ALLHAT Validation of Heart Failure Events in ALLHAT














- Slides: 37

ALLHAT Validation of Heart Failure Events in ALLHAT Participants Assigned to Doxazosin and Chlorthalidone L. B. Piller, B. R. Davis, J. A. Cutler, W. C. Cushman, J. T. Wright, J. D. Williamson, F. H. Leenen, O. Randall, J. S. Golden The University of Texas School of Public Health Houston, Texas

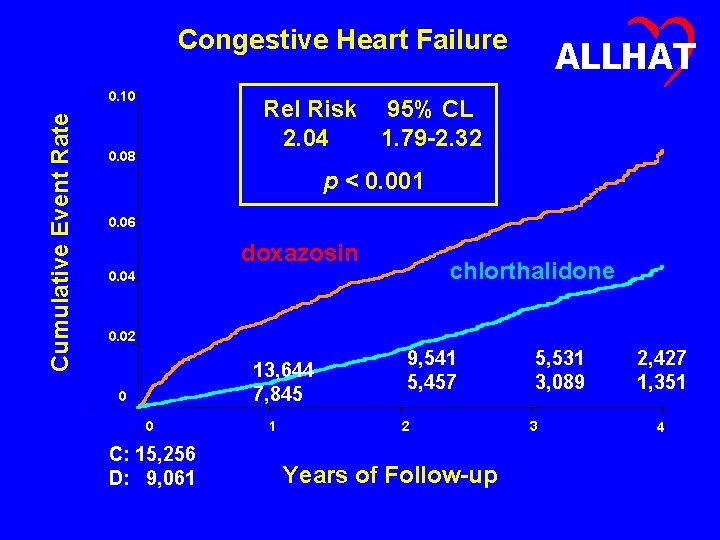
ALLHAT At Issue The doxazosin group showed a doubling of risk of CHF compared to the chlorthalidone group (RR, 2. 04; 95% CI, 1. 792. 32).

ALLHAT Objectives • To describe the process by which the clinical reports of heart failure events were validated • To describe the statistical analyses which led to the cessation of the doxazosin arm

Congestive Heart Failure Cumulative Event Rate 0. 10 ALLHAT Rel Risk 95% CL 2. 04 1. 79 -2. 32 0. 08 p < 0. 001 0. 06 doxazosin chlorthalidone 0. 04 0. 02 13, 644 7, 845 0 0 C: 15, 256 D: 9, 061 1 9, 541 5, 457 2 Years of Follow-up 5, 531 3, 089 3 2, 427 1, 351 4

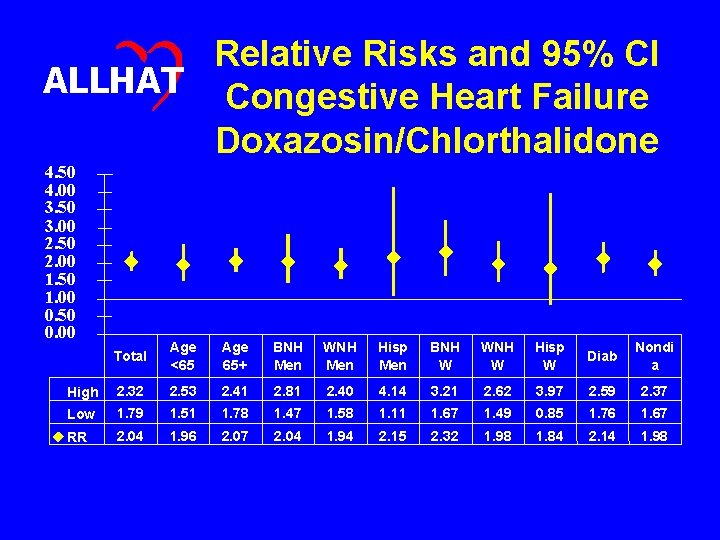
Relative Risks and 95% CI ALLHAT Congestive Heart Failure Doxazosin/Chlorthalidone 4. 50 4. 00 3. 50 3. 00 2. 50 2. 00 1. 50 1. 00 0. 50 0. 00 Total Age <65 Age 65+ BNH Men WNH Men Hisp Men BNH W WNH W Hisp W Diab Nondi a High 2. 32 2. 53 2. 41 2. 81 2. 40 4. 14 3. 21 2. 62 3. 97 2. 59 2. 37 Low 1. 79 1. 51 1. 78 1. 47 1. 58 1. 11 1. 67 1. 49 0. 85 1. 76 1. 67 RR 2. 04 1. 96 2. 07 2. 04 1. 94 2. 15 2. 32 1. 98 1. 84 2. 14 1. 98

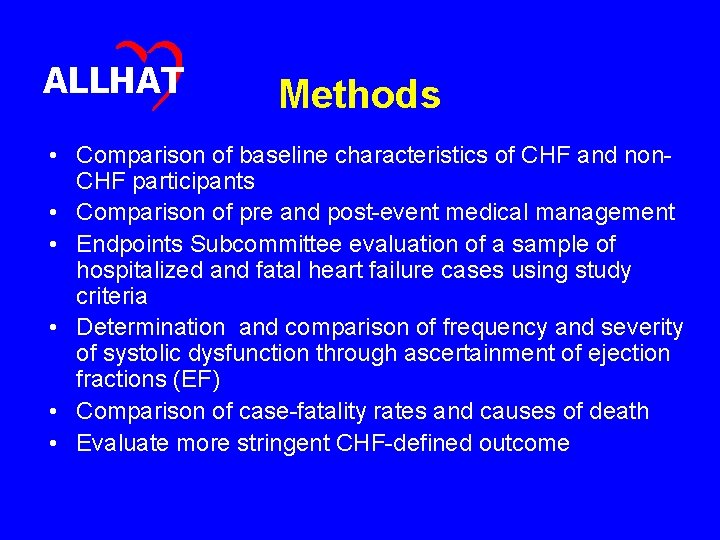
ALLHAT Methods • Comparison of baseline characteristics of CHF and non. CHF participants • Comparison of pre and post-event medical management • Endpoints Subcommittee evaluation of a sample of hospitalized and fatal heart failure cases using study criteria • Determination and comparison of frequency and severity of systolic dysfunction through ascertainment of ejection fractions (EF) • Comparison of case-fatality rates and causes of death • Evaluate more stringent CHF-defined outcome

