2019 Neurodevelopmental Disorders Research Retreat Power Point Presentations






















- Slides: 65

2019 Neurodevelopmental Disorders Research Retreat Power. Point Presentations

Thank YOU for joining us! Retreat Organizing Committee: Sponsors: Manny Casanova (USC-Greenville) Chris Cowan (MUSC) Perry Halushka (MUSC) Jane Joseph (MUSC) Dayan Ranwala (MUSC) Jane Roberts (USC) Rich Steet (GGC) Jeff Twiss (USC) Michael Watson (MUSC) • • • Office of the Provost, MUSC SC Commission on Higher Education Office of Research, USC Greenwood Genetics Center Neuroscience Institute, MUSC South Carolina Clinical and Translational Research Institute, MUSC (NIH - UL 1 TR 001450)

South Carolina Autism and Neurodevelopmental Disorders (SCAND) Consortium – 2 nd Annual retreat Mission: to drive research excellence in South Carolina for clinical, translational and mechanistic studies aimed at understanding and treating neurodevelopmental and autism spectrum disorders through a state-wide, multi-disciplinary research consortium. South Carolina institutions represented today: College of Charleston, The Citadel, Claflin Univ, Clemson Univ, Furman Univ, Greenwood Genetic Center, USC and USC-Greenville School of Medicine

From Bench to Bedside and Back Again: integrated approach to studying the genetics of a neurodevelopmental disorder • At the Bench: MEF 2 proteins regulate activity-dependent synapse development Christopher W. Cowan, Ph. D, MUSC • At the Bedside: human genome sequencing links MEF 2 C to syndromic autism Steven Skinner, MD, Greenwood Genetics Center • Back to the Bench: modeling MEF 2 C haploinsufficiency syndrome in mice Adam Harrington, Ph. D, MUSC • Into the Future: Insights into brain pathology and potential therapeutic strategies Catherine Bridges, BS, MUSC Question and Answer period

At the Bench: MEF 2 Proteins Regulate Activity-Dependent Synapse Development Christopher W. Cowan, Ph. D Professor of Neuroscience William E Murray Smart. State Endowed Chair in Neuroscience

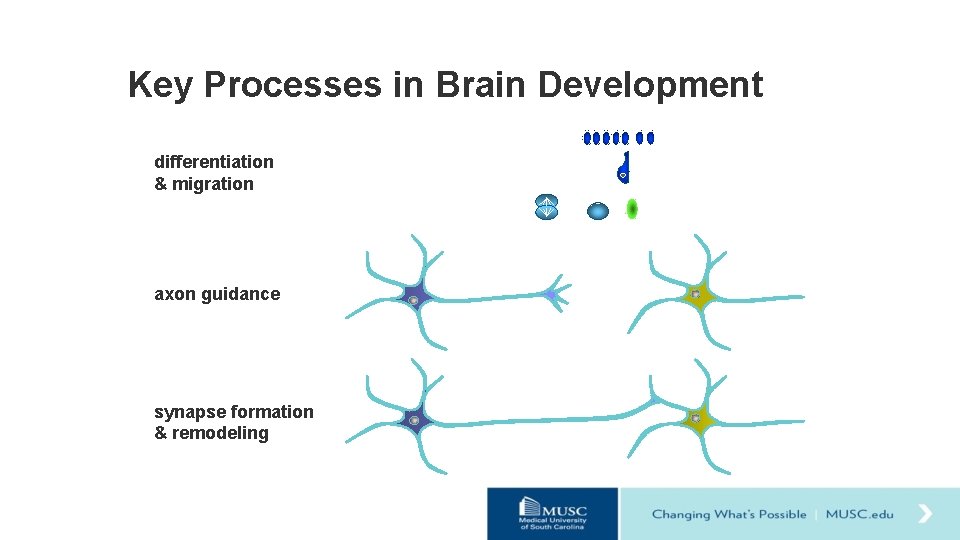
Key Processes in Brain Development differentiation & migration axon guidance synapse formation & remodeling

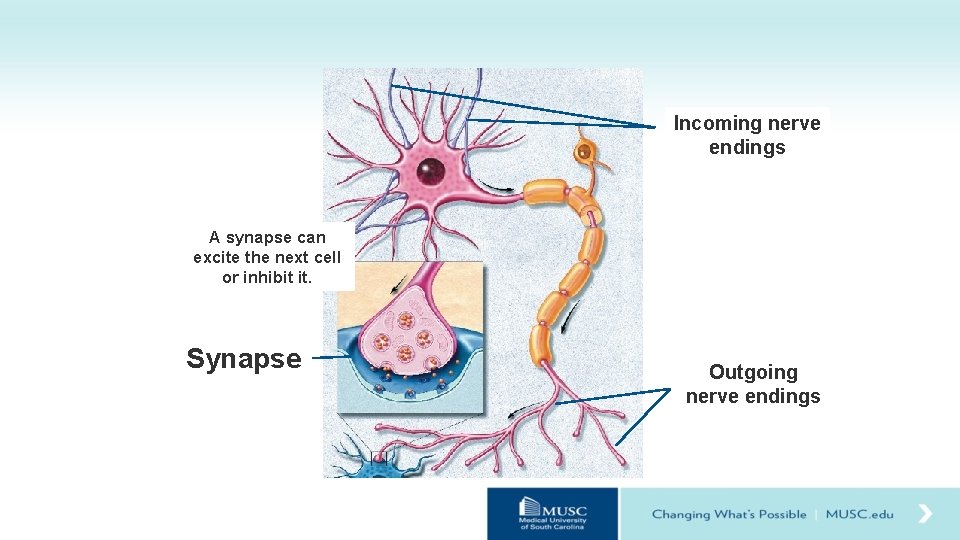
Incoming nerve endings A synapse can excite the next cell or inhibit it. Synapse Outgoing nerve endings

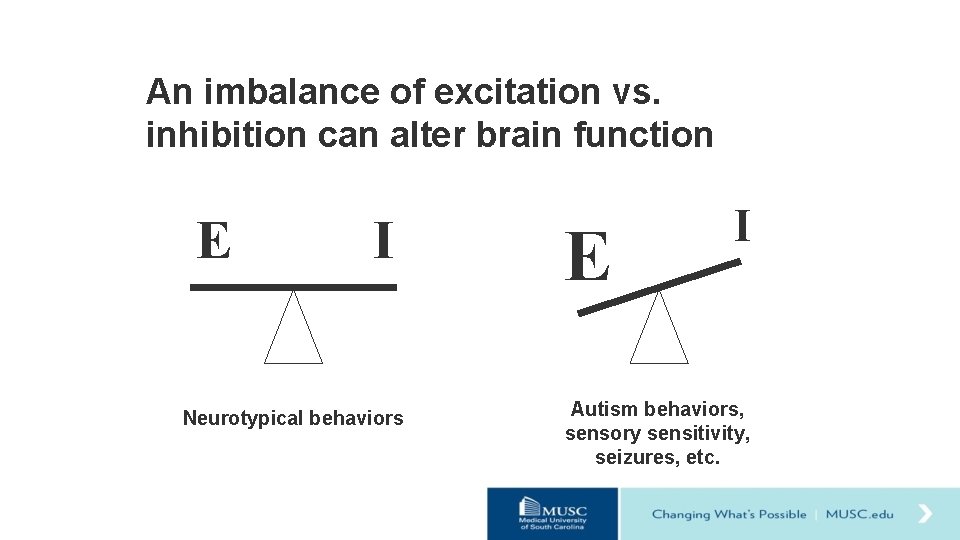
An imbalance of excitation vs. inhibition can alter brain function E I Neurotypical behaviors E I Autism behaviors, sensory sensitivity, seizures, etc.

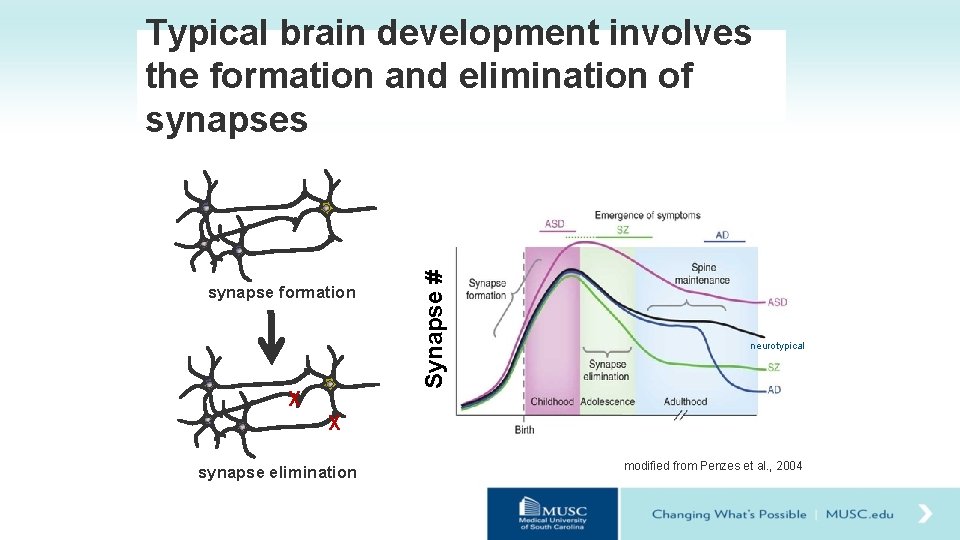
synapse formation X Synapse # Typical brain development involves the formation and elimination of synapses neurotypical X synapse elimination modified from Penzes et al. , 2004

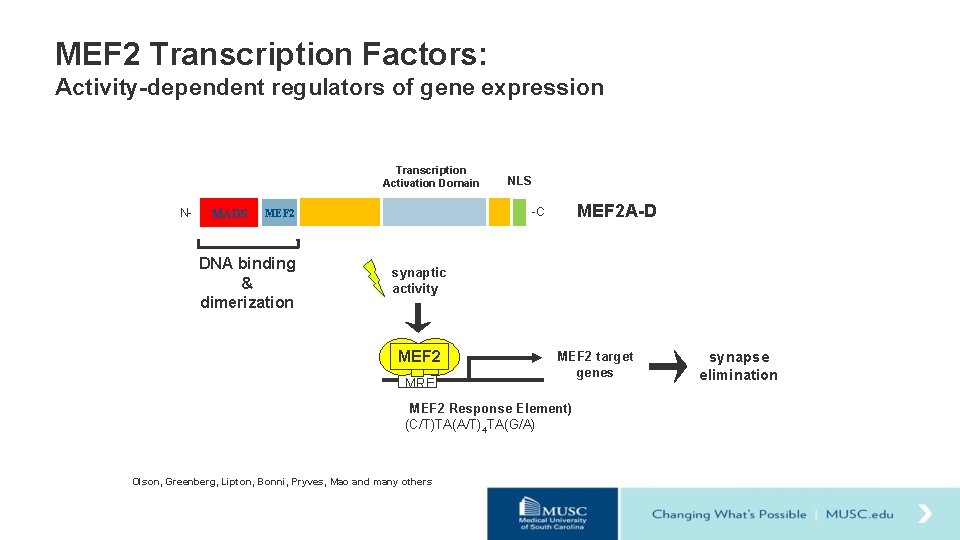
MEF 2 Transcription Factors: Activity-dependent regulators of gene expression Transcription Activation Domain N- MADS MEF 2 A-D -C MEF 2 DNA binding & dimerization NLS synaptic activity MEF 2 MRE MEF 2 target genes (MEF 2 Response Element) (C/T)TA(A/T)4 TA(G/A) Olson, Greenberg, Lipton, Bonni, Pryves, Mao and many others synapse elimination

