A Quantum Chemical Study of HZn CH 3






- Slides: 33

A Quantum Chemical Study of HZn. CH 3 A Transition Metal Compound with 4 s 2 Recoupled Pair Bonding David E. Woon & Thom H. Dunning, Jr. RI 12

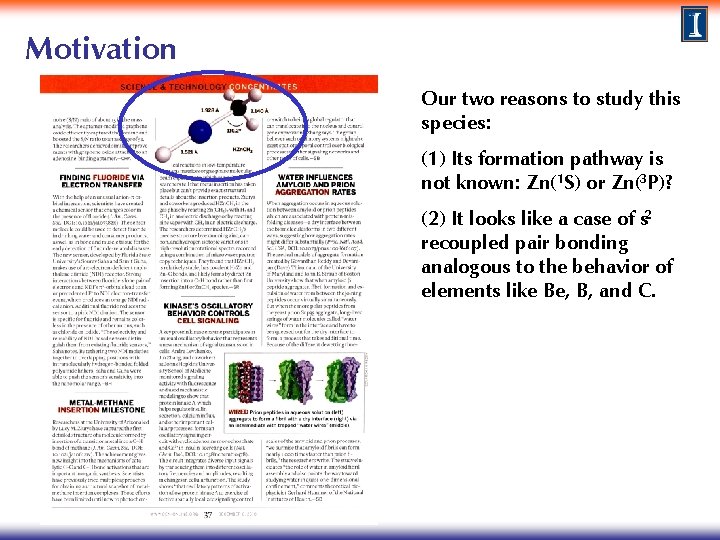
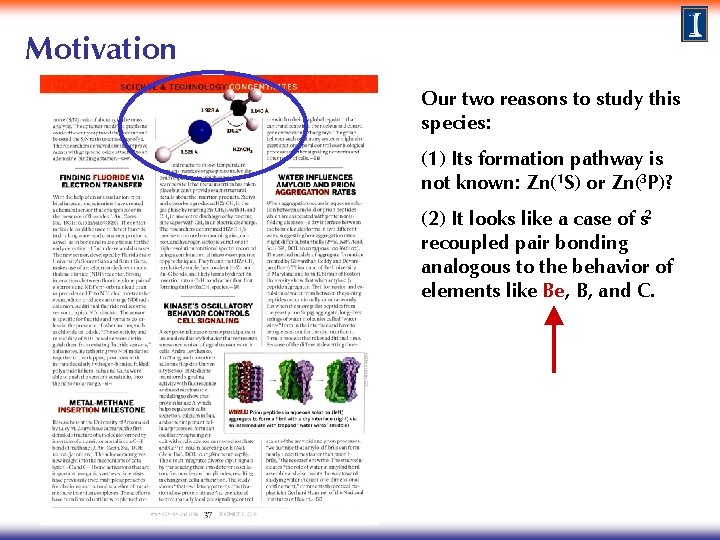
Motivation In late 2010, Flory et al. reported new experimental results for HZn. CH 3 (methyl zinc hydride), which is thought to be formed by direct insertion of Zn into a methane CH bond. M. A. Flory, A. J. Apponi, L. N. Zack, and L. M. Ziurys, J Am Chem Soc 132, 17186, 2010. featured in the 6 December 2010 issue of Chemical & Engineering News

Motivation Our two reasons to study this species: (1) Its formation pathway is not known: Zn(1 S) or Zn(3 P)? (2) It looks like a case of s 2 recoupled pair bonding analogous to the behavior of elements like Be, B, and C.

Motivation Our two reasons to study this species: (1) Its formation pathway is not known: Zn(1 S) or Zn(3 P)? (2) It looks like a case of s 2 recoupled pair bonding analogous to the behavior of elements like Be, B, and C.

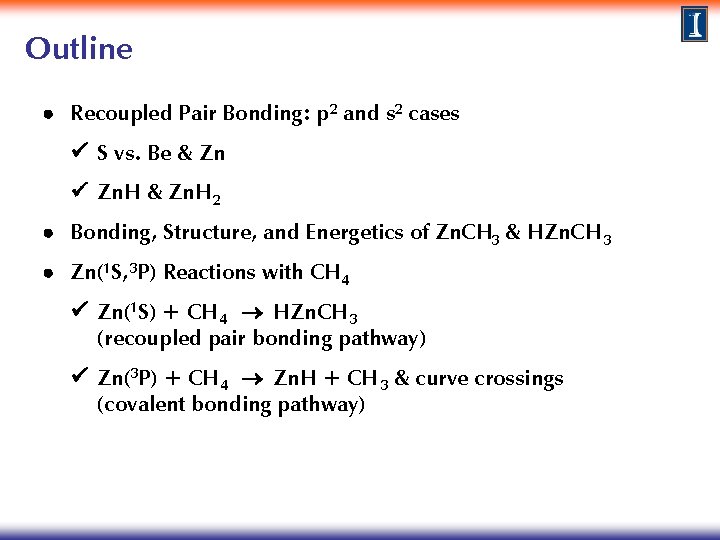
Outline Recoupled Pair Bonding: p 2 and s 2 cases S vs. Be & Zn Zn. H & Zn. H 2 Bonding, Structure, and Energetics of Zn. CH 3 & HZn. CH 3 Zn(1 S, 3 P) Reactions with CH 4 Zn(1 S) + CH 4 HZn. CH 3 (recoupled pair bonding pathway) Zn(3 P) + CH 4 Zn. H + CH 3 & curve crossings (covalent bonding pathway)