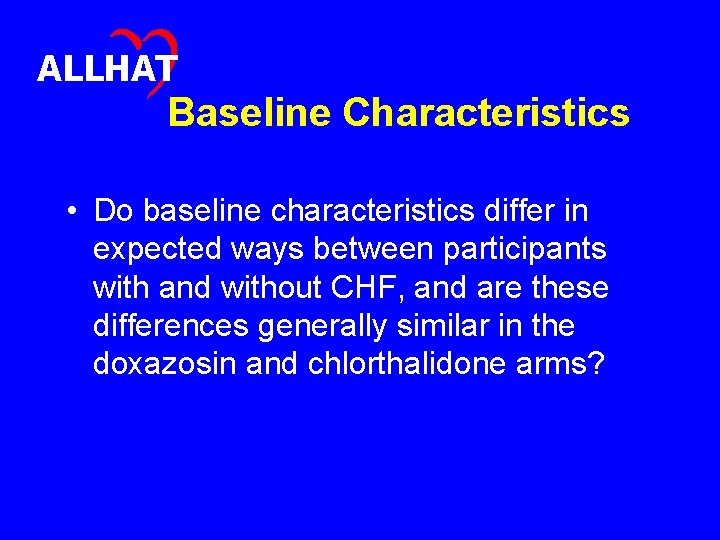
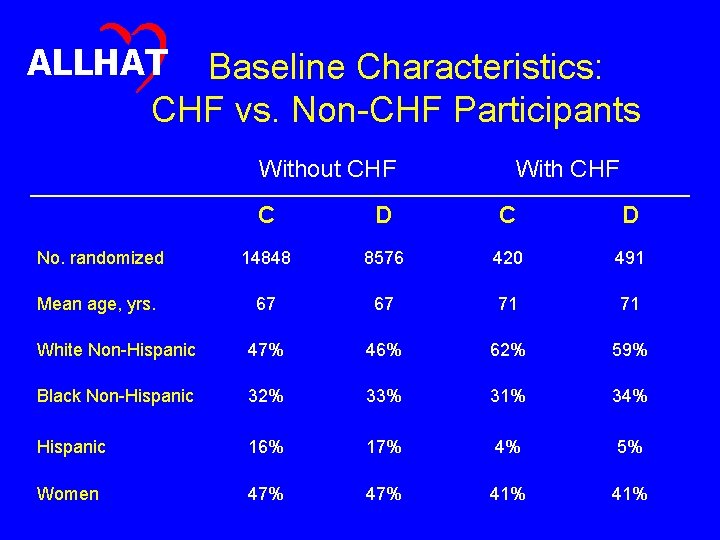
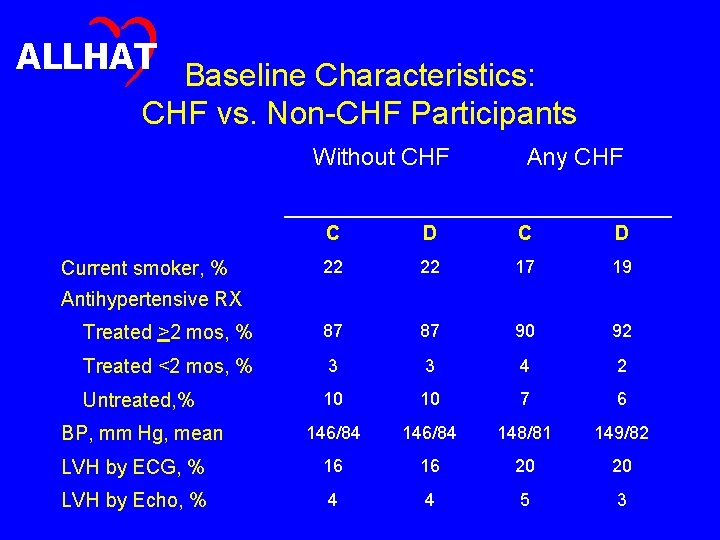
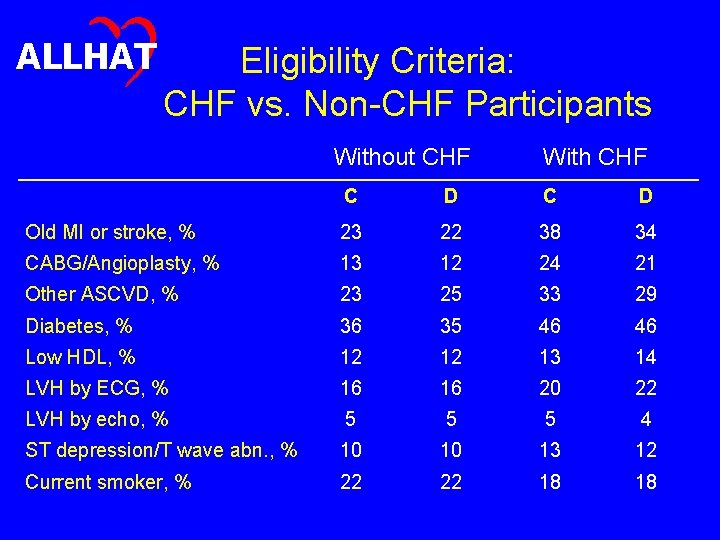
ALLHAT Baseline Characteristics • Do baseline characteristics differ in expected ways between participants with and without CHF, and are these differences generally similar in the doxazosin and chlorthalidone arms?