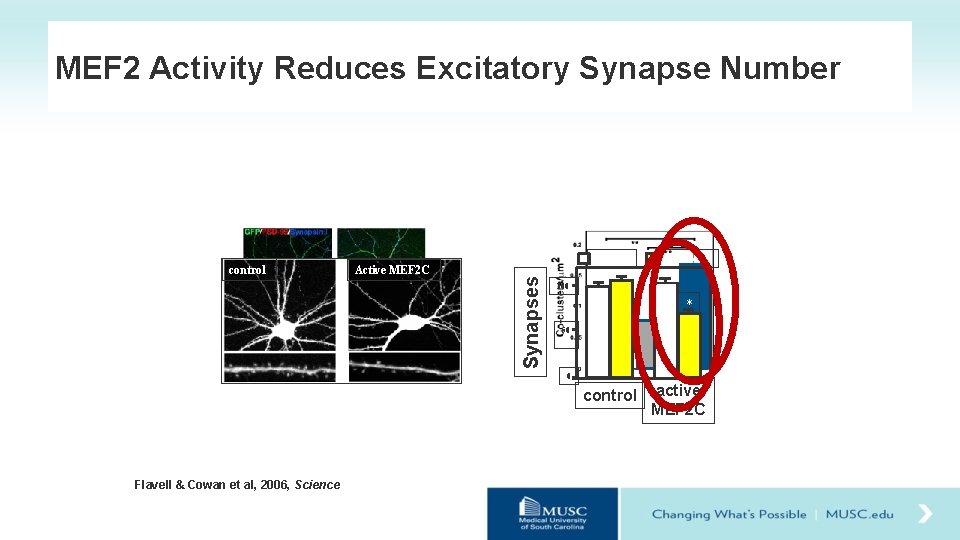
MEF 2 Activity Reduces Excitatory Synapse Number vehicle Active MEF 2 C Synapses control tamoxifen 100 * 50 0 controls active Reduced control MEF 2 C MEF 2 Flavell & Cowan et al, 2006, Science

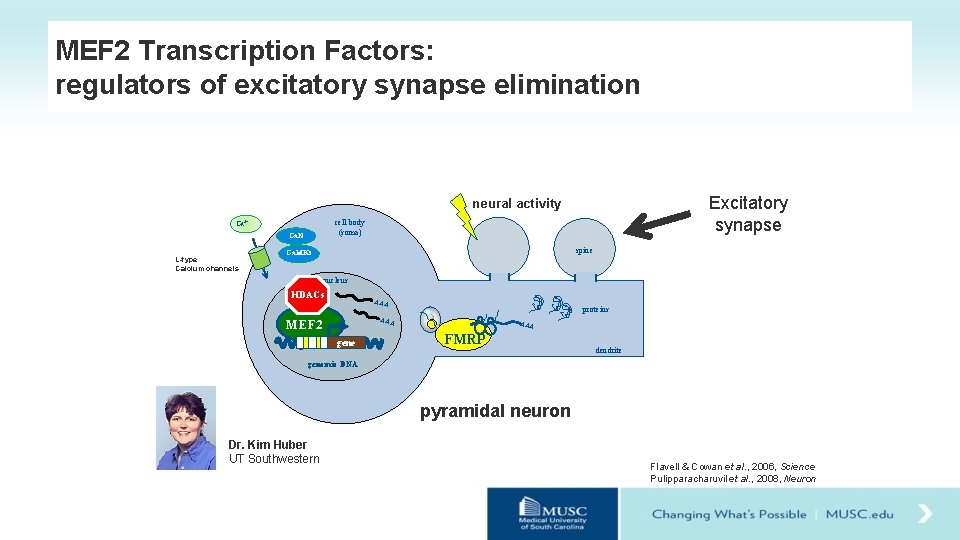
MEF 2 Transcription Factors: regulators of excitatory synapse elimination Excitatory synapse neural activity cell body (soma) Ca 2+ Ca. N L-type Calcium channels spine Ca. MKs nucleus HDACs AAA MEF 2 gene proteins AAA FMRP dendrite genomic DNA pyramidal neuron Dr. Kim Huber UT Southwestern Flavell & Cowan et al. , 2006, Science Pulipparacharuvil et al. , 2008, Neuron

Fragile X Syndrome Trinucleotide repeat expansion in the FMR 1 gene that causes transcriptional silencing Leading genetic cause of Autism and ID Sensory hypersensitivity, ADHD, social anxiety & epilepsy

Increased synapse # in Fragile X Syndrome HUMAN control FXS Irwin et al. , 2001 MOUSE wildtype Fmr 1 KO Dolen et al. , 2007

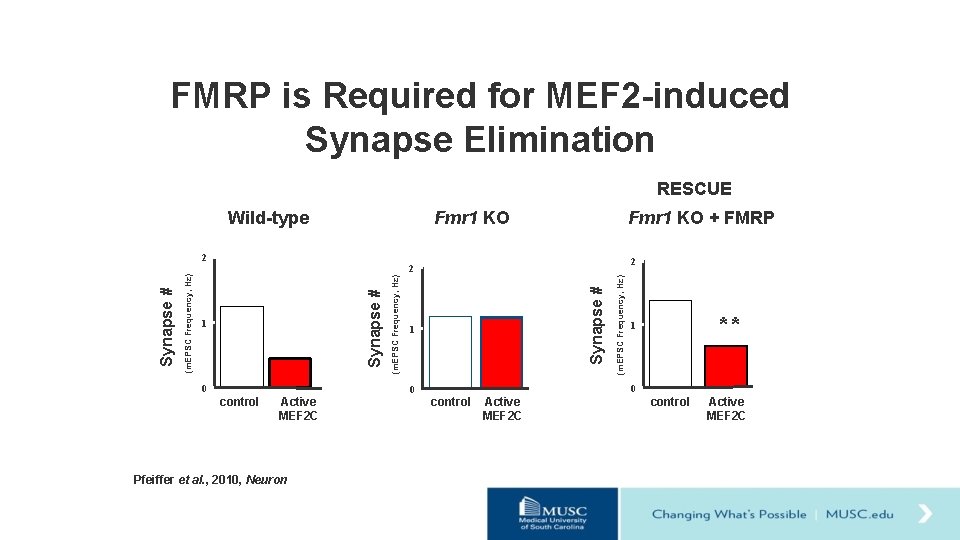
FMRP is Required for MEF 2 -induced Synapse Elimination RESCUE Fmr 1 KO Wild-type 2 0 1 Active MEF 2 C Pfeiffer et al. , 2010, Neuron ** 1 0 0 control (m. EPSC Frequency, Hz) * Synapse # 1 (m. EPSC Frequency, Hz) 2 Synapse # (m. EPSC Frequency, Hz) Synapse # 2 Fmr 1 KO + FMRP control Active MEF 2 C

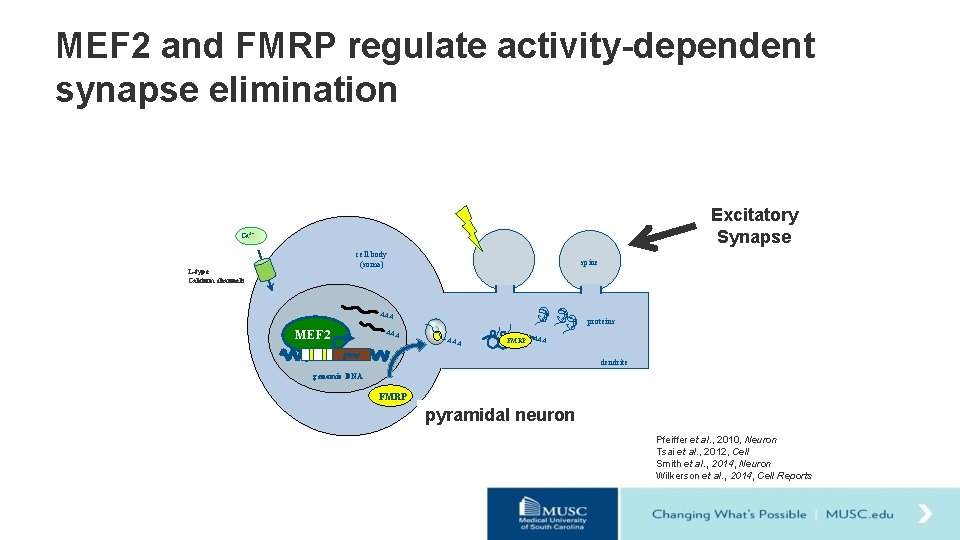
MEF 2 and FMRP regulate activity-dependent synapse elimination Excitatory Synapse neural activity Ca 2+ cell body (soma) L-type Calcium channels spine AAA MEF 2 AAA proteins AAA FMRP AAA gene dendrite genomic DNA FMRP pyramidal neuron Pfeiffer et al. , 2010, Neuron Tsai et al. , 2012, Cell Smith et al. , 2014, Neuron Wilkerson et al. , 2014, Cell Reports

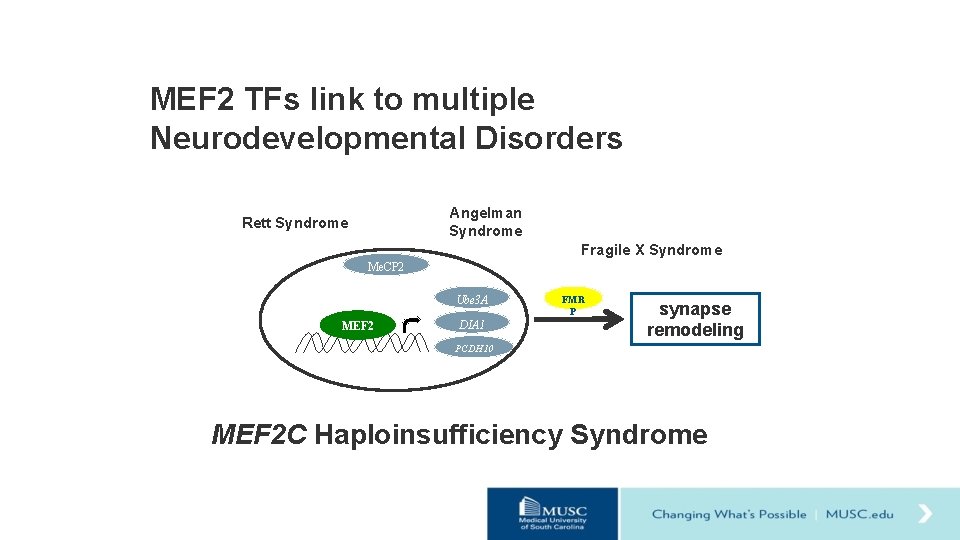
MEF 2 TFs link to multiple Neurodevelopmental Disorders Angelman Syndrome Rett Syndrome Fragile X Syndrome Me. CP 2 Ube 3 A MEF 2 DIA 1 FMR P synapse remodeling PCDH 10 MEF 2 C Haploinsufficiency Syndrome