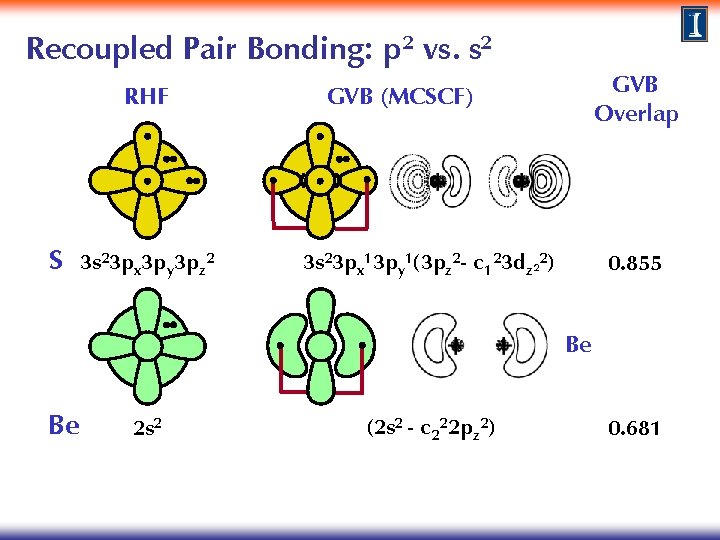
Recoupled Pair Bonding: p 2 vs. s 2 RHF S 3 s 23 px 3 py 3 pz 2 GVB Overlap GVB (MCSCF) 3 s 23 px 13 py 1(3 pz 2 - c 123 dz 22) 0. 855 Be Be 2 s 2 (2 s 2 - c 222 pz 2) 0. 681

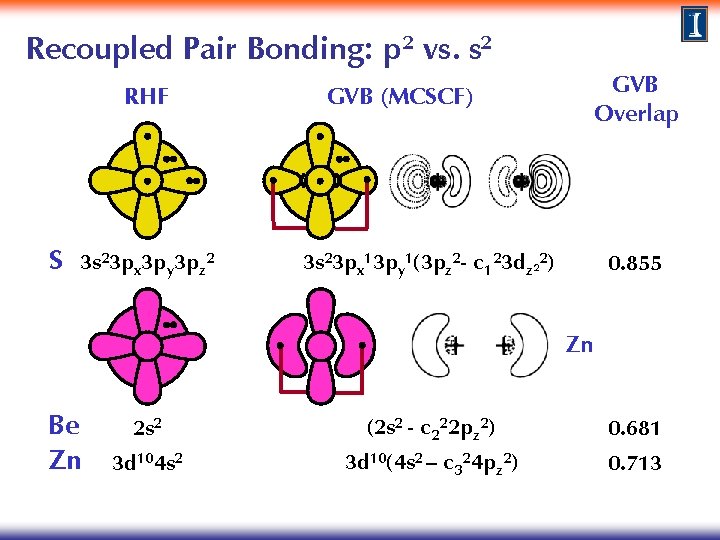
Recoupled Pair Bonding: p 2 vs. s 2 RHF S 3 s 23 px 3 py 3 pz 2 GVB (MCSCF) GVB Overlap 3 s 23 px 13 py 1(3 pz 2 - c 123 dz 22) 0. 855 Zn Be Zn 2 s 2 (2 s 2 - c 222 pz 2) 0. 681 3 d 104 s 2 3 d 10(4 s 2 – c 324 pz 2) 0. 713