ALLHAT Baseline Characteristics: CHF vs. Non-CHF Participants Without CHF With CHF C D No. randomized 14848 8576 420 491 Mean age, yrs. 67 67 71 71 White Non-Hispanic 47% 46% 62% 59% Black Non-Hispanic 32% 33% 31% 34% Hispanic 16% 17% 4% 5% Women 47% 41%

ALLHAT Baseline Characteristics: CHF vs. Non-CHF Participants Without CHF Any CHF C D 22 22 17 19 Treated >2 mos, % 87 87 90 92 Treated <2 mos, % 3 3 4 2 Untreated, % 10 10 7 6 BP, mm Hg, mean 146/84 148/81 149/82 LVH by ECG, % 16 16 20 20 LVH by Echo, % 4 4 5 3 Current smoker, % Antihypertensive RX

ALLHAT Eligibility Criteria: CHF vs. Non-CHF Participants Without CHF With CHF C D Old MI or stroke, % 23 22 38 34 CABG/Angioplasty, % 13 12 24 21 Other ASCVD, % 23 25 33 29 Diabetes, % 36 35 46 46 Low HDL, % 12 12 13 14 LVH by ECG, % 16 16 20 22 LVH by echo, % 5 5 5 4 ST depression/T wave abn. , % 10 10 13 12 Current smoker, % 22 22 18 18

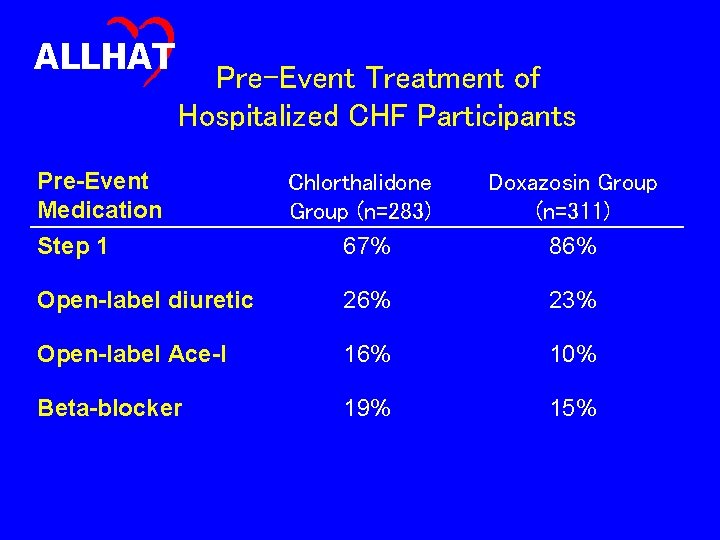
ALLHAT Pre and Post-Event Treatment of Heart Failure Participants • Was pre-heart failure event step 1 treatment compliance equivalent in the chlorthalidone and doxazosin groups? • Were the heart failure participants in the chlorthalidone and doxazosin groups managed similarly following the heart failure events?

ALLHAT Pre-Event Treatment of Hospitalized CHF Participants Pre-Event Medication Step 1 Chlorthalidone Group (n=283) 67% Doxazosin Group (n=311) 86% Open-label diuretic 26% 23% Open-label Ace-I 16% 10% Beta-blocker 19% 15%

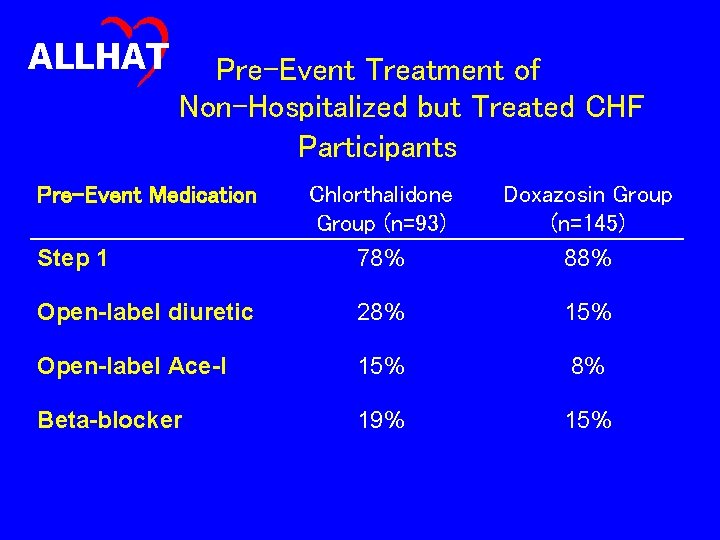
ALLHAT Pre-Event Treatment of Non-Hospitalized but Treated CHF Participants Pre-Event Medication Chlorthalidone Group (n=93) 78% Doxazosin Group (n=145) 88% Open-label diuretic 28% 15% Open-label Ace-I 15% 8% Beta-blocker 19% 15% Step 1