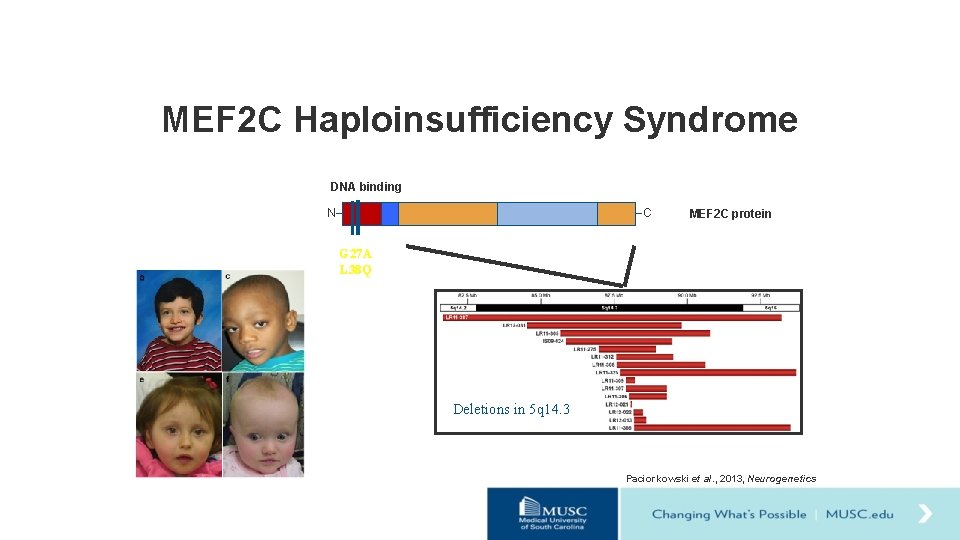
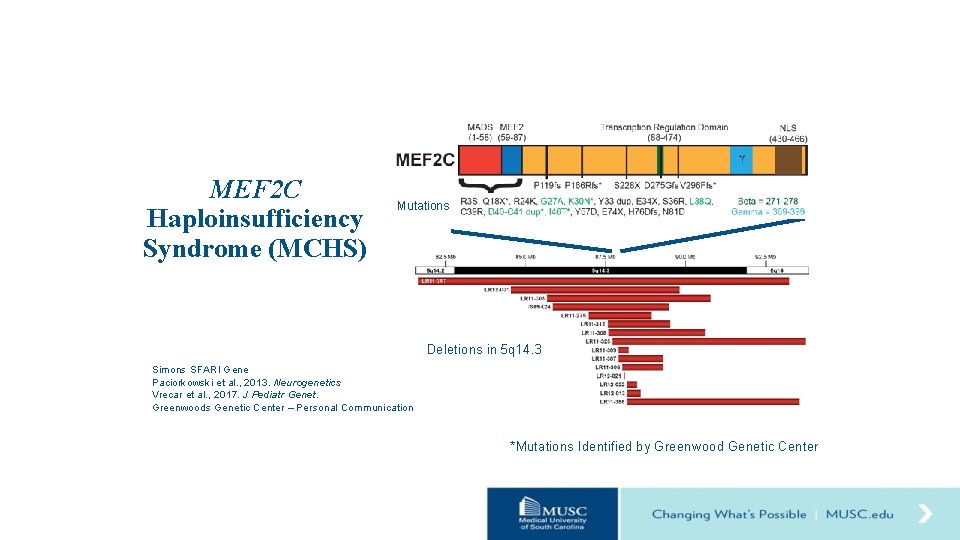
MEF 2 C Haploinsufficiency Syndrome DNA binding N C MEF 2 C protein G 27 A L 38 Q Deletions in 5 q 14. 3 Paciorkowski et al. , 2013, Neurogenetics

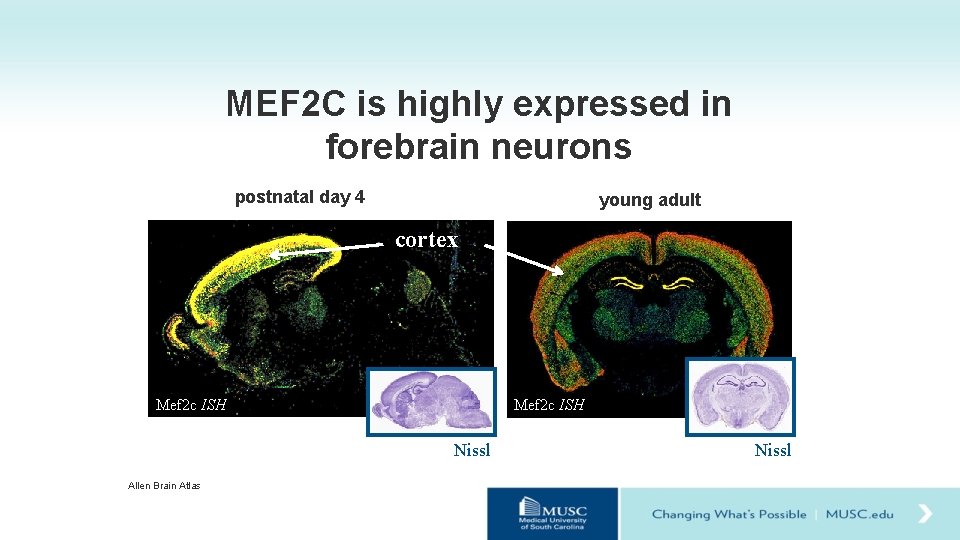
MEF 2 C is highly expressed in forebrain neurons postnatal day 4 young adult cortex Mef 2 c ISH Nissl Allen Brain Atlas Nissl

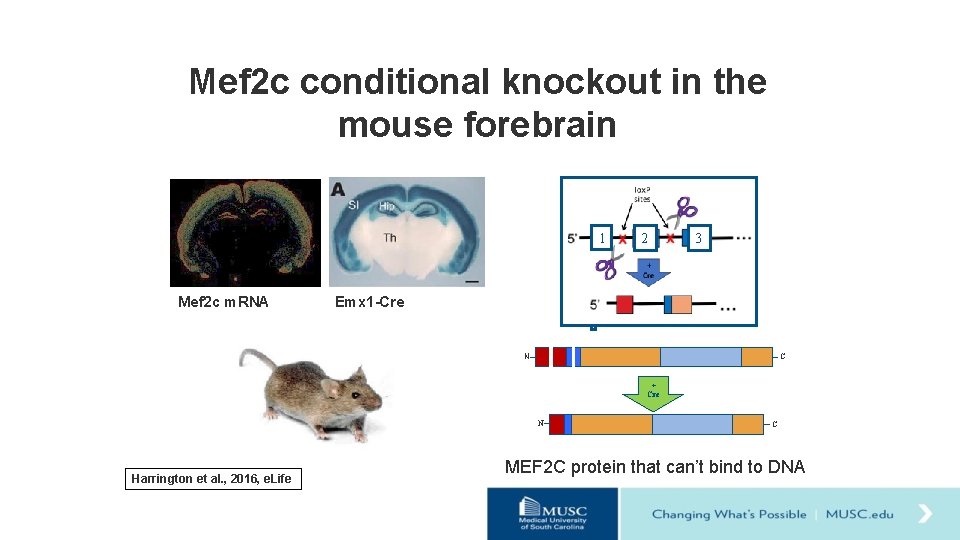
Mef 2 c conditional knockout in the mouse forebrain 1 Mef 2 c m. RNA 2 3 Emx 1 -Cre N C + Cre N Harrington et al. , 2016, e. Life C MEF 2 C protein that can’t bind to DNA

Mef 2 c conditional knockout mice display numerous NDD-related behaviors 1. Reduced social interaction 2. Reduced ultrasonic vocalizations (social-related communication) 3. Increased repetitive motor movements 4. Motor hyperactivity 5. Severe deficits in learning and memory 6. Altered Anxiety-like behavior Harrington et al. , 2016, e. Life

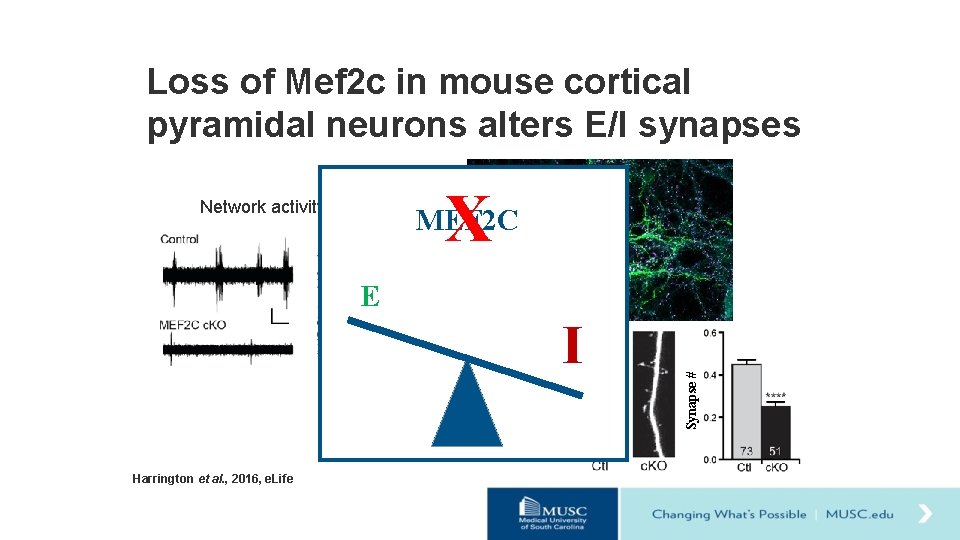
Loss of Mef 2 c in mouse cortical pyramidal neurons alters E/I synapses X Network activity MEF 2 C I Harrington et al. , 2016, e. Life Synapse # E