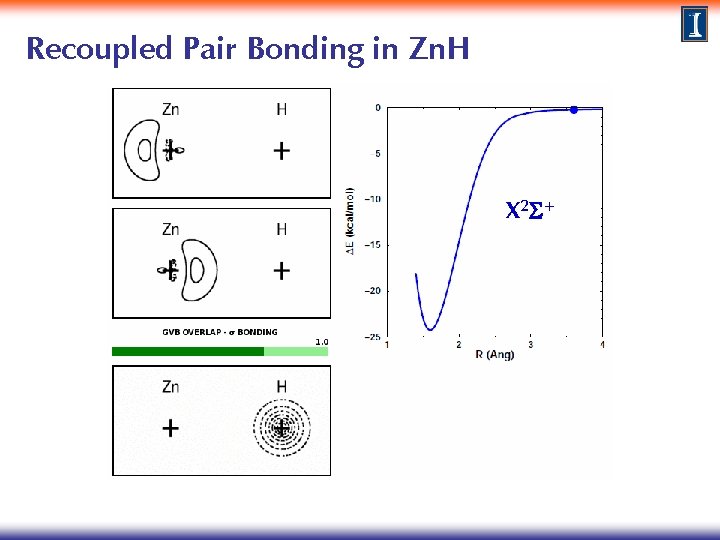
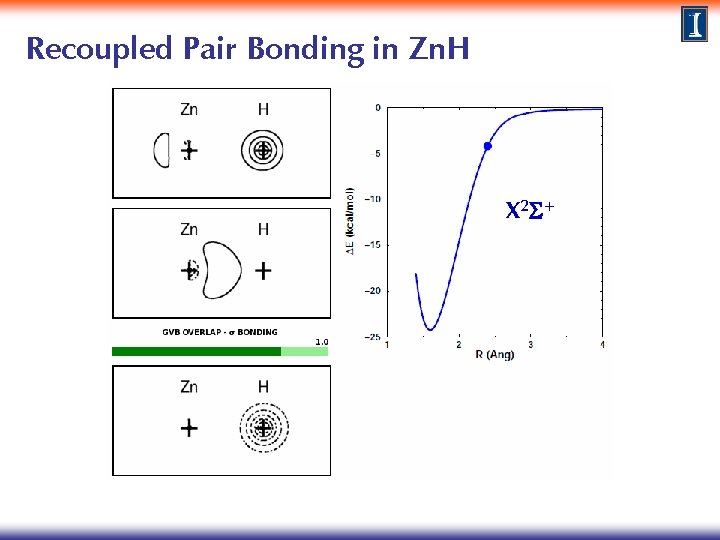
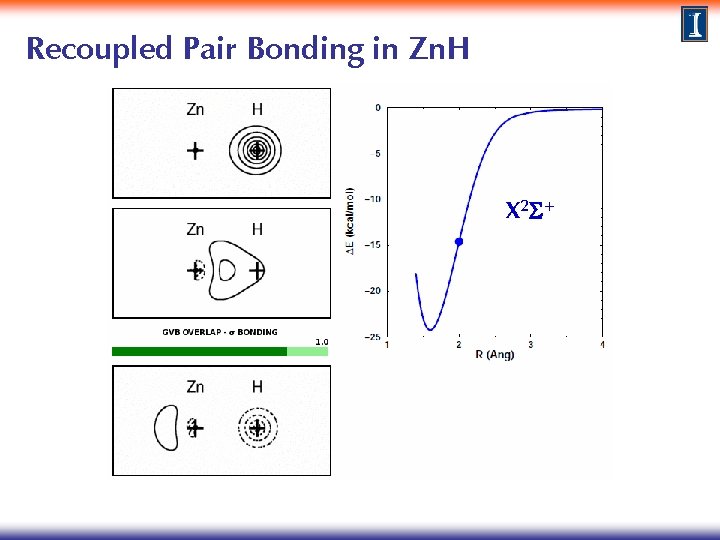
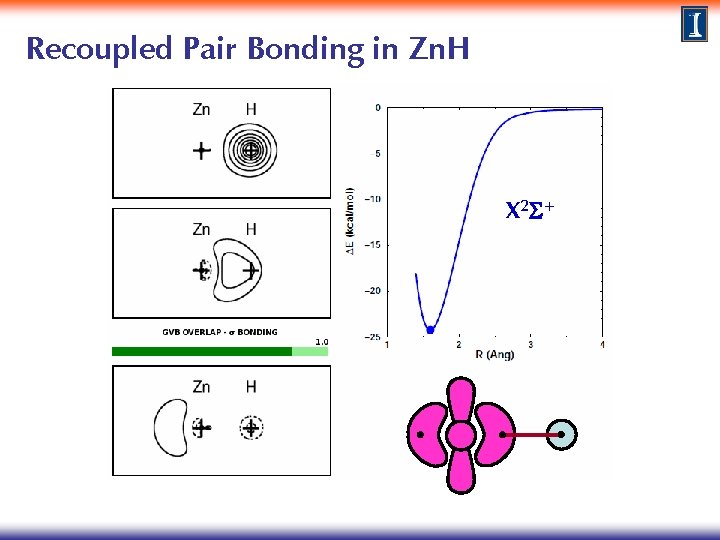
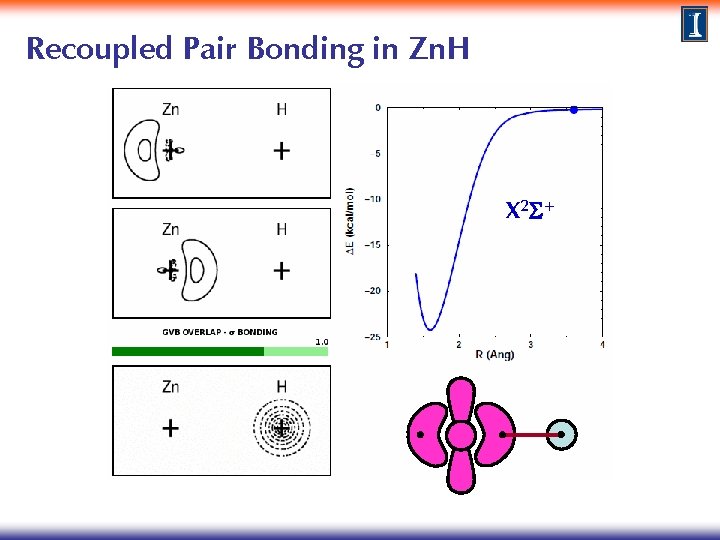
Recoupled Pair Bonding in Zn. H X 2 S+