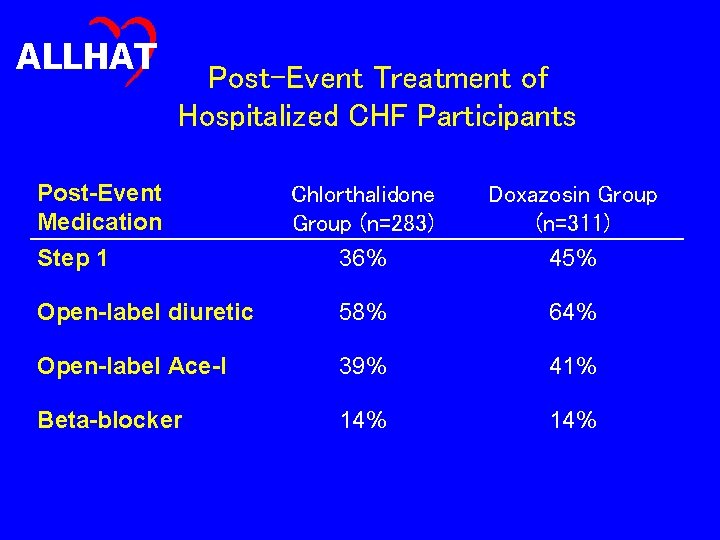
ALLHAT Post-Event Treatment of Hospitalized CHF Participants Post-Event Medication Chlorthalidone Group (n=283) 36% Doxazosin Group (n=311) 45% Open-label diuretic 58% 64% Open-label Ace-I 39% 41% Beta-blocker 14% Step 1

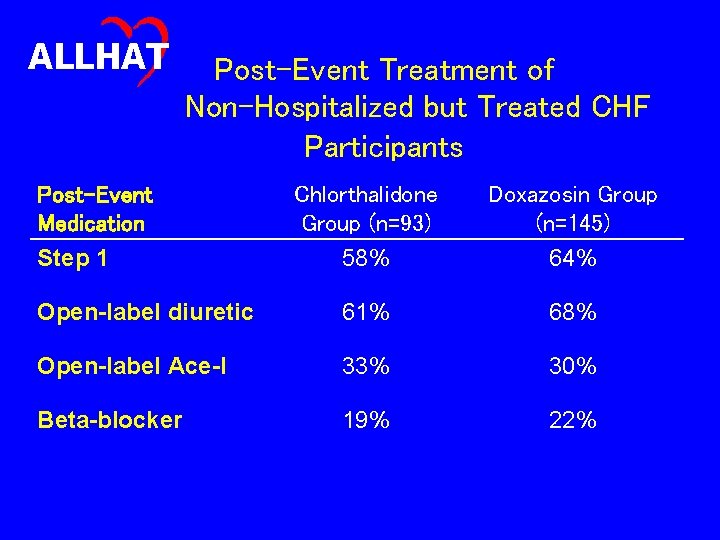
ALLHAT Post-Event Treatment of Non-Hospitalized but Treated CHF Participants Post-Event Medication Step 1 Chlorthalidone Group (n=93) 58% Doxazosin Group (n=145) 64% Open-label diuretic 61% 68% Open-label Ace-I 33% 30% Beta-blocker 19% 22%

ALLHAT Heart Failure Event Reporting • Did the reports of hospitalized or fatal heart failure adhere to the study criteria?

ALLHAT Study Events Documentation • Documentation required: – Discharge summaries for all hospitalizations – Death certificates for all deaths • Additional QC documentation for random 10% sample of MI’s, strokes and fatal CHD Management • Routinely reviewed for accuracy and appropriateness • Queries sent to the sites for clarification of discrepancies • A random 10% of MI’s, strokes and fatal CHD reports reevaluated by the Endpoints Subcommittee for quality control

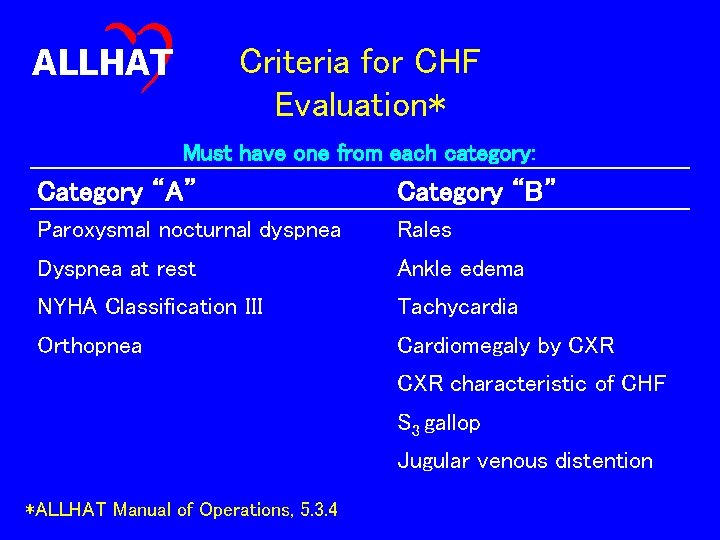
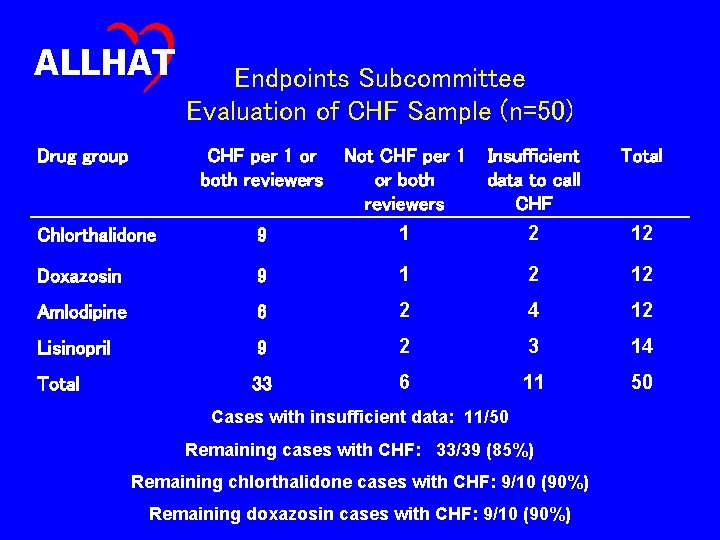
ALLHAT Endpoint Subcommittee Evaluation of CHF Events • 50 cases of reported hospitalized and/or fatal CHF • Each case reviewed by two Subcommittee members • Criteria for confirmation of CHF as described in the Manual of Operations

