Mitochondrial DNA and neurodevelopmental disorders in HEU children





- Slides: 27

Mitochondrial DNA and neurodevelopmental disorders in HEU children – observations from the CARMA cohort 3 rd HIV Exposed Uninfected (HEU) Child Workshop July 23, 2017 Hélène Côté, Ph. D Pathology & Laboratory Medicine The University of British Columbia Canada

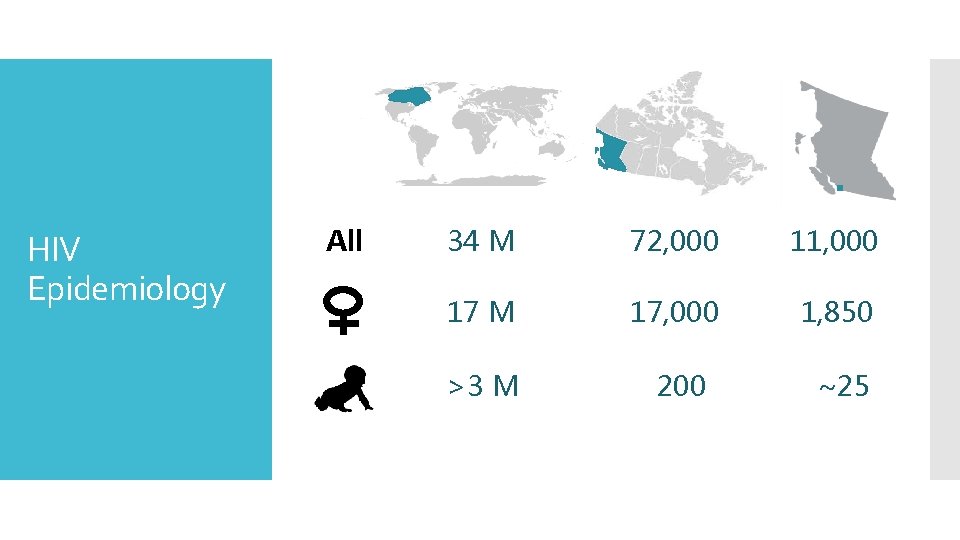
HIV Epidemiology All 34 M 72, 000 11, 000 17 M 17, 000 1, 850 >3 M 200 ~25

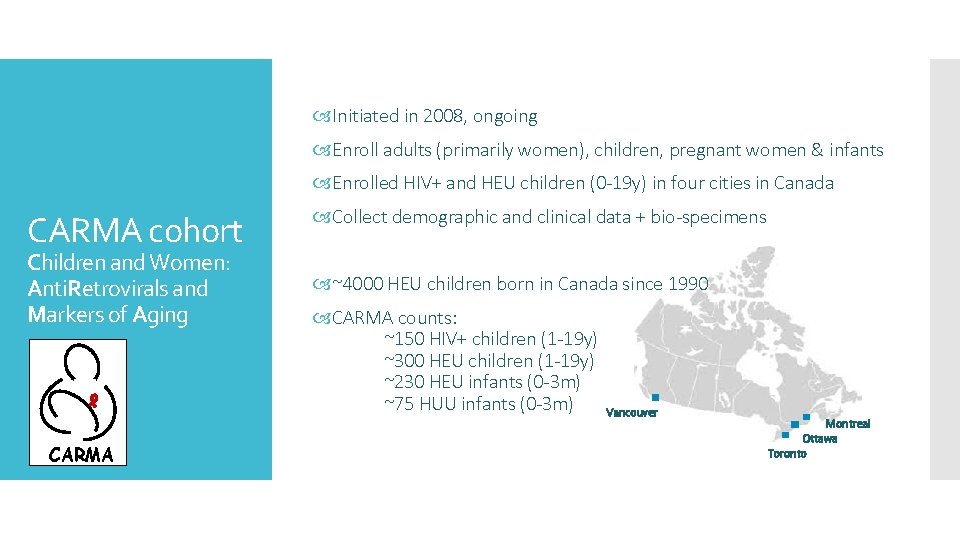
Initiated in 2008, ongoing Enroll adults (primarily women), children, pregnant women & infants Enrolled HIV+ and HEU children (0 -19 y) in four cities in Canada CARMA cohort Children and Women: Anti. Retrovirals and Markers of Aging CARMA Collect demographic and clinical data + bio-specimens ~4000 HEU children born in Canada since 1990 CARMA counts: ~150 HIV+ children (1 -19 y) ~300 HEU children (1 -19 y) ~230 HEU infants (0 -3 m) ~75 HUU infants (0 -3 m) Vancouver Montreal Ottawa Toronto

Neurodevelopmental disorder Diagnosed by 2 -3 years of age Heterogeneous symptoms Autism spectrum disorder (ASD) Multi-hit (genetic/environment) Prevalence in general population ~1. 5% Variable severity Asperger’s Syndrome Childhood disintegrative disorder Classic autism