Recoupled Pair Bonding in Zn. H X 2 S+

Recoupled Pair Bonding in Zn. H X 2 S+

Recoupled Pair Bonding in Zn. H X 2 S+

Recoupled Pair Bonding in Zn. H X 2 S+

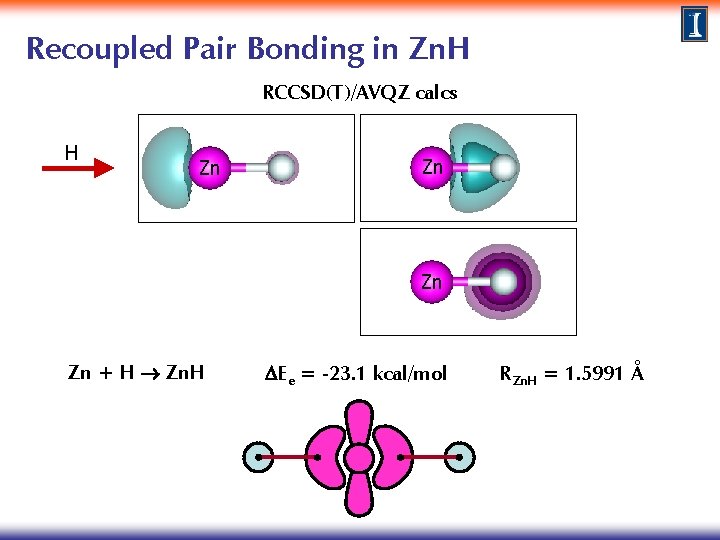
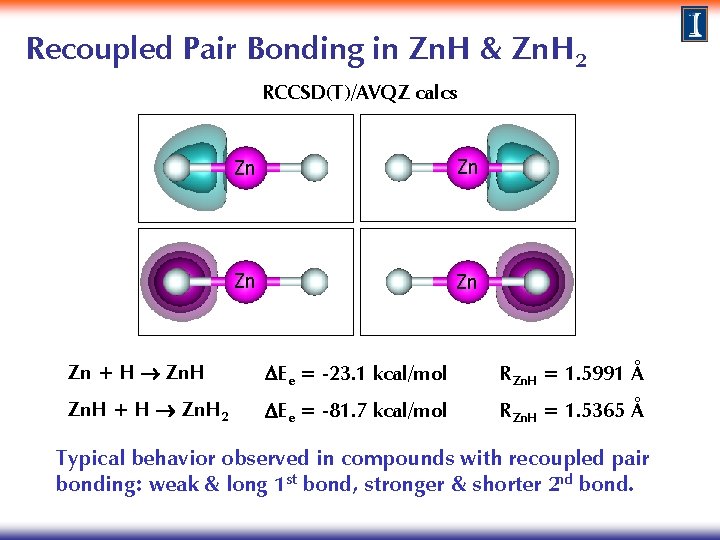
Recoupled Pair Bonding in Zn. H RCCSD(T)/AVQZ calcs H Zn Zn + H Zn. H DEe = -23. 1 kcal/mol RZn. H = 1. 5991 Å

Recoupled Pair Bonding in Zn. H & Zn. H 2 RCCSD(T)/AVQZ calcs Zn Zn Zn + H Zn. H DEe = -23. 1 kcal/mol RZn. H = 1. 5991 Å Zn. H + H Zn. H 2 DEe = -81. 7 kcal/mol RZn. H = 1. 5365 Å Typical behavior observed in compounds with recoupled pair bonding: weak & long 1 st bond, stronger & shorter 2 nd bond.

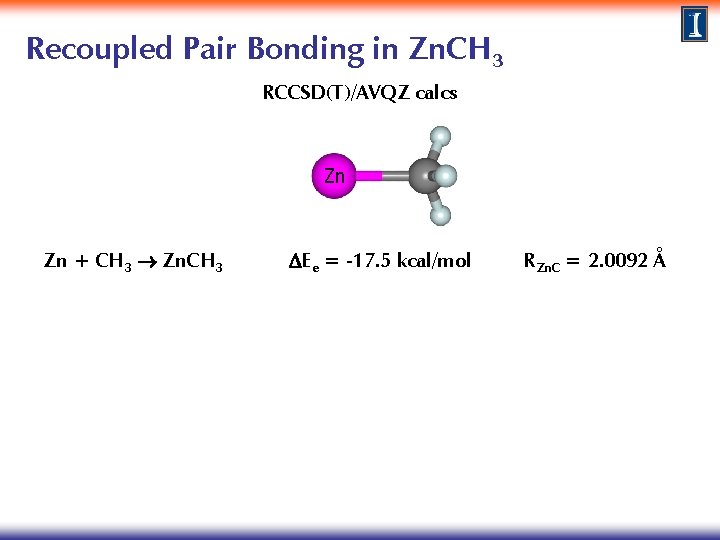
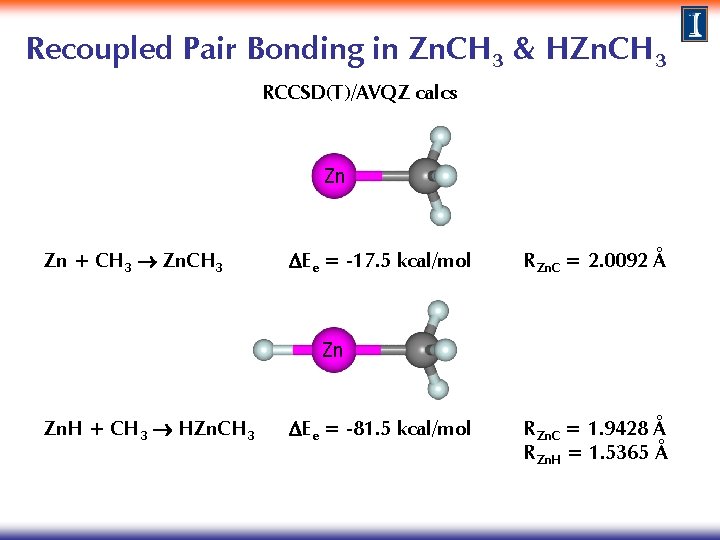
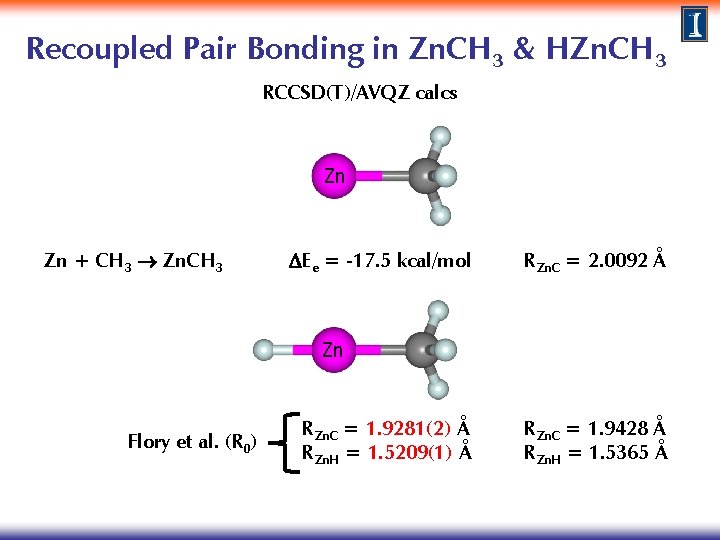
Recoupled Pair Bonding in Zn. CH 3 RCCSD(T)/AVQZ calcs Zn Zn + CH 3 Zn. CH 3 DEe = -17. 5 kcal/mol RZn. C = 2. 0092 Å