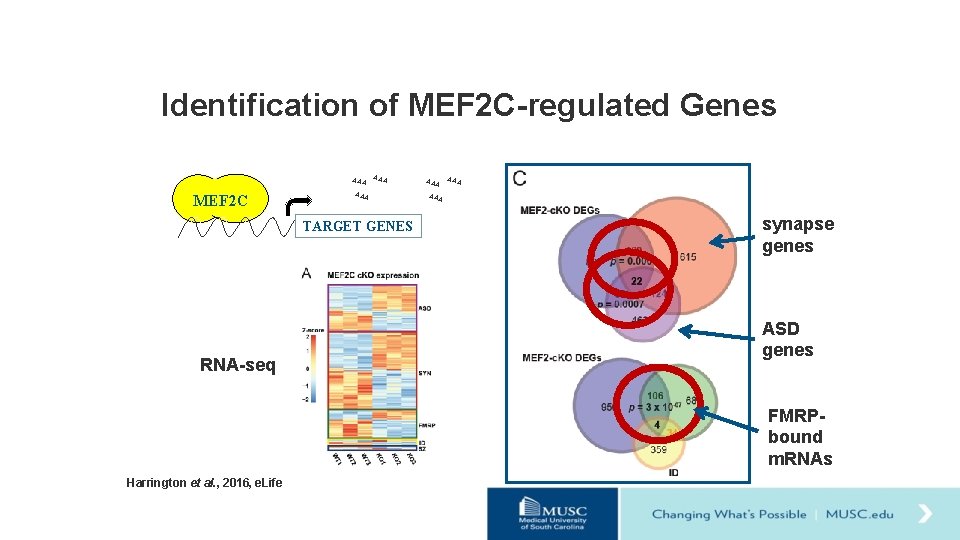
Identification of MEF 2 C-regulated Genes AAA MEF 2 C AAA TARGET GENES RNA-seq AAA AAA synapse genes ASD genes FMRPbound m. RNAs Harrington et al. , 2016, e. Life

Summary 1. MEF 2 and FMRP work together to regulate excitatory synapse elimination 2. Deletions and mutations in MEF 2 C are associated with an emerging neurodevelopmental disorder (MCHS) 3. Selective loss of MEF 2 C in mouse cortex dysregulates hundreds of neuronal genes, including risk genes for ASD. 4. …and it produces behaviors reminiscent of ASD and NDD symptoms 5. MEF 2 C regulates E/I synapse balance in the developing cortex

At the bedside: Human Genome Sequencing Links MEF 2 C to Syndromic Autism Steven A. Skinner, MD Hannah Warren, MS CGC March 1, 2019

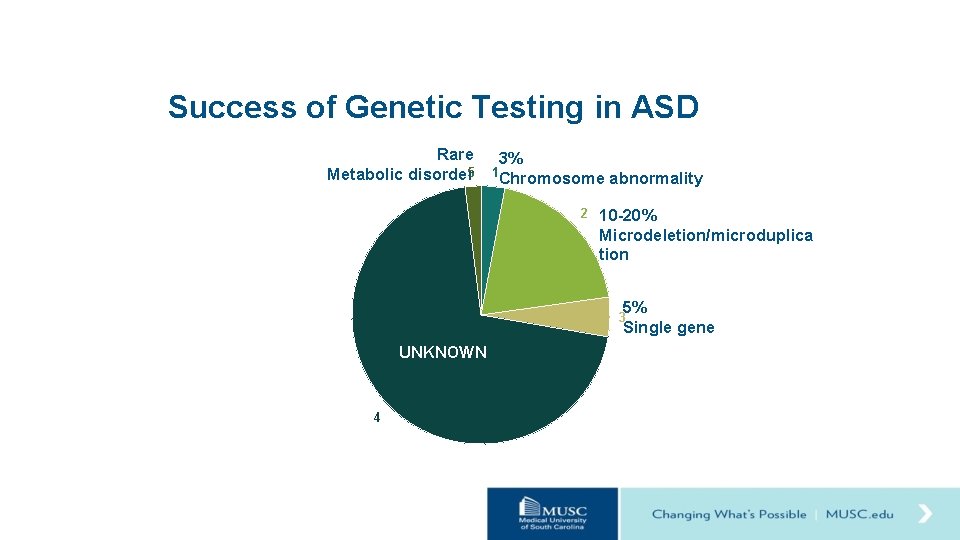
Success of Genetic Testing in ASD Rare 3% Metabolic disorder 5 1 Chromosome abnormality 2 10 -20% Microdeletion/microduplica tion 5% Single gene 3 UNKNOWN 4

MEF 2 C Originally identified as critical gene in 5 q 14. 3 microdeletion syndrome in 2010 Pathogenic variants within the gene have since been described MEF 2 C haploinsufficiency appears to impair central nervous system function Common clinical findings: › › › › Intellectual disability Autism spectrum disorder Epilepsy Absence of speech Limited walking abilities Hypotonia Stereotypic movements Minor brain malformations Rocha H. , et al. 2016. EJMG. 59, 478 -482. Zweier, M. , Rauch, A. 2011. Mol. Syndromol. 2, 164 -170.

Genotype-Phenotype Correlation Variability noted in symptoms associated with different alterations impacting the MEF 2 C gene › However, limited numbers overall Haploinsufficiency generally associated with severe intellectual disability, epilepsy, stereotypic movements, and brain abnormalities Missense variants and smaller deletions seem to be associated with a higher chance of independent walking, a lower risk of refractory seizures, and some limited speech Duplications seem to be associated with a milder phenotype of mild cognitive impairment, speech disorder, and microcephaly Wang et al. 2018. BMC Med Genet. 19: 191. Rocha H. , et al. 2016. EJMG. 59, 478 -482. Zweier, M. , Rauch, A. 2011. Mol. Syndromol. 2, 164 -170. Vrecar et al. 2017. J Pediatr Genet. 6, 129 -141.

Patient 1 Presented at age 9 y 8 m with global developmental delay Medical history: › History of grand mal seizures, well controlled with medication › Abnormal brain MRI with areas of heterotopia in left lateral region Developmental history: › Ambulatory with toe walking › Babbles with a few words, mostly nonverbal communication › Sleeping difficulties requiring medication, containment at night Age 9 y 8 m Behavioral abnormalities: › Repetitive hand movements including flipping pages in a book, hand flapping, hand mouthing › Some hyperventilation › Truncal rocking › Love of water › High pain tolerance Age 13 y 11 m

Patient 1 Numerous genetic and biochemical tests that were normal Whole exome sequencing pursued in 2014 › In frame duplication identified in MEF 2 C gene › c. 120_125 dup. CTATGA › Paternally inherited- clinically significant? Follow-up family studies showed father to be mosaic

Patient 2 Presented at age 14 m for developmental delay and seizures Medical history: › Seizures developed at 4 m › Normal brain MRI › Low muscle tone › Tremulous › Two small indentations with hair on the midline neck Developmental history: › Not sitting unsupported at evaluation › Some babbling › No behavioral concerns Age 16 m

Patient 2 Chromosomal microarray identified a 5 q 14. 3 microdeletion of 109 kb › Includes exons 1 and 2 of MEF 2 C gene › De novo

Patient 3 Presented at age 17 m for developmental delay and seizures Medical history: › Seizures developed at 6 m › Abnormal MRI with asymmetric appearance of the hippocampi › Low muscle tone › Some twitching movements Developmental history: › › › Was not sitting unsupported at initial evaluation Some noises with no babbling Bruxism, hyperextending of legs Potentially some pauses in breathing Reduced eye contact Age 17 m

Patient 3 Chromosomal microarray identified a maternally inherited variant of uncertain significance 145 -gene Epilepsy Panel identified a missense variant in MEF 2 C › c. 90 G>T (p. K 30 N) › Not previously reported › De novo

Patient 4 Presented at age 16 m for global developmental delay and 5 q 14. 3 microdeletion Medical history: › Abnormal EEG showing seizure activity at 14 m › Microcephaly › Low muscle tone › Failure to thrive Developmental history: › Not sitting unsupported at evaluation › Few single words › Repetitive hand movements with hand wringing Age 16 m

Patient 4 Chromosomal microarray identified 5 q 14. 3 microdeletion of 1264 kb › Encompasses entire MEF 2 C gene › Not maternally inherited, father yet to be tested › Presumed de novo

The Faces of MEF 2 C

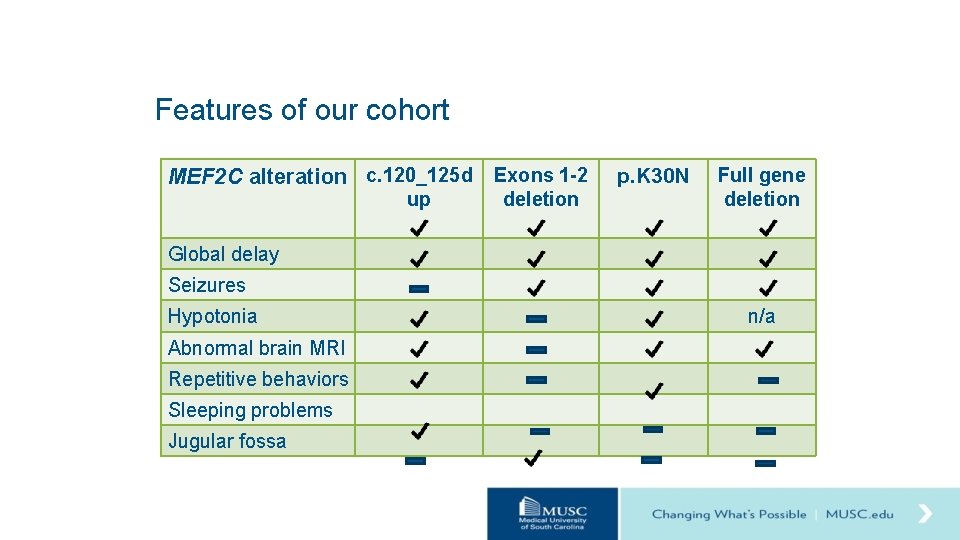
Features of our cohort MEF 2 C alteration c. 120_125 d Exons 1 -2 up deletion p. K 30 N Full gene deletion Global delay Seizures Hypotonia Abnormal brain MRI Repetitive behaviors Sleeping problems Jugular fossa n/a

Future Plans Developing parent questionnaire to expand clinical knowledge regarding alterations involving MEF 2 C

Back to the Bench: Modeling MEF 2 C Haploinsufficiency in Mice Adam Harrington, Ph. D Postdoctoral Scholar Cowan lab; Dept of Neuroscience Medical University of South Carolina

MEF 2 C Haploinsufficiency Syndrome (MCHS) Mutations Deletions in 5 q 14. 3 Simons SFARI Gene Paciorkowski et al. , 2013. Neurogenetics Vrecar et al. , 2017. J Pediatr Genet. Greenwoods Genetic Center – Personal Communication *Mutations Identified by Greenwood Genetic Center

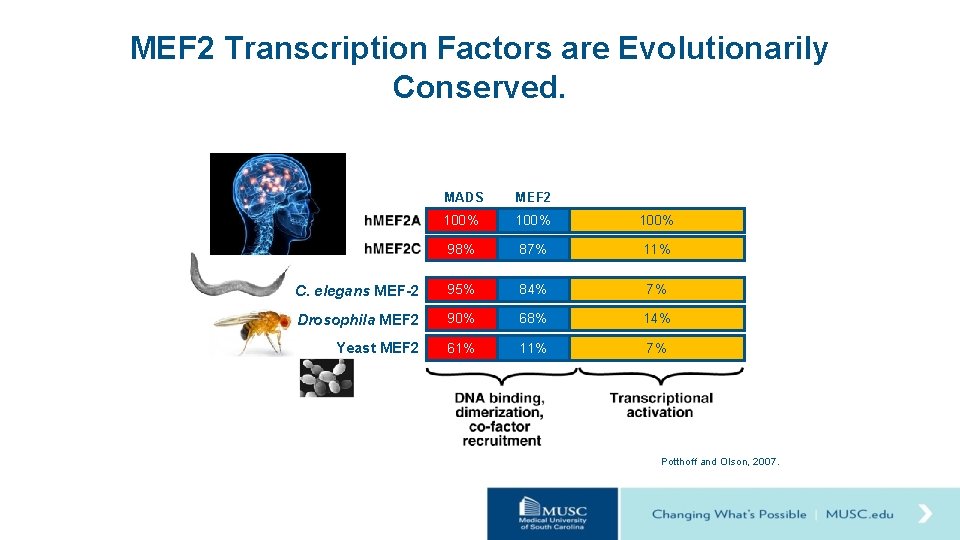
MEF 2 Transcription Factors are Evolutionarily Conserved. MADS MEF 2 100% 98% 87% 11% C. elegans MEF-2 95% 84% 7% Drosophila MEF 2 90% 68% 14% Yeast MEF 2 61% 11% 7% Potthoff and Olson, 2007.