• Substance use during pregnancy • Maternal infections • ↑ age Factors associated with ASD • • • Sex Gastrointestinal abnormalities Hypotonia Intellectual disability Seizures/epilepsy • Mitochondrial dysfunction Genetic susceptibility

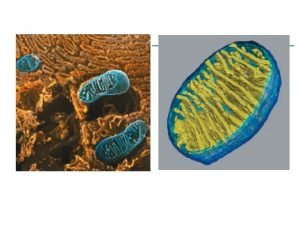
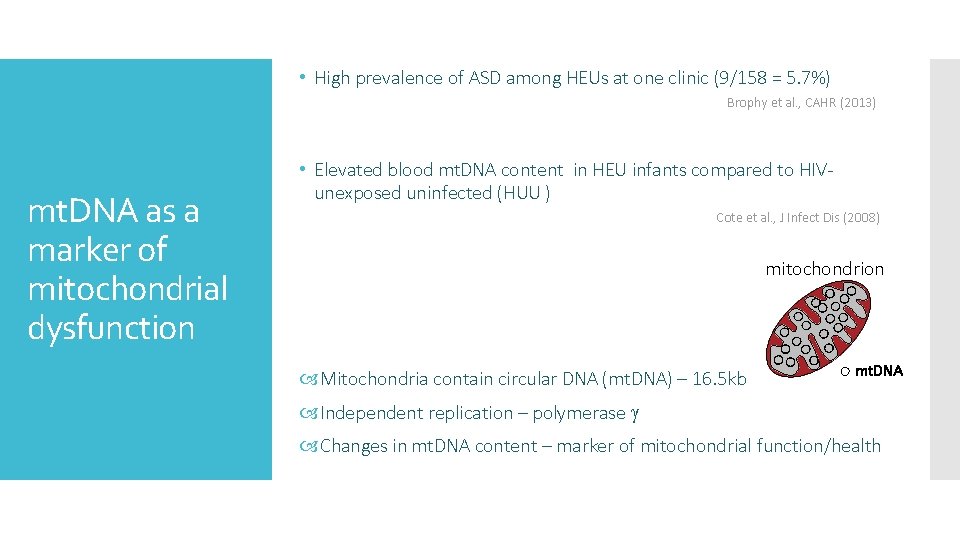
• High prevalence of ASD among HEUs at one clinic (9/158 = 5. 7%) Brophy et al. , CAHR (2013) mt. DNA as a marker of mitochondrial dysfunction • Elevated blood mt. DNA content in HEU infants compared to HIVunexposed uninfected (HUU ) Cote et al. , J Infect Dis (2008) mitochondrion Mitochondria contain circular DNA (mt. DNA) – 16. 5 kb mt. DNA Independent replication – polymerase Changes in mt. DNA content – marker of mitochondrial function/health

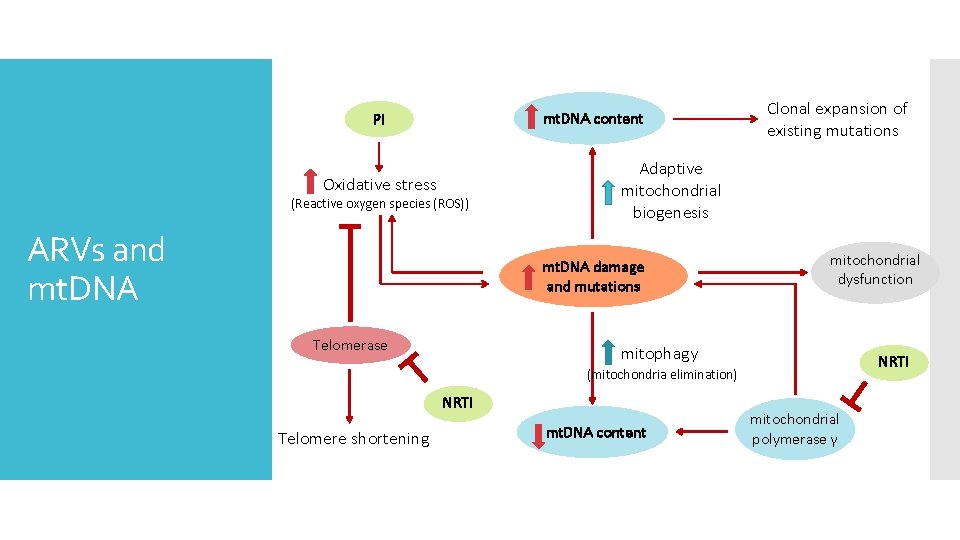
mt. DNA content PI Oxidative stress (Reactive oxygen species (ROS)) ARVs and mt. DNA Adaptive mitochondrial biogenesis mt. DNA damage and mutations Telomerase Clonal expansion of existing mutations mitochondrial dysfunction mitophagy NRTI (mitochondria elimination) NRTI Telomere shortening mt. DNA content mitochondrial polymerase γ