Recoupled Pair Bonding in Zn. CH 3 & HZn. CH 3 RCCSD(T)/AVQZ calcs Zn Zn + CH 3 Zn. CH 3 DEe = -17. 5 kcal/mol RZn. C = 2. 0092 Å Zn Zn. H + CH 3 HZn. CH 3 DEe = -81. 5 kcal/mol RZn. C = 1. 9428 Å RZn. H = 1. 5365 Å

Recoupled Pair Bonding in Zn. CH 3 & HZn. CH 3 RCCSD(T)/AVQZ calcs Zn Zn + CH 3 Zn. CH 3 DEe = -17. 5 kcal/mol RZn. C = 2. 0092 Å Zn Flory et al. (R 0) RZn. C = 1. 9281(2) Å RZn. H = 1. 5209(1) Å RZn. C = 1. 9428 Å RZn. H = 1. 5365 Å

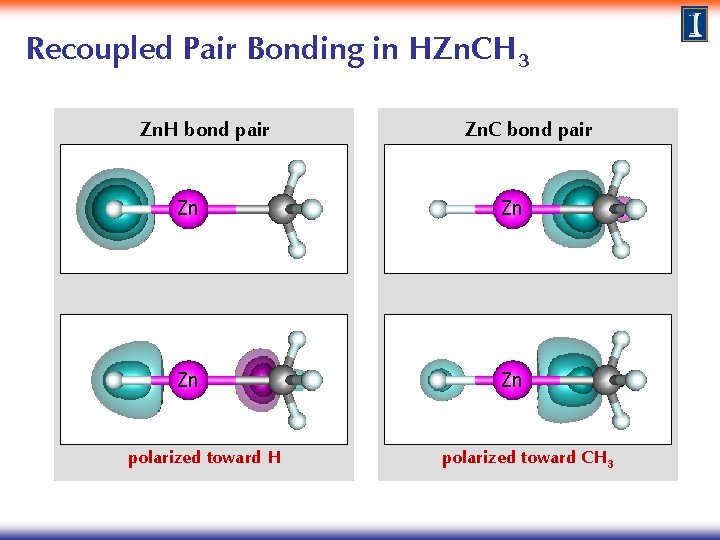
Recoupled Pair Bonding in HZn. CH 3 Zn. H bond pair Zn. C bond pair Zn Zn polarized toward H polarized toward CH 3

Recoupled Pair Bonding in HZn. CH 3 H orbital CH 3 singly occupied orbital Zn Zn Zn left lobe orbital Zn right lobe orbital Zn Zn GVB overlap s=0. 704

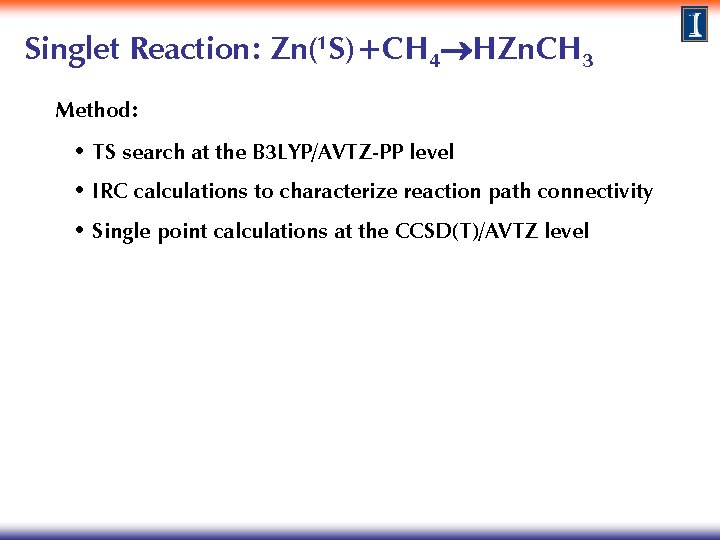
Singlet Reaction: Zn(1 S)+CH 4 HZn. CH 3 Method: • TS search at the B 3 LYP/AVTZ-PP level • IRC calculations to characterize reaction path connectivity • Single point calculations at the CCSD(T)/AVTZ level

