss vasculitis has been seen in patients started on leukotriene modifiers who were on systemic steroids • It is unlikely that this is a direct effect of leukotriene inhibition in these patients, and is more probably due to preexisting CSS unmasked as a result of steroid withdrawal

Chromones

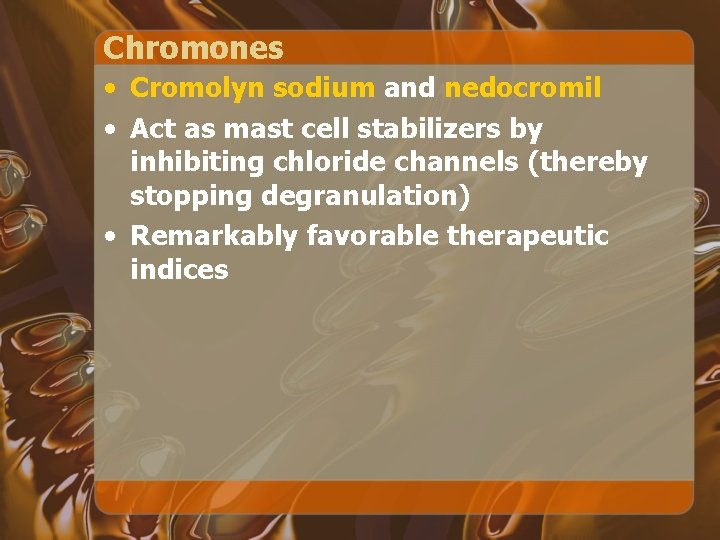
Chromones • Cromolyn sodium and nedocromil • Act as mast cell stabilizers by inhibiting chloride channels (thereby stopping degranulation) • Remarkably favorable therapeutic indices

Use of Chromones • Children and young adults with mild to moderate asthma requiring an antiinflammatory agent • Patients with a strong allergic component to their asthma • Patients with exercise-induced bronchospasm • Patients who require an antiinflammatory agent but for whom ICS are contraindicated or undesirable

Anti-Ig. E Therapy

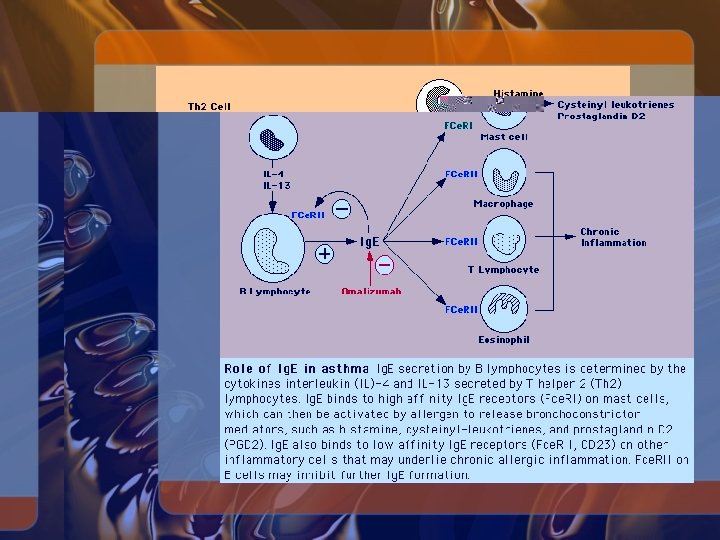
Anti-Ig. E Therapy • Most asthmatic patients have elevated circulating Ig. E concentrations when levels are adjusted for age • Ig. E is produced by B lymphocytes

Anti-Ig. E Therapy • Recombinant humanized antibody omalizumab (Xolair) binds Ig. E with high affinity • Developed for the treatment of allergic diseases • Binds to the C-epsilon-3 domain of circulating Ig. E but does not bind to Fcepsilon-RI, and therefore does not activate mast cells or basophils • Specific to Ig. E; does not bind to Ig. G or Ig. A


Omalizumab • SQ injection every 2 to 4 weeks • Dose determined by levels of serum Ig. E • Considered as an add-on therapy to reduce or discontinue treatment with oral corticosteroids • May also be indicated in patients who have severe allergic symptoms of asthma and rhinitis and who have very high circulating levels of Ig. E

Omalizumab • Circulating Ig. E-omalizumab complexes are small • No evidence of immune complex formation • Elimination half-life is 1 to 4 weeks • Aerosolized omalizumab (10 mg) is ineffective in protecting against allergen challenge and has no effect on circulating Ig. E