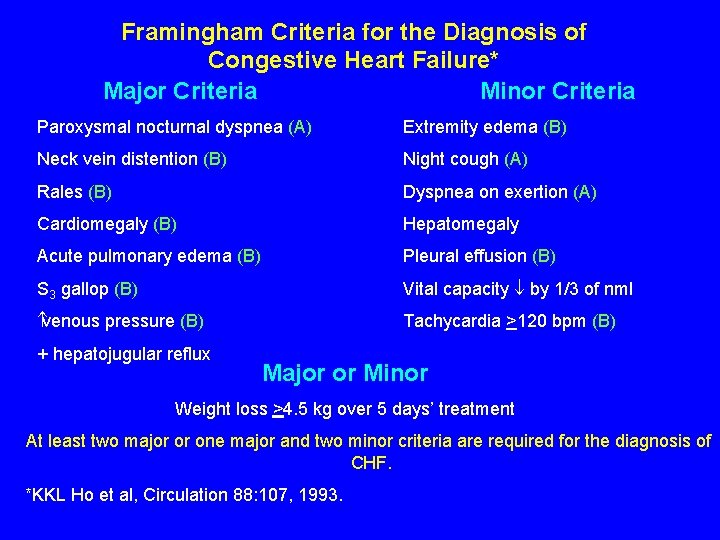
ALLHAT Criteria for CHF Evaluation* Must have one from each category: Category “A” Category “B” Paroxysmal nocturnal dyspnea Rales Dyspnea at rest Ankle edema NYHA Classification III Tachycardia Orthopnea Cardiomegaly by CXR characteristic of CHF S 3 gallop Jugular venous distention *ALLHAT Manual of Operations, 5. 3. 4

New York Heart Association Functional Classification of Congestive Heart Failure* I No limitations of activity; ordinary activity does not cause undue fatigue, palpitations, dyspnea or anginal pain II Slight limitations of activity; asymptomatic at rest; ordinary activity results in fatigue, palpitations, dyspnea or anginal pain III Marked limitations of activity; usually asymptomatic at rest; less than ordinary activity causes fatigue, palpitations, dyspnea or anginal pain IV Inability to carry on any physical activity without discomfort; symptoms at rest; increased discomfort with any physical activity * Criteria Committee, New York Heart Association. Diseases of the heart and blood vessels. Nomenclature and criteria for diagnosis. 6 th ed. 1964: 114.

Framingham Criteria for the Diagnosis of Congestive Heart Failure* Major Criteria Minor Criteria Paroxysmal nocturnal dyspnea (A) Extremity edema (B) Neck vein distention (B) Night cough (A) Rales (B) Dyspnea on exertion (A) Cardiomegaly (B) Hepatomegaly Acute pulmonary edema (B) Pleural effusion (B) S 3 gallop (B) Vital capacity by 1/3 of nml venous pressure (B) Tachycardia >120 bpm (B) + hepatojugular reflux Major or Minor Weight loss >4. 5 kg over 5 days’ treatment At least two major or one major and two minor criteria are required for the diagnosis of CHF. *KKL Ho et al, Circulation 88: 107, 1993.

ALLHAT Drug group Endpoints Subcommittee Evaluation of CHF Sample (n=50) CHF per 1 or both reviewers Not CHF per 1 or both reviewers Insufficient data to call CHF Total Chlorthalidone 9 1 2 12 Doxazosin 9 1 2 12 Amlodipine 6 2 4 12 Lisinopril 9 2 3 14 Total 33 6 11 50 Cases with insufficient data: 11/50 Remaining cases with CHF: 33/39 (85%) Remaining chlorthalidone cases with CHF: 9/10 (90%) Remaining doxazosin cases with CHF: 9/10 (90%)

ALLHAT Severity of Heart Failure • What is the frequency of systolic dysfunction in doxazosin and chlorthalidone participants with heart failure? • Is the systolic dysfunction equally severe in the chlorthalidone and doxazosin participants with heart failure?

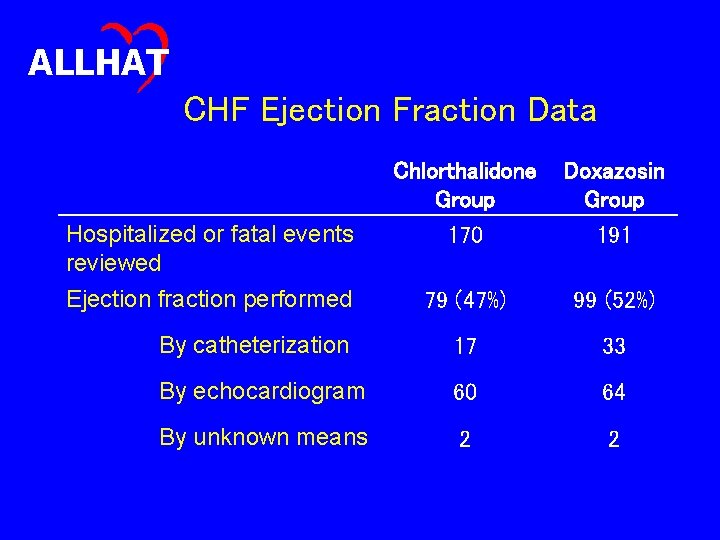
ALLHAT Assessment of the Severity of Hospitalized and Fatal Heart Failure • Half of hospitalized or fatal heart failure events reviewed for ejection fractions (EF) • If EF documented, further information sought – How was it obtained? – Was the EF equally severe across drug groups? – Given possible differences between VA and non. VA sites in levels of diagnostic testing, was there a difference between the two groups in EF data?