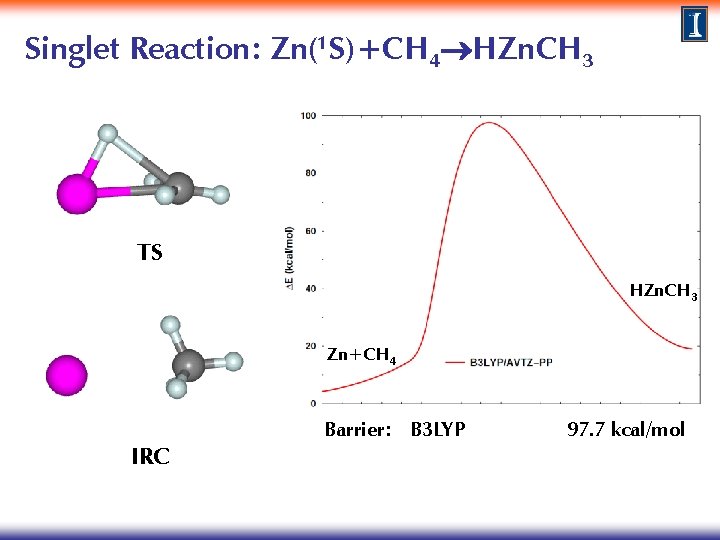
Singlet Reaction: Zn(1 S)+CH 4 HZn. CH 3 TS HZn. CH 3 Zn+CH 4 IRC Barrier: B 3 LYP 97. 7 kcal/mol

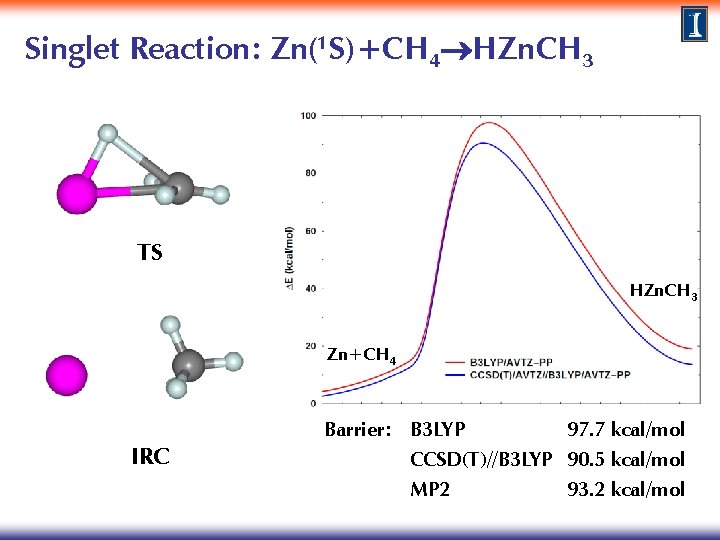
Singlet Reaction: Zn(1 S)+CH 4 HZn. CH 3 TS HZn. CH 3 Zn+CH 4 IRC Barrier: B 3 LYP 97. 7 kcal/mol CCSD(T)//B 3 LYP 90. 5 kcal/mol MP 2 93. 2 kcal/mol

Singlet Reaction: Zn(1 S)+CH 4 HZn. CH 3 The barrier for direct insertion on the singlet surface is very large. What happens on the triplet surface? Does it lead to a more efficient pathway to forming HZn. CH 3?