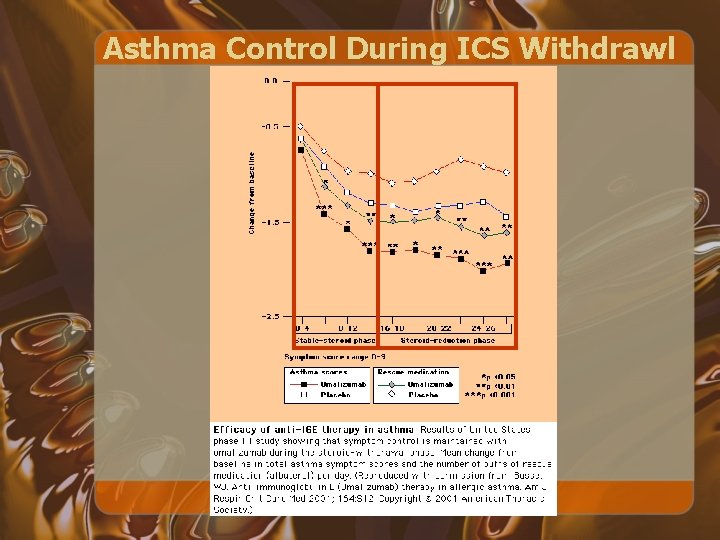
Asthma Control During ICS Withdrawl

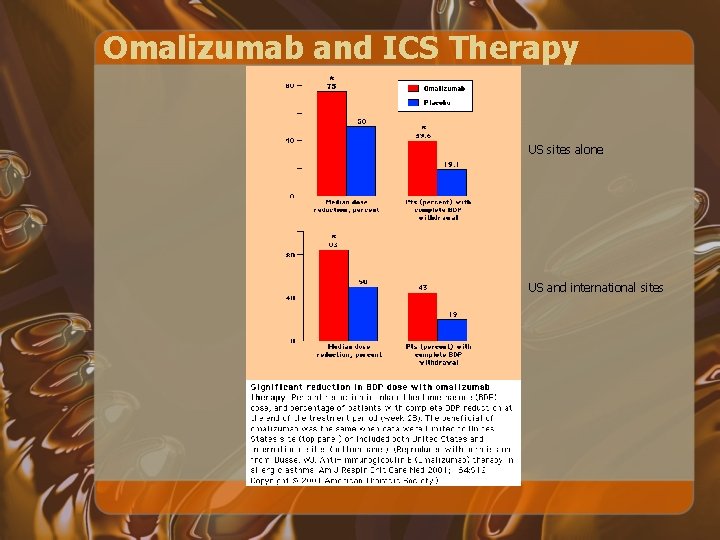
Omalizumab and ICS Therapy US sites alone US and international sites

Omalizumab • Necessary to obtain almost complete disappearance of circulating Ig. E in order to achieve clinical efficacy • Dose of 150 to 375 mcg every 2 to 4 weeks • Peak serum concentration is reached in 7 to 8 days • Should be treated for a minimum of 12 weeks • Cost is $10, 000 to $12, 000 per year • Well tolerated

Exhaled Nitric Oxide

Nitric Oxide Effects in the Lung • Regulates vascular and bronchial tone, promoting dilation of both vessels and airway • Helps to facilitate the coordinated beating of ciliated epithelial cells • Important neurotransmitter for non-adrenergic, non-cholinergic neurons which run in the bronchial wall

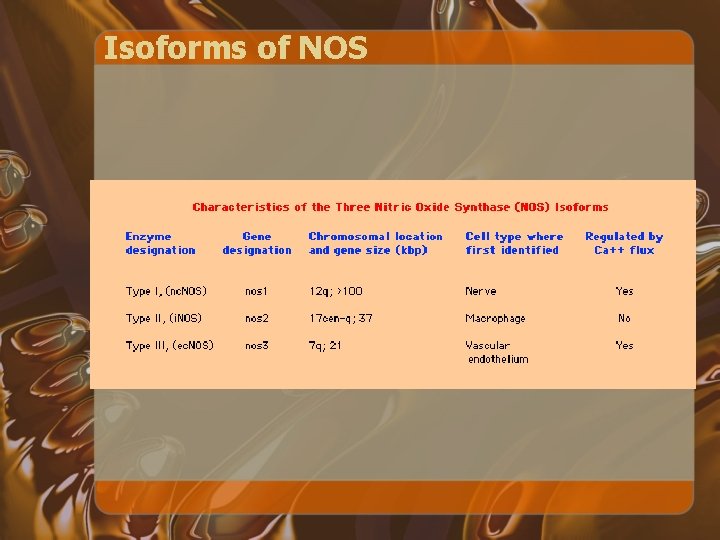
Isoforms of NOS

NO Formation • n. NOS and e. NOS are usually constitutively active and produce low amounts of NO • i. NOS has the capacity to generate large quantities of NO when transcriptionally upregulated by the inflammatory cytokines TNF alpha, IL-1 beta, and IFN-gamma