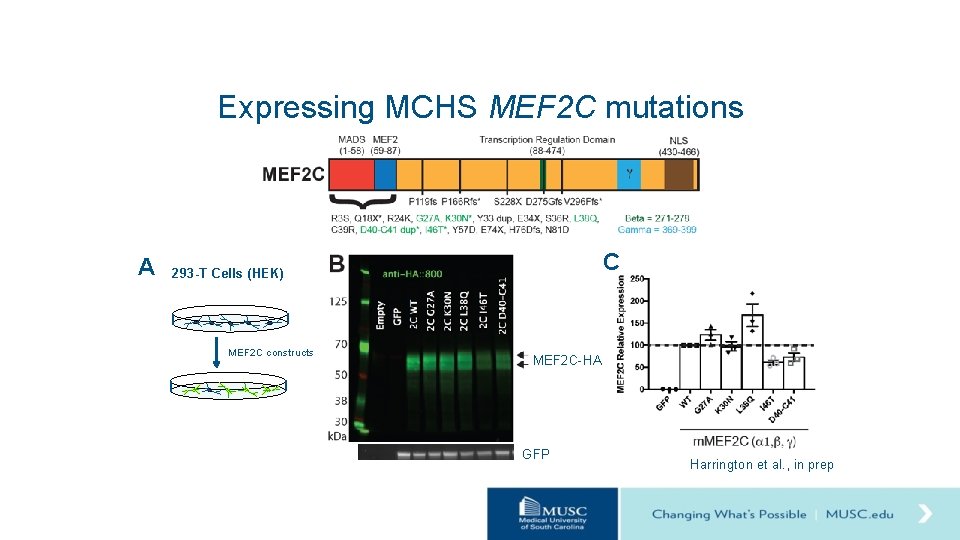
Expressing MCHS MEF 2 C mutations A C 293 -T Cells (HEK) MEF 2 C constructs MEF 2 C-HA GFP Harrington et al. , in prep

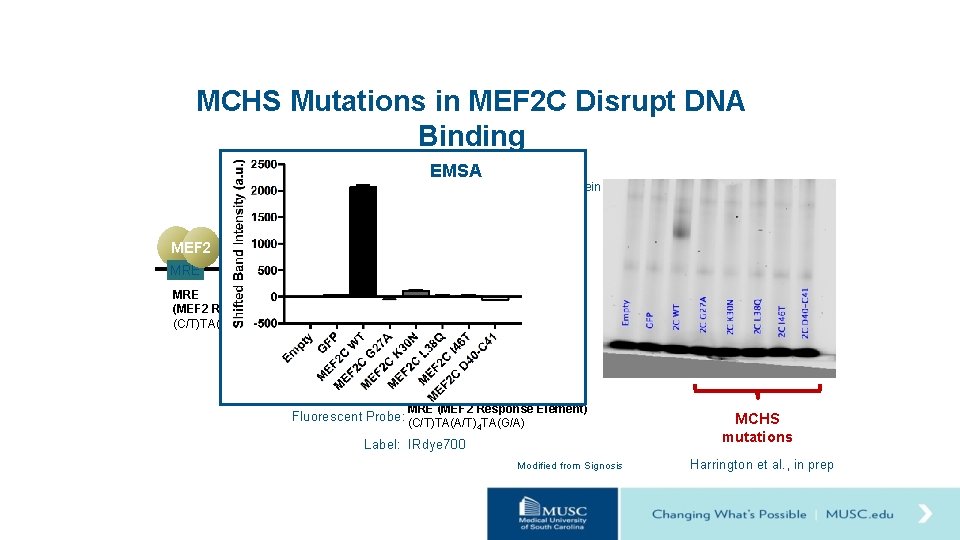
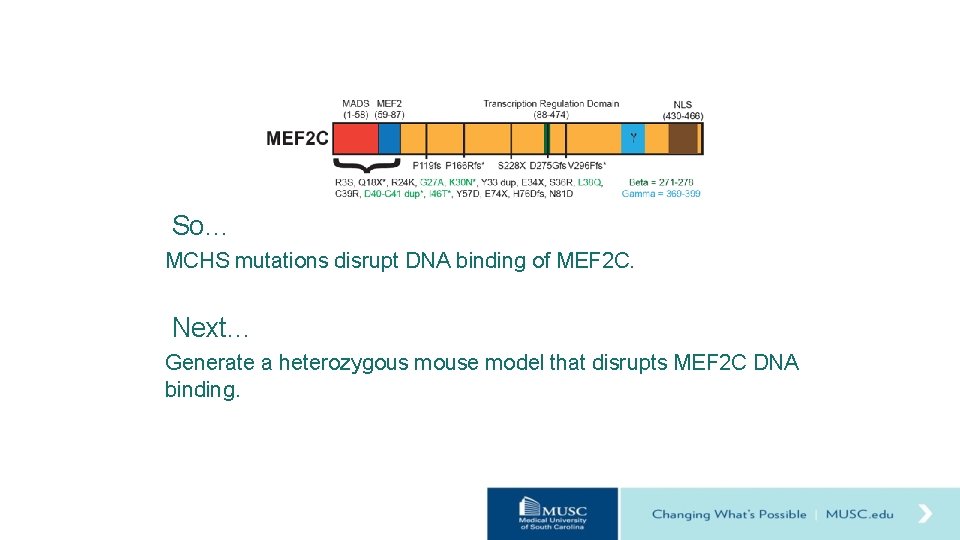
MCHS Mutations in MEF 2 C Disrupt DNA Binding EMSA MEF 2 + MEF 2 C Protein MEF 2 target genes MRE (MEF 2 Response Element) (C/T)TA(A/T)4 TA(G/A) MRE (MEF 2 Response Element) Fluorescent Probe: (C/T)TA(A/T) TA(G/A) 4 Label: IRdye 700 Modified from Signosis MCHS mutations Harrington et al. , in prep

So… MCHS mutations disrupt DNA binding of MEF 2 C. Next… Generate a heterozygous mouse model that disrupts MEF 2 C DNA binding.

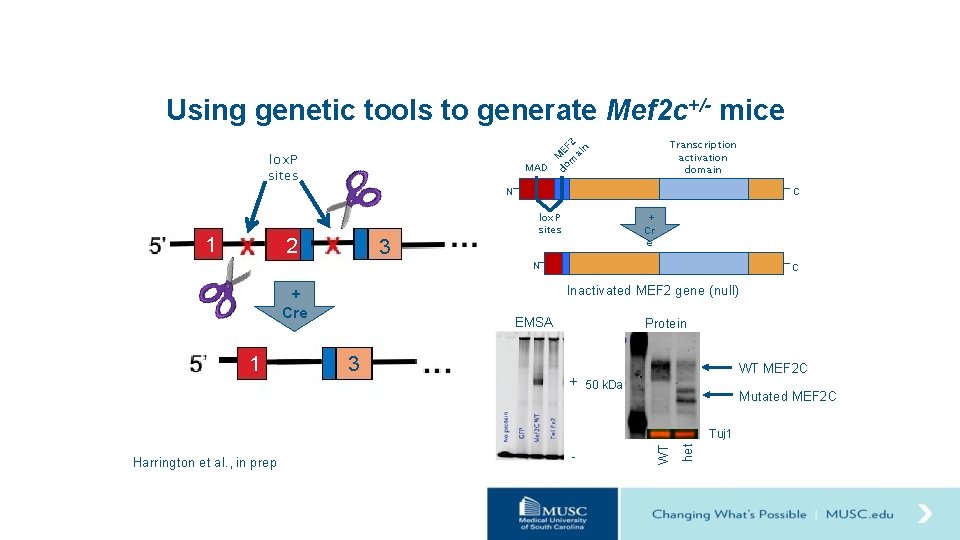
Using genetic tools to generate Mef 2 c+/- mice lox. P sites 1 N 2 3 2 EF in M ma MAD do S lox. P sites Transcription activation domain C + Cr e N Inactivated MEF 2 gene (null) + Cre 1 C EMSA 3 Protein WT MEF 2 C + 50 k. Da Mutated MEF 2 C - het Harrington et al. , in prep WT Tuj 1