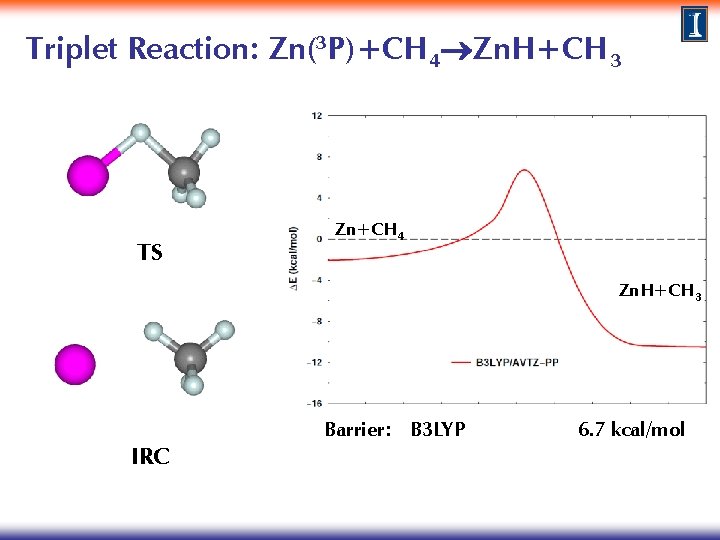
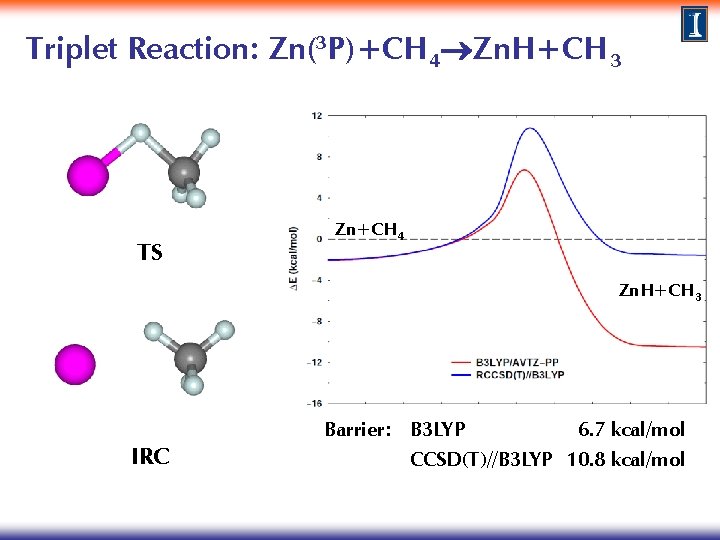
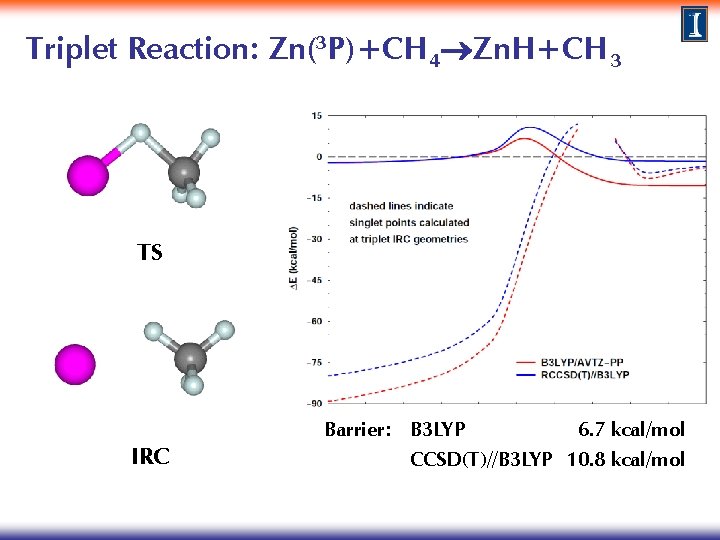
Triplet Reaction: Zn(3 P)+CH 4 Zn. H+CH 3 Method: TS search at the B 3 LYP/AVTZ-PP level IRC calculations to characterize reaction path connectivity Single point calculations at the CCSD(T)/AVTZ level · Perform singlet calculations at triplet IRC geometries for both methods to explore the existence of surface crossings and the possibility of surface hopping

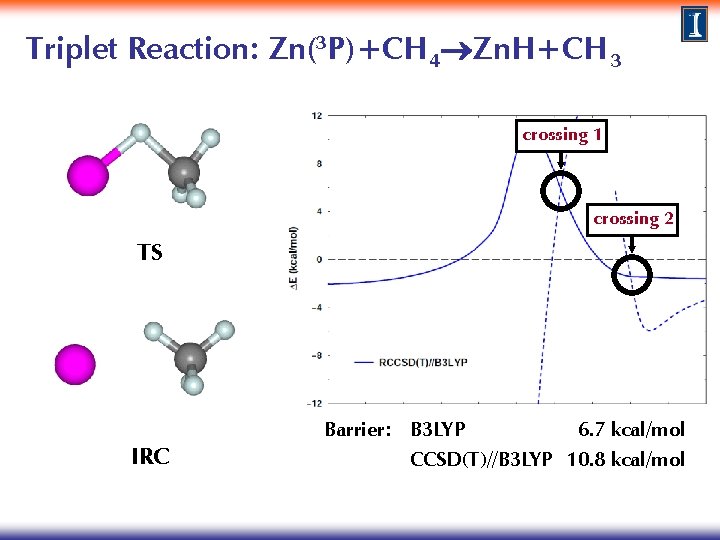
Triplet Reaction: Zn(3 P)+CH 4 Zn. H+CH 3 TS Zn+CH 4 Zn. H+CH 3 IRC Barrier: B 3 LYP 6. 7 kcal/mol

Triplet Reaction: Zn(3 P)+CH 4 Zn. H+CH 3 TS Zn+CH 4 Zn. H+CH 3 IRC Barrier: B 3 LYP 6. 7 kcal/mol CCSD(T)//B 3 LYP 10. 8 kcal/mol

Triplet Reaction: Zn(3 P)+CH 4 Zn. H+CH 3 TS IRC Barrier: B 3 LYP 6. 7 kcal/mol CCSD(T)//B 3 LYP 10. 8 kcal/mol

Triplet Reaction: Zn(3 P)+CH 4 Zn. H+CH 3 crossing 1 crossing 2 TS IRC Barrier: B 3 LYP 6. 7 kcal/mol CCSD(T)//B 3 LYP 10. 8 kcal/mol