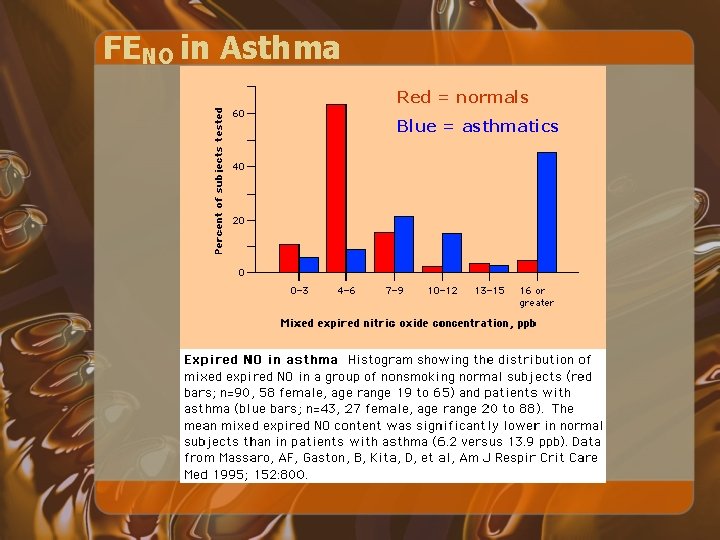
FENO in Asthma Red = normals Blue = asthmatics

FENO • Asthmatics have higher FENO than do similar non-asthmatic subjects • The magnitude of FENO in increased in proportion to bronchial wall inflammation, induced-sputum eosinophils, and airway hyperresponsiveness

FENO • Increased FENO levels are associated with deterioration in asthma control • FENO is reduced in a dose-dependent manner with antiinflammatory treatment • Prospective randomized single-blind placebo controlled trial showed tha following FENO levels can significantly decrease the dose of ICS without a significant change in rate of asthma exacerbations, use of oral steroids, or in level of airway inflammation* *Smith et al, NEJM May 2005; 352(21): 2163 -73

Pregnancy and Asthma

Beta 2 Agonists • Beta agonists are safe • More experience with older medications • Also used as tocolytic agents

Theophylline • Methylxanthines, especially theophylline and aminophylline, are considered safe • Clinical use is limited • Decreased protein binding during pregancy; should use levels 8 to 12 µg/m. L • Fetal serum levels same as maternal • Decreased drug clearance during the third trimester • Can inhibit uterine contraction

Inhaled Anticholinergics • Safe, especially ipatropium • No adverse development in fetus

Corticosteroids • Small risk during pregnacy, and should be withheld unless indicated • Three potential areas of concern have been raised: congenital malformations, primarily cleft palate; low birth weight; and neonatal adrenal insufficiency • No data in humans to show increased risk of cleft palate in humans • Palatial closure in 12 th week

Corticosteroids • Some studies show a slight decrease in birth weight with systemic steroids • Slight increased risk of prematurity in studies, but may be related to severity of asthma rather than use of steroids

Inhaled Corticosteroids • ICS, especially beclomethasone, triamcinolone, and budesonide, have been studied • The only randomized, controlled trial assessed 105 asthma exacerbations in 84 pregnant women who were managed with or without inhaled beclomethasone • Associated with a decreased rate of readmission for asthma (12 vs 33%), and no adverse events or outcomes were noted* *Wendel et al, Am J Obstet Gynecol 1996 Jul; 175(1): 150 -4

Inhaled Corticosteroids • Registry-based cohort study of 2, 014 Swedish women who used inhaled budesonide during early pregnancy • Rate of congenital malformations was no different from that of the general population (3. 8 vs 3. 5%)* • Based largely upon these findings, budesonide is currently the only inhaled corticosteroid with a pregnancy category B rating *Kallen et al, Obstet Gynecol 1999 Mar; 93(3): 392 -5

Chromones • Animal data and experience in 296 human pregnancies have not demonstrated an increase in fetal malformations or other adverse effects with cromolyn sodium • Human data are not available for nedocromil, although preclinical animal studies suggest that this drug is also safe