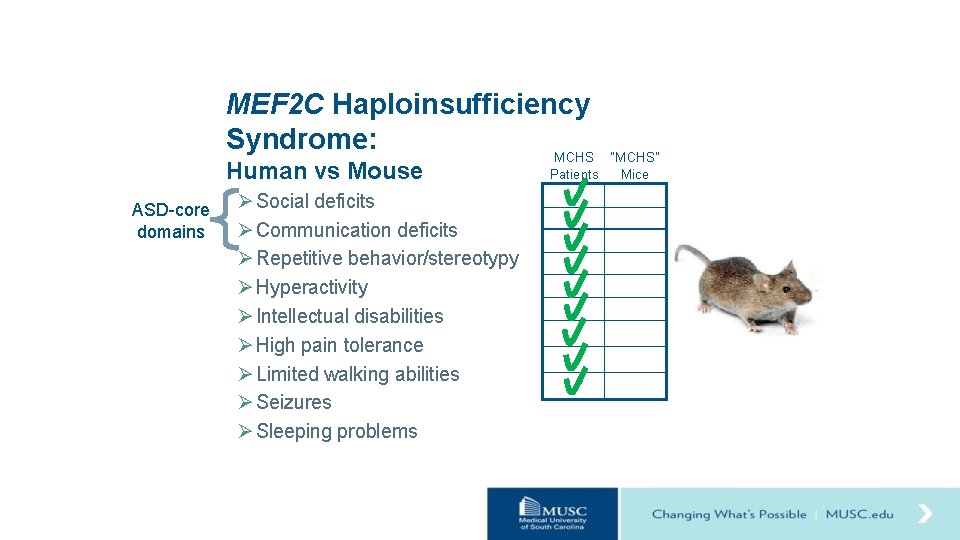
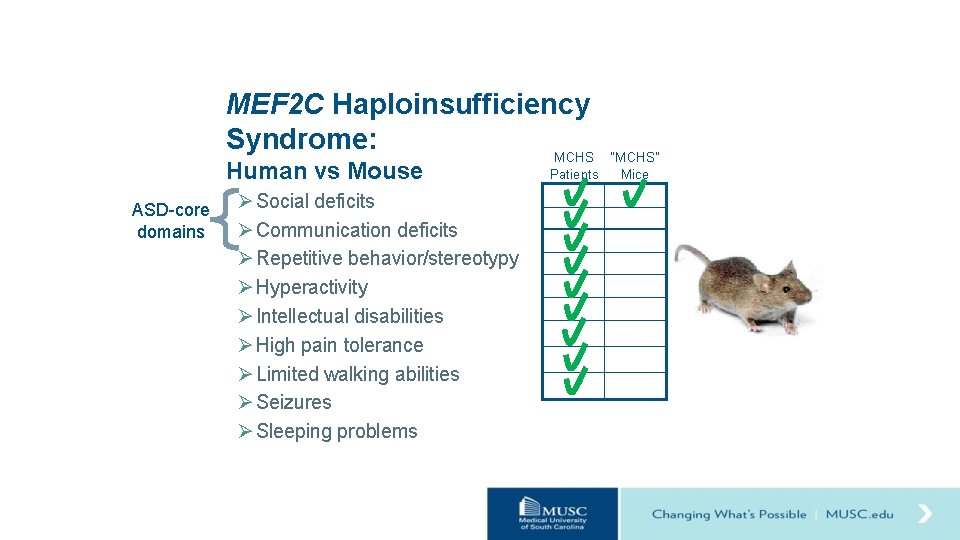
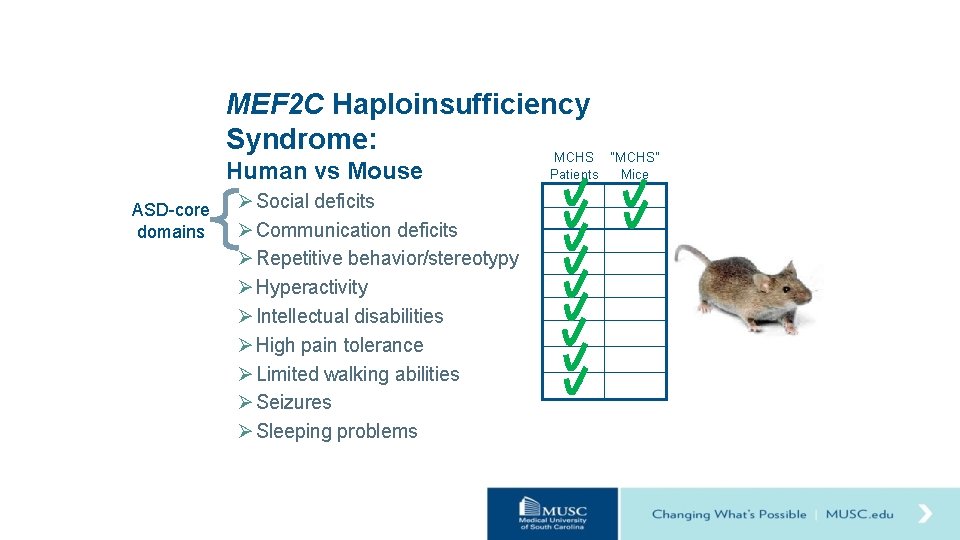
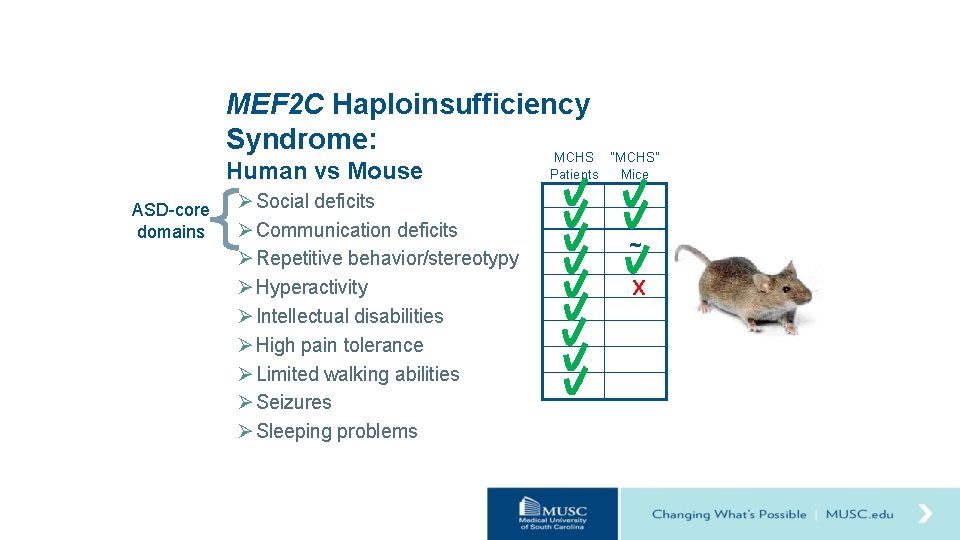
Hypothesis Loss of one copy of Mef 2 c (Mef 2 c+/-) in mice will result in behavioral changes relevant to MCHS. Ø Social deficits Ø Communication deficits Ø Repetitive behavior/stereotypy Ø Hyperactivity Ø Intellectual disabilities Ø High pain tolerance Ø Limited walking abilities Ø Seizures Ø Sleeping problems

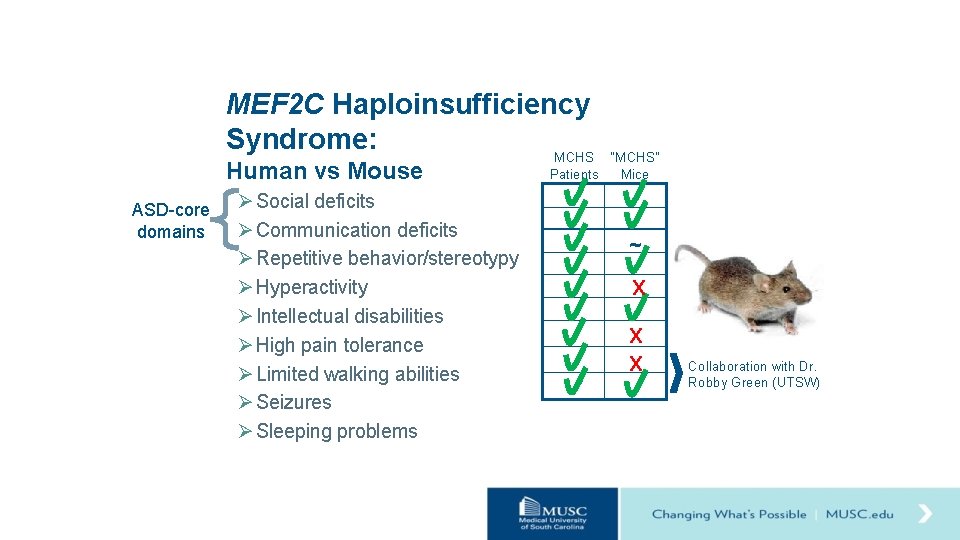
MEF 2 C Haploinsufficiency Syndrome: MCHS Human vs Mouse ASD-core domains Ø Social deficits Ø Communication deficits Ø Repetitive behavior/stereotypy Ø Hyperactivity Ø Intellectual disabilities Ø High pain tolerance Ø Limited walking abilities Ø Seizures Ø Sleeping problems “MCHS” Patients Mice

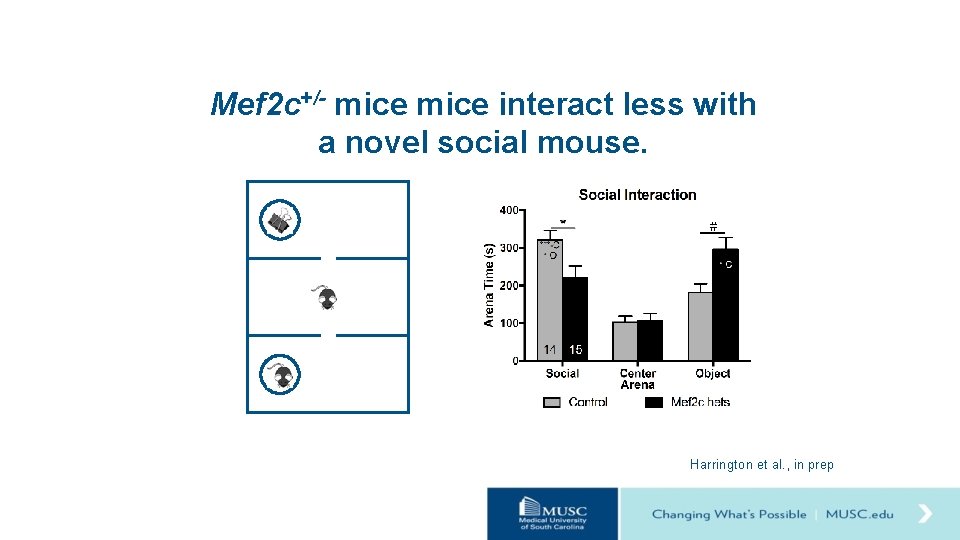
Mef 2 c+/- mice interact less with a novel social mouse. Harrington et al. , in prep

MEF 2 C Haploinsufficiency Syndrome: MCHS Human vs Mouse ASD-core domains Ø Social deficits Ø Communication deficits Ø Repetitive behavior/stereotypy Ø Hyperactivity Ø Intellectual disabilities Ø High pain tolerance Ø Limited walking abilities Ø Seizures Ø Sleeping problems “MCHS” Patients Mice