ALLHAT CHF Ejection Fraction Data Chlorthalidone Group Doxazosin Group Hospitalized or fatal events reviewed 170 191 Ejection fraction performed 79 (47%) 99 (52%) By catheterization 17 33 By echocardiogram 60 64 By unknown means 2 2

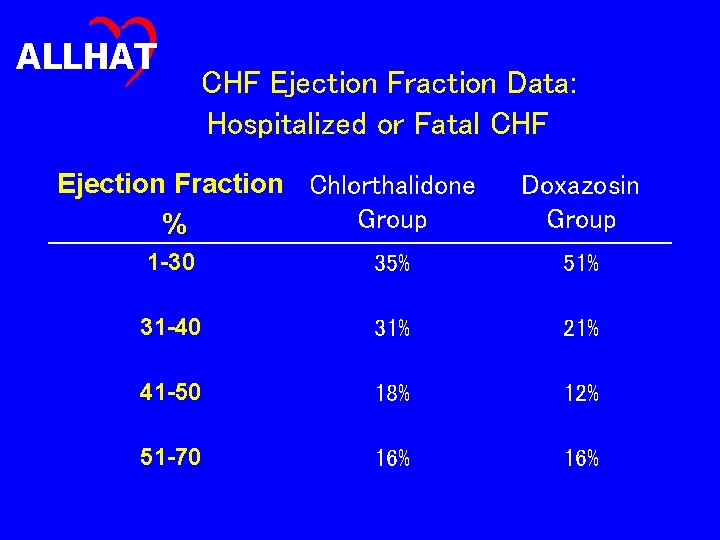
ALLHAT CHF Ejection Fraction Data: Hospitalized or Fatal CHF Ejection Fraction Chlorthalidone Group % Doxazosin Group 1 -30 35% 51% 31 -40 31% 21% 41 -50 18% 12% 51 -70 16%

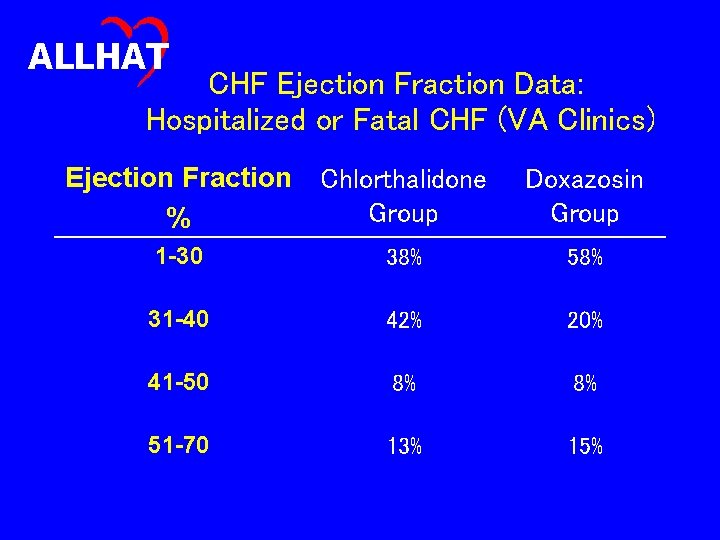
ALLHAT CHF Ejection Fraction Data: Hospitalized or Fatal CHF (VA Clinics) Ejection Fraction Chlorthalidone Group % Doxazosin Group 1 -30 38% 58% 31 -40 42% 20% 41 -50 8% 8% 51 -70 13% 15%

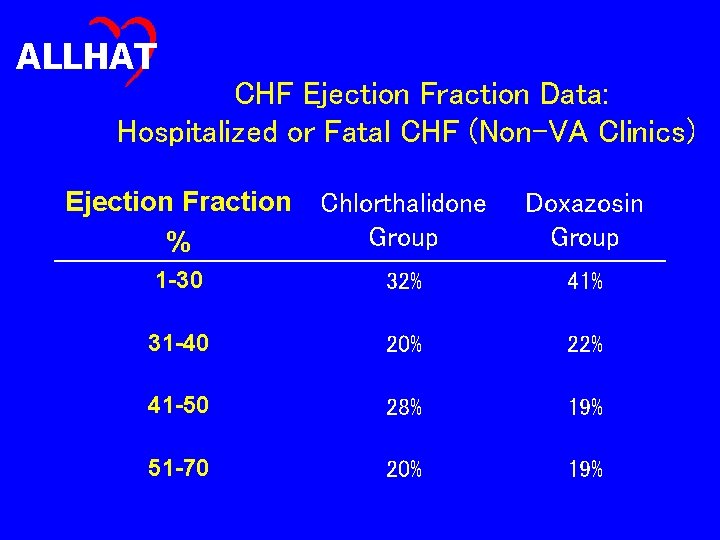
ALLHAT CHF Ejection Fraction Data: Hospitalized or Fatal CHF (Non-VA Clinics) Ejection Fraction Chlorthalidone Group % Doxazosin Group 1 -30 32% 41% 31 -40 20% 22% 41 -50 28% 19% 51 -70 20% 19%

ALLHAT Fatality Rates and Cause of Death • Is all-cause mortality similar for the chlorthalidone and doxazosin participants? • Are case-fatality rates similar and as high as expected for chlorthalidone and doxazosin participants hospitalized for heart failure? • Are causes of deaths for chlorthalidone and doxazosin participants previously hospitalized for heart failure similarly distributed?