Leukotriene Modifiers • Animal data on zafirlukast have shown no teratogenicity at oral doses up to 160 times the maximum human daily oral dose on a mg/m 2 basis • No teratogenicity has been observed with montelukast given to rats or rabbits at doses greater than 300 times the maximum human daily oral dose on a mg/m 2 basis • Adequate studies in pregnant human women have not been performed




Conclusion

Horse hospitals
 Pathophysiology definition
Pathophysiology definition ........ is an alternative of log based recovery.
........ is an alternative of log based recovery. Ashok kumar pandey iith
Ashok kumar pandey iith Goyal definition
Goyal definition Mohak sukhwani
Mohak sukhwani Bryn mawr college computer science
Bryn mawr college computer science Preethika kumar
Preethika kumar Kamal kumar ips
Kamal kumar ips G ravindra kumar
G ravindra kumar Kalyan kumar hcl
Kalyan kumar hcl Ravi kumar kopparapu
Ravi kumar kopparapu Uiuc ece 445
Uiuc ece 445 Ranjana kumar
Ranjana kumar Pavan kumar vijay
Pavan kumar vijay Kumar mangalam birla committee
Kumar mangalam birla committee Kumar mangalam birla committee
Kumar mangalam birla committee Dr anuj kumar tripathi neurosurgeon
Dr anuj kumar tripathi neurosurgeon Kumar venkitanarayanan
Kumar venkitanarayanan Kumar is producing the photoelectric effect by using
Kumar is producing the photoelectric effect by using Kumar raj kharel
Kumar raj kharel Ulnar grasp
Ulnar grasp Kumar n. sivarajan
Kumar n. sivarajan Emani prize
Emani prize Cics training
Cics training Akshay kumar assistant
Akshay kumar assistant Eprg
Eprg Medisep hospitals in kerala
Medisep hospitals in kerala Dr pulin kumar
Dr pulin kumar Rohtash kumar vs state of haryana
Rohtash kumar vs state of haryana Rakesh kumar ntnu
Rakesh kumar ntnu Tan steinbach kumar
Tan steinbach kumar Jay kumar kar
Jay kumar kar Senthil kumar palanisamy
Senthil kumar palanisamy Saturn
Saturn Ravi kumar sacramento
Ravi kumar sacramento Santosh kumar swain kiit
Santosh kumar swain kiit Ca sripriya kumar
Ca sripriya kumar Management assertions definition
Management assertions definition Kumar
Kumar Dr v kumar
Dr v kumar Dr. pradip kumar khastagir
Dr. pradip kumar khastagir Dr sunesh kumar aiims
Dr sunesh kumar aiims Kumar satish ravi
Kumar satish ravi Dr alok kumar pandey
Dr alok kumar pandey Akshay kumar
Akshay kumar Mahendra kumar fiji
Mahendra kumar fiji Dilip kumar
Dilip kumar Anil kumar nmr
Anil kumar nmr Bedford interview skills
Bedford interview skills Alok kumar jagadev
Alok kumar jagadev Alok kumar jagadev
Alok kumar jagadev Chapter 9 kumar steinbach tan
Chapter 9 kumar steinbach tan Kumar
Kumar Meena patel life of pi
Meena patel life of pi Cholegogue
Cholegogue Pyelomyotomy
Pyelomyotomy Manas kumar chaudhuri
Manas kumar chaudhuri Vikas kumar
Vikas kumar Hamiltonian formulation of general relativity
Hamiltonian formulation of general relativity Kumar samrudhi society
Kumar samrudhi society Manas kumar chaudhuri
Manas kumar chaudhuri Association analysis advanced concepts
Association analysis advanced concepts Rfm
Rfm Dr ashish kumar bhutani
Dr ashish kumar bhutani Chetan prakash vs met institute
Chetan prakash vs met institute Hari ram kumar
Hari ram kumar Rajesh kumar bhagat
Rajesh kumar bhagat Kumar
Kumar Vikas kumar
Vikas kumar Kumar
Kumar Samir kumar khanal
Samir kumar khanal Nirupama prakash kumar
Nirupama prakash kumar Arwin kumar
Arwin kumar Srinidhi sampath kumar
Srinidhi sampath kumar Iihs moodle
Iihs moodle Subrata kumar panda
Subrata kumar panda Light house project indore
Light house project indore Saroj kumar dash
Saroj kumar dash Kumar kom jet
Kumar kom jet