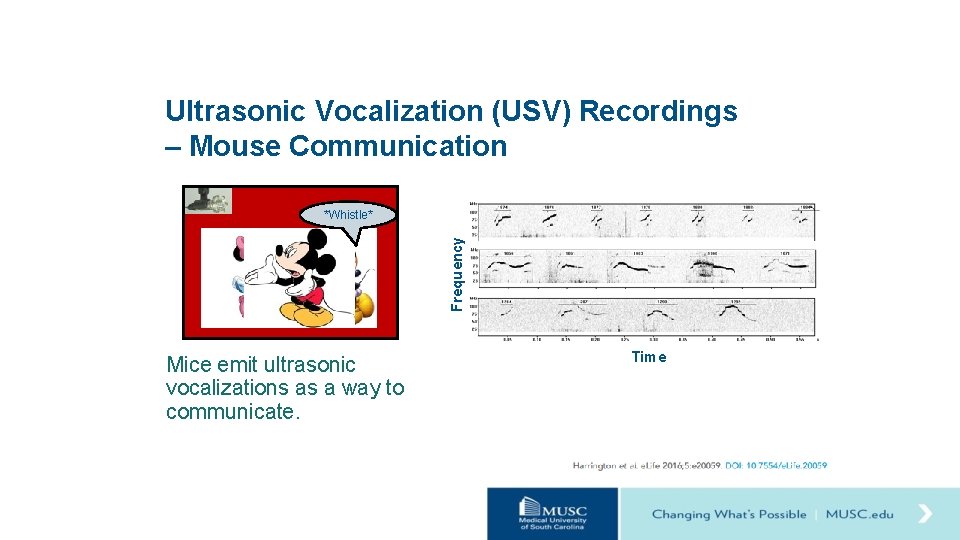
Ultrasonic Vocalization (USV) Recordings – Mouse Communication Frequency *Whistle* Mice emit ultrasonic vocalizations as a way to communicate. Time

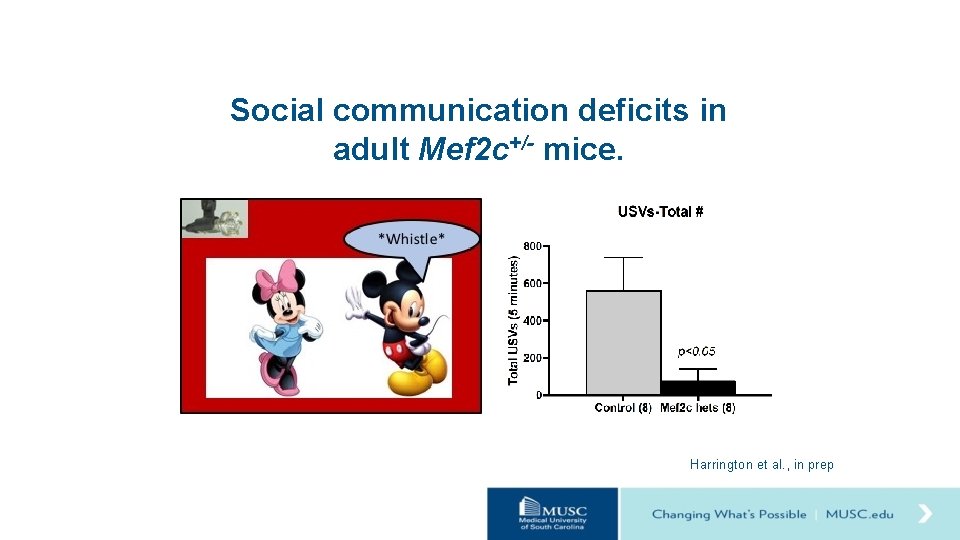
Social communication deficits in adult Mef 2 c+/- mice. Harrington et al. , in prep

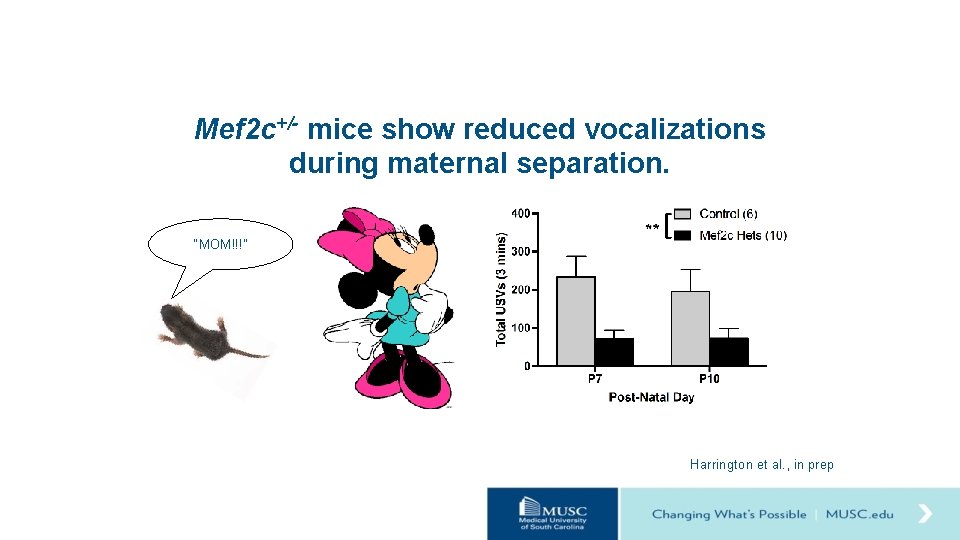
Mef 2 c+/- mice show reduced vocalizations during maternal separation. “MOM!!!” Harrington et al. , in prep

MEF 2 C Haploinsufficiency Syndrome: MCHS Human vs Mouse ASD-core domains Ø Social deficits Ø Communication deficits Ø Repetitive behavior/stereotypy Ø Hyperactivity Ø Intellectual disabilities Ø High pain tolerance Ø Limited walking abilities Ø Seizures Ø Sleeping problems “MCHS” Patients Mice

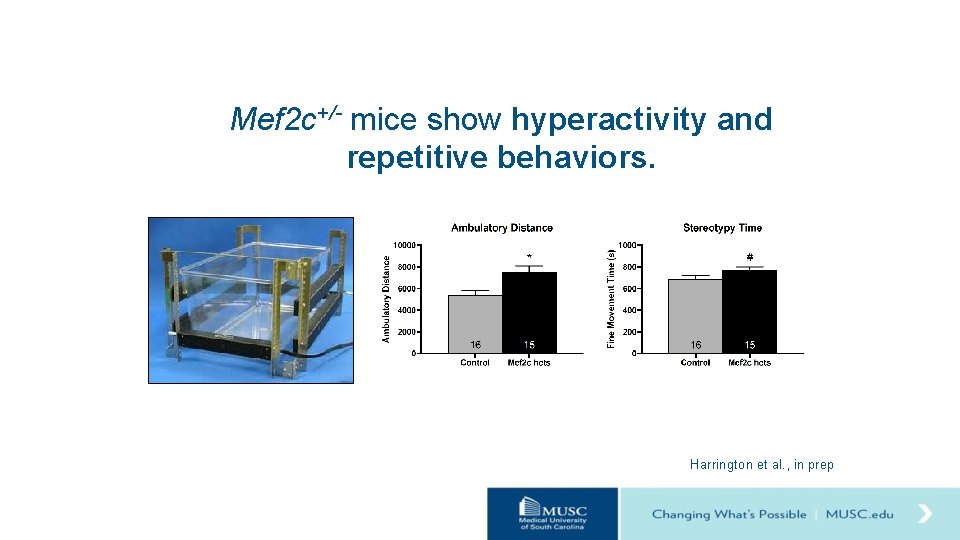
Mef 2 c+/- mice show hyperactivity and repetitive behaviors. Harrington et al. , in prep

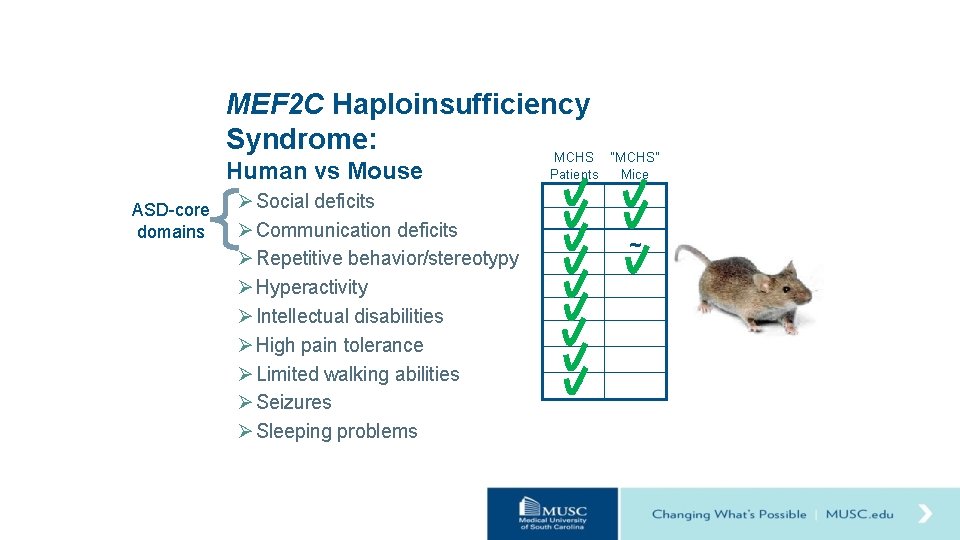
MEF 2 C Haploinsufficiency Syndrome: MCHS Human vs Mouse ASD-core domains Ø Social deficits Ø Communication deficits Ø Repetitive behavior/stereotypy Ø Hyperactivity Ø Intellectual disabilities Ø High pain tolerance Ø Limited walking abilities Ø Seizures Ø Sleeping problems “MCHS” Patients Mice ~

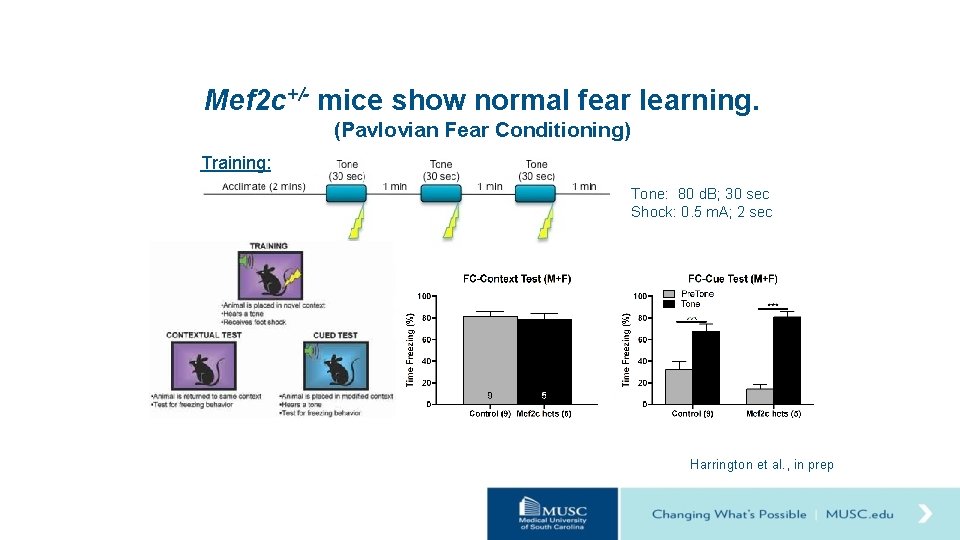
Mef 2 c+/- mice show normal fear learning. (Pavlovian Fear Conditioning) Training: Tone: 80 d. B; 30 sec Shock: 0. 5 m. A; 2 sec Harrington et al. , in prep