All-Cause Mortality ALLHAT Cumulative Event Rate Rel Risk 95% CL 1. 03 0. 90 -1. 15 p = 0. 56 doxazosin chlorthalidone 13, 739 8, 054 C: 15, 268 D: 9, 067 10, 513 6, 118 Years of Follow-up 5, 702 3, 287 2, 530 1, 481

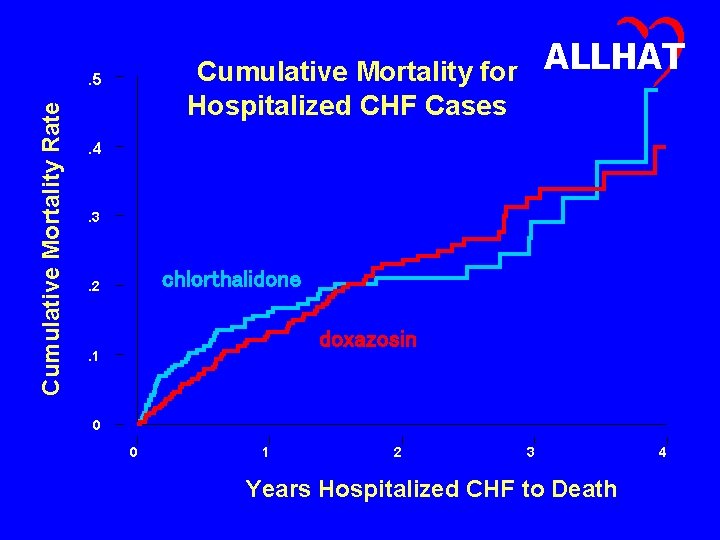
. 5 Cumulative Mortality Rate ALLHAT Cumulative Mortality for Hospitalized CHF Cases . 4 . 3 chlorthalidone . 2 doxazosin . 1 0 0 1 2 3 Years Hospitalized CHF to Death 4

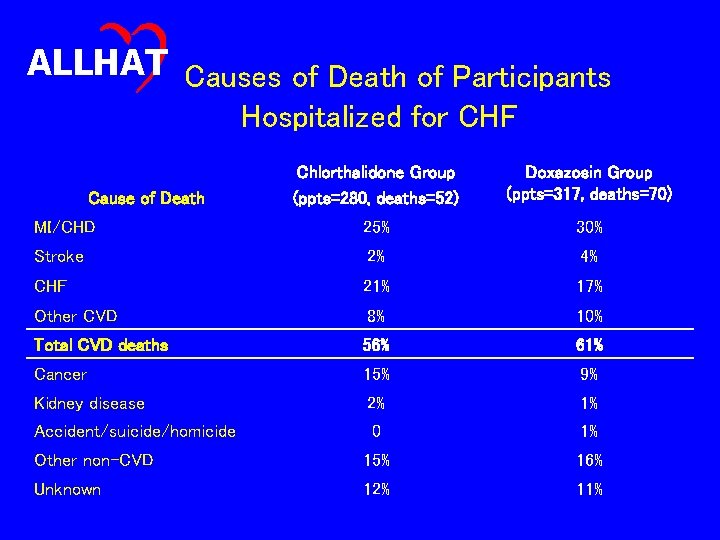
ALLHAT Causes of Death of Participants Hospitalized for CHF Chlorthalidone Group (ppts=280, deaths=52) Doxazosin Group (ppts=317, deaths=70) MI/CHD 25% 30% Stroke 2% 4% CHF 21% 17% Other CVD 8% 10% Total CVD deaths 56% 61% Cancer 15% 9% Kidney disease 2% 1% Accident/suicide/homicide 0 1% Other non-CVD 15% 16% Unknown 12% 11% Cause of Death

ALLHAT Fatal and Hospitalized CHF: Increased Risk? Does the more stringent CHF outcome, fatal and hospitalized CHF, show a similarly increased CHF risk in the doxazosin group compared to the chlorthalidone group?

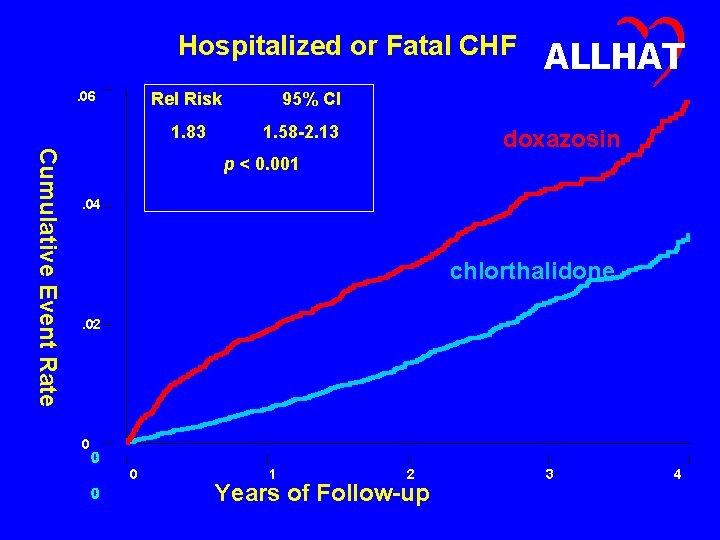
Hospitalized or Fatal CHF. 06 Rel Risk 1. 83 ALLHAT 95% CI 1. 58 -2. 13 Cumulative Event Rate doxazosin p < 0. 001. 04 chlorthalidone. 02 0 0 1 2 Years of Follow-up 3 4