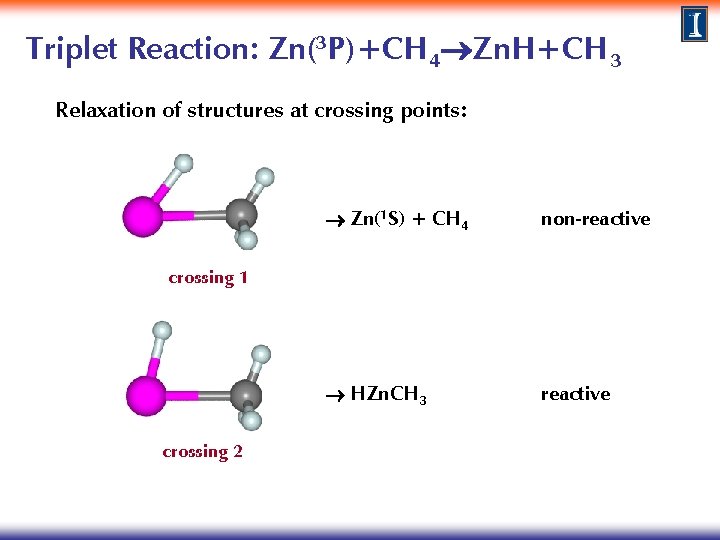
Triplet Reaction: Zn(3 P)+CH 4 Zn. H+CH 3 Relaxation of structures at crossing points: Zn(1 S) + CH 4 non-reactive HZn. CH 3 reactive crossing 1 crossing 2

Singlet vs. Triplet Reactions The singlet surface reaction leads to direct insertion, but the barrier is very high. The triplet surface reaction leads to insertion if hopping occurs to the singlet surface late in the IRC. There is still a modest barrier to overcome, but surface hopping may or may not be very efficient. Harvey (Phys Chem Phys 9, 331, 2007) found that reaction kinetics favor spin-allowed pathways at high temperatures.

Loose Ends Verify singlet and triplet transition states at full RCCSD(T) and/or MRCI levels of theory Calculate triplet to singlet surface hopping rates Explore the Zn(1 S) + CH 4 Zn. H + CH 3 abstraction reaction: if it occurs, is it competitive with the insertion reaction? Compare to prior theory for both spin surfaces.

Conclusions § Zinc species such as Zn. H, Zn. H 2, Zn. CH 3, and HZn. CH 3 all arise from recoupling the 4 s 2 pair in a manner similar to recoupling s 2 pairs in early p-block elements (Be-C, Mg-Si, etc. ) and p 2 pairs in late p-block elements (S-Cl, etc. ). § Singlet and triplet pathways to HZn. CH 3 were found: while there is a large barrier on the singlet pathway, may still be the dominant pathway if the surface hopping rate is very slow. it

Acknowledgement Funding: The Distinguished Chair for Research Excellence in Chemistry at the University of Illinois at Urbana-Champaign L-R: Lina Chen, Jeff Leiding, Beth Lindquist, Thom Dunning, Lu Xu, David Woon, Tyler Takeshita
 Classical mechanics
Classical mechanics Quantum physics vs quantum mechanics
Quantum physics vs quantum mechanics Chapter 27 quantum theory study guide answers
Chapter 27 quantum theory study guide answers Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 1 chemical changes
Section 1 chemical changes Formula of love
Formula of love Are kc and kp equal
Are kc and kp equal Chemical reactions study guide
Chemical reactions study guide Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Baking chemical change
Baking chemical change Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions What is case series
What is case series Retrospective cohort study vs prospective cohort study
Retrospective cohort study vs prospective cohort study What is method study
What is method study Marty lobdell
Marty lobdell In area
In area Time study objectives
Time study objectives Differentiate between time study and motion study
Differentiate between time study and motion study What is time
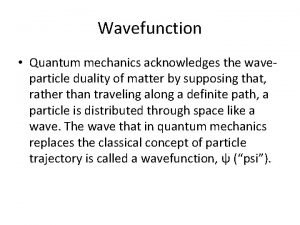
What is time Eigenfunction
Eigenfunction Heisenberg uncertainty principle
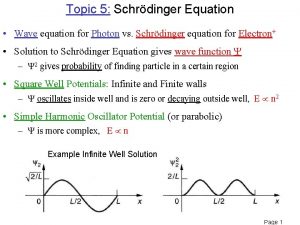
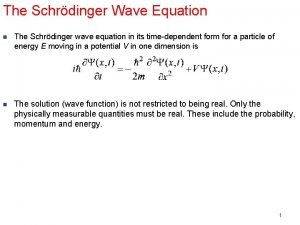
Heisenberg uncertainty principle Schrodinger wave equation
Schrodinger wave equation Physics topic 12
Physics topic 12 Chirozone
Chirozone Expectation value in quantum mechanics
Expectation value in quantum mechanics Expectation value of energy in quantum mechanics
Expectation value of energy in quantum mechanics Quantum mechanical model atom
Quantum mechanical model atom Gil kalai quantum computing
Gil kalai quantum computing The limits of quantum computers
The limits of quantum computers Turing test
Turing test Quantum mechanical model vs bohr model
Quantum mechanical model vs bohr model Quantum numbers and electron configuration
Quantum numbers and electron configuration