Mef 2 c+/- mice do not show intellectual disabilities. Other cognitive test: Spatial learning (Barnes maze) Working memory (Novel object recognition; y-maze) Operant learning (Sucrose self-administration (SA)) Discrimination learning (Sucrose SA) Extinction learning (Sucrose SA) Harrington et al. , in prep

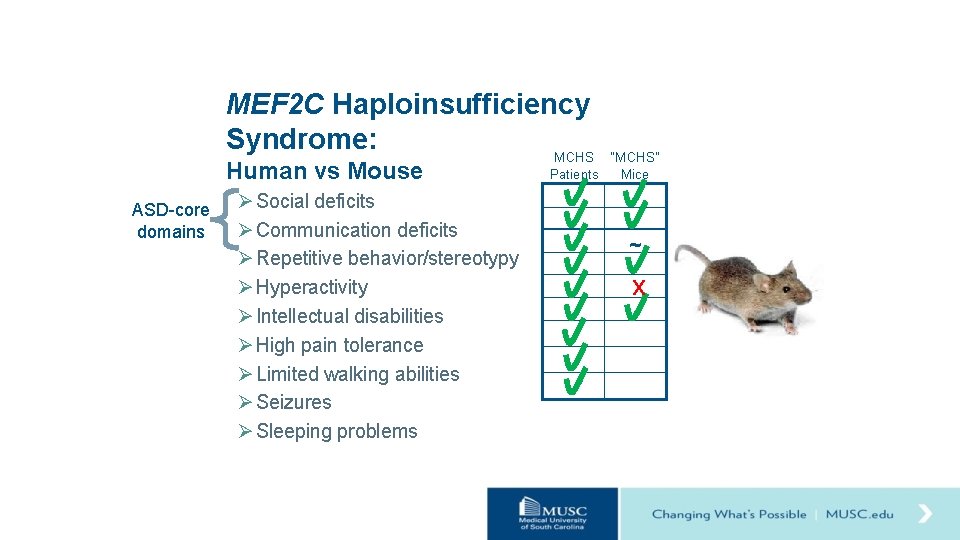
MEF 2 C Haploinsufficiency Syndrome: MCHS Human vs Mouse ASD-core domains Ø Social deficits Ø Communication deficits Ø Repetitive behavior/stereotypy Ø Hyperactivity Ø Intellectual disabilities Ø High pain tolerance Ø Limited walking abilities Ø Seizures Ø Sleeping problems “MCHS” Patients Mice ~ X

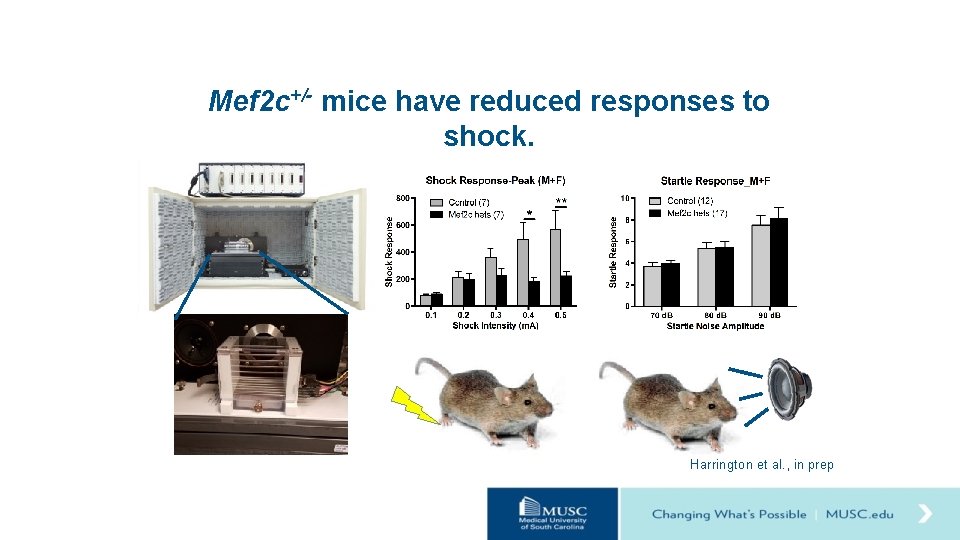
Mef 2 c+/- mice have reduced responses to shock. Harrington et al. , in prep

MEF 2 C Haploinsufficiency Syndrome: MCHS Human vs Mouse ASD-core domains Ø Social deficits Ø Communication deficits Ø Repetitive behavior/stereotypy Ø Hyperactivity Ø Intellectual disabilities Ø High pain tolerance Ø Limited walking abilities Ø Seizures Ø Sleeping problems “MCHS” Patients Mice ~ X

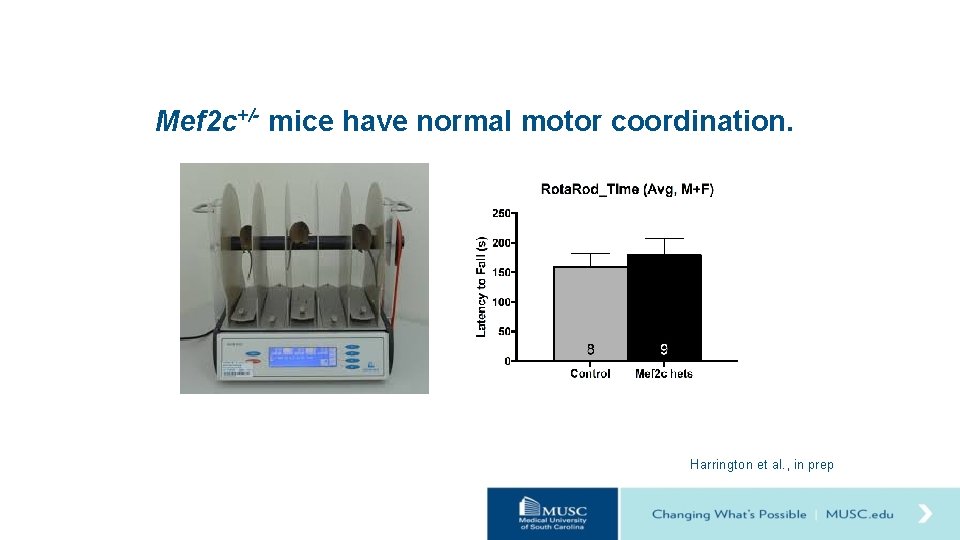
Mef 2 c+/- mice have normal motor coordination. Harrington et al. , in prep

MEF 2 C Haploinsufficiency Syndrome: MCHS Human vs Mouse ASD-core domains Ø Social deficits Ø Communication deficits Ø Repetitive behavior/stereotypy Ø Hyperactivity Ø Intellectual disabilities Ø High pain tolerance Ø Limited walking abilities Ø Seizures Ø Sleeping problems “MCHS” Patients Mice ~ X X X Collaboration with Dr. Robby Green (UTSW)

Summary Mef 2 c+/- mice display some behaviors similar to those reported in MEF 2 C Haploinsufficiency Syndrome (MCHS) individuals. Mef 2 c+/- mice may be a relevant animal model for studying brain and behavioral dysfunction in MCHS and for testing candidate therapeutics.

Challenges Heterogeneity of symptoms in MCHS individuals › Refining the phenotype of MCHS MEF 2 C deletions vs point mutations Genetic variation in humans › Polygenic risk alleles Environmental influences A mouse in NOT a human…
 Chapter 11 childhood and neurodevelopmental disorders
Chapter 11 childhood and neurodevelopmental disorders Neurodevelopmental disorders dsm-5 ppt
Neurodevelopmental disorders dsm-5 ppt Major neurocognitive disorder criteria
Major neurocognitive disorder criteria Neurodevelopmental disorders
Neurodevelopmental disorders Neurodevelopmental treatment for stroke
Neurodevelopmental treatment for stroke Neurodevelopmental therapy principles
Neurodevelopmental therapy principles Decreto 1330 de 2019 power point
Decreto 1330 de 2019 power point Power triangle diagram
Power triangle diagram Power bi power point
Power bi power point Point point power
Point point power Foot fire dribble adalah
Foot fire dribble adalah Olc technical services retreat
Olc technical services retreat Retreat at rtp
Retreat at rtp Retreat evaluation form
Retreat evaluation form Difference between slope decline and slope retreat
Difference between slope decline and slope retreat Vipassana 10 day silent retreat substance abuse
Vipassana 10 day silent retreat substance abuse Physician burnout retreat
Physician burnout retreat River types disposed to waywardness crossword clue
River types disposed to waywardness crossword clue Synthesis plural
Synthesis plural Covetousness
Covetousness Atmana bhava vindate
Atmana bhava vindate Craft of scientific presentations
Craft of scientific presentations Introduction to mental health awareness presentation
Introduction to mental health awareness presentation Worst presentation ever
Worst presentation ever To maintain audience interest in a multimedia presentation
To maintain audience interest in a multimedia presentation Slidetodoc
Slidetodoc Areas donde se utilizan las presentaciones electronicas
Areas donde se utilizan las presentaciones electronicas Developing oral and online presentation
Developing oral and online presentation Verbal support in presentations
Verbal support in presentations Tok presentations
Tok presentations World's worst presentation
World's worst presentation Business presentations bristol
Business presentations bristol Ria seminar system
Ria seminar system Boardworks ltd
Boardworks ltd Scqa analysis
Scqa analysis Internet presentations
Internet presentations Who is lazarus in the most dangerous game
Who is lazarus in the most dangerous game Note card for presentation
Note card for presentation The end pictures for presentations
The end pictures for presentations Useful phrases for presentations
Useful phrases for presentations Powerpoint add in efficient elements
Powerpoint add in efficient elements Yourexec
Yourexec Catchy titles for science projects
Catchy titles for science projects Research project title
Research project title Cooking merit badge powerpoint
Cooking merit badge powerpoint Customer service presentations
Customer service presentations Switch stacking concept
Switch stacking concept Bad powerpoint presentations examples
Bad powerpoint presentations examples Really bad powerpoint presentations
Really bad powerpoint presentations You exec presentations
You exec presentations Roof ppt presentations
Roof ppt presentations Yoursite com
Yoursite com Horse topics for presentations
Horse topics for presentations Types of oral presentations
Types of oral presentations Research methods for business students 2019
Research methods for business students 2019 Solar power satellites and microwave power transmission
Solar power satellites and microwave power transmission Potential power
Potential power Flex power power supply
Flex power power supply Unit of dispersive power of grating
Unit of dispersive power of grating Power of a power property
Power of a power property General power rule vs power rule
General power rule vs power rule Power angle curve in power system stability
Power angle curve in power system stability Power absorbed or supplied
Power absorbed or supplied Evangelio del domingo en power point
Evangelio del domingo en power point Como hacer una ova
Como hacer una ova Powerpoint boutique
Powerpoint boutique