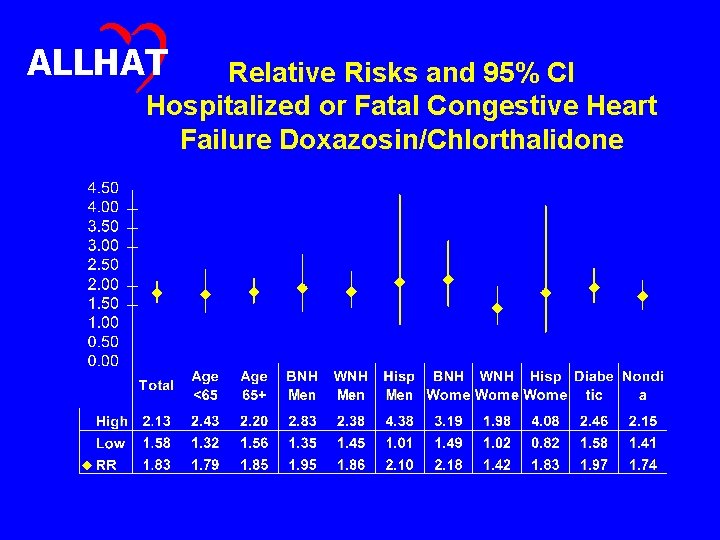
ALLHAT Relative Risks and 95% CI Hospitalized or Fatal Congestive Heart Failure Doxazosin/Chlorthalidone

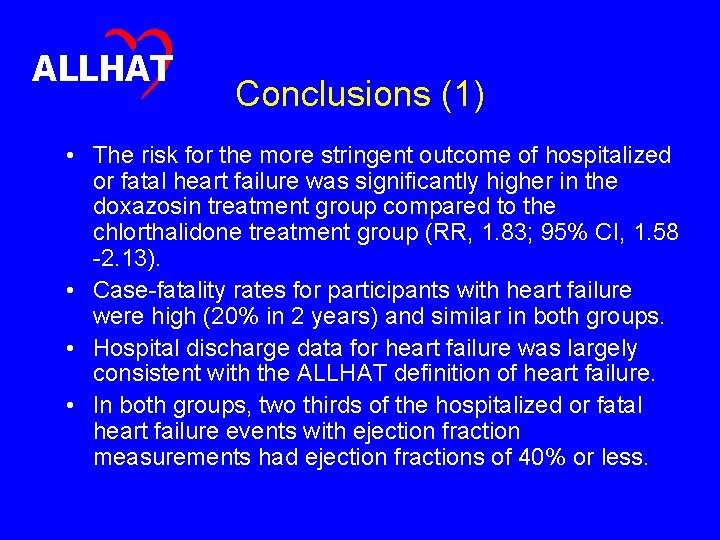
ALLHAT Conclusions (1) • The risk for the more stringent outcome of hospitalized or fatal heart failure was significantly higher in the doxazosin treatment group compared to the chlorthalidone treatment group (RR, 1. 83; 95% CI, 1. 58 -2. 13). • Case-fatality rates for participants with heart failure were high (20% in 2 years) and similar in both groups. • Hospital discharge data for heart failure was largely consistent with the ALLHAT definition of heart failure. • In both groups, two thirds of the hospitalized or fatal heart failure events with ejection fraction measurements had ejection fractions of 40% or less.

ALLHAT Conclusions (2) • The treatment following the heart failure events in both groups was consistent with recommended treatment of heart failure in the community. • Similar percentages of participants in both treatment groups were on step 1 medication prior to the heart failure event. • Participants who developed heart failure had a greater history of coronary heart disease than participants who did not develop heart failure.
 Failure to sense pacemaker
Failure to sense pacemaker Brittle vs ductile fracture
Brittle vs ductile fracture Ventricular escape rhythm
Ventricular escape rhythm Pathophysiology of valvular heart disease
Pathophysiology of valvular heart disease Diabetes and heart failure
Diabetes and heart failure Congestive heart failure zones for management
Congestive heart failure zones for management Right vs left-sided heart failure chart
Right vs left-sided heart failure chart Heart failure defined
Heart failure defined Acute pulmonary congestion histology
Acute pulmonary congestion histology Heart failure complications
Heart failure complications Compensatory mechanism of heart failure
Compensatory mechanism of heart failure Lmnop heart failure
Lmnop heart failure New york scale heart failure
New york scale heart failure Compensatory mechanism of heart failure
Compensatory mechanism of heart failure Chapter 24 heart failure drugs
Chapter 24 heart failure drugs Right sided heart failure
Right sided heart failure Lvedp normal range
Lvedp normal range Donkey analogy heart failure
Donkey analogy heart failure Keith rn heart failure case study
Keith rn heart failure case study Edema assessment
Edema assessment Diabetes and heart failure
Diabetes and heart failure Heart failure
Heart failure Acute vs chronic heart failure
Acute vs chronic heart failure Heart failure definition
Heart failure definition Congestive heart failure symtoms
Congestive heart failure symtoms Mutually exclusive events vs not mutually exclusive events
Mutually exclusive events vs not mutually exclusive events Sheep heart anatomy
Sheep heart anatomy Stars plowhorses puzzles dogs
Stars plowhorses puzzles dogs Heart border percussion of heart
Heart border percussion of heart Human validation process
Human validation process Is unit testing verification or validation
Is unit testing verification or validation Uc doorways validation
Uc doorways validation Clinical validation query example
Clinical validation query example Validation partielle
Validation partielle Verification and validation
Verification and validation Apic cleaning validation guidelines 2014
Apic cleaning validation guidelines 2014 Testing types in software engineering
Testing types in software engineering Validation checks ict
Validation checks ict