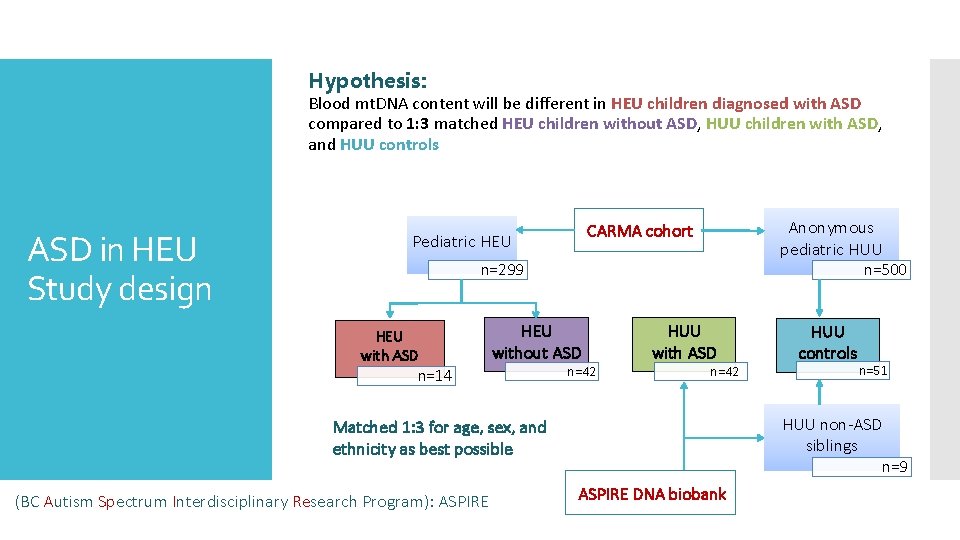
Hypothesis: Blood mt. DNA content will be different in HEU children diagnosed with ASD compared to 1: 3 matched HEU children without ASD, HUU children with ASD, and HUU controls ASD in HEU Study design Anonymous pediatric HUU CARMA cohort Pediatric HEU n=299 HEU with ASD n=500 HEU without ASD n=14 n=42 HUU with ASD n=42 n=51 HUU non-ASD siblings Matched 1: 3 for age, sex, and ethnicity as best possible (BC Autism Spectrum Interdisciplinary Research Program): ASPIRE HUU controls n=9 ASPIRE DNA biobank

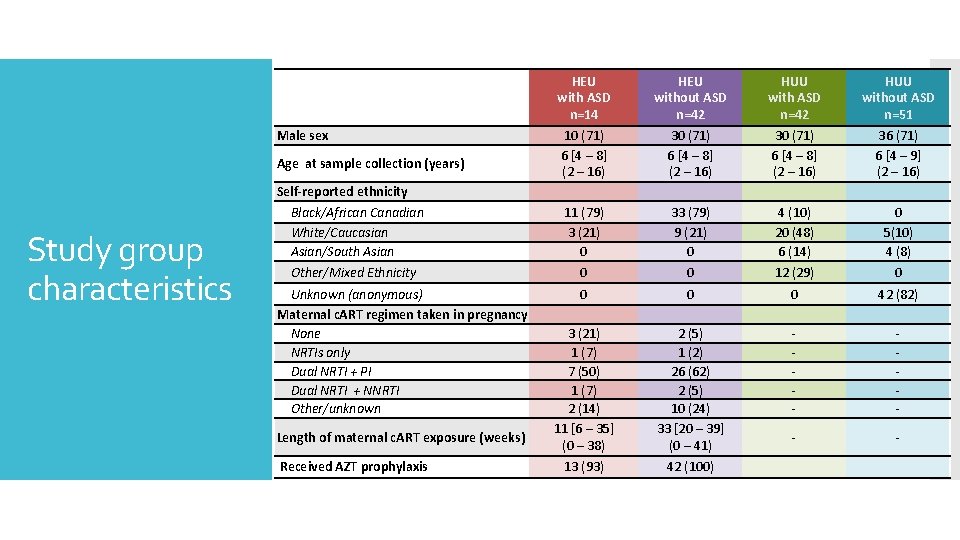
Male sex Age at sample collection (years) Study group characteristics Self-reported ethnicity Black/African Canadian White/Caucasian Asian/South Asian Other/Mixed Ethnicity Unknown (anonymous) Maternal c. ART regimen taken in pregnancy None NRTIs only Dual NRTI + PI Dual NRTI + NNRTI Other/unknown Length of maternal c. ART exposure (weeks) Received AZT prophylaxis HEU with ASD n=14 10 (71) 6 [4 – 8] (2 – 16) 11 (79) 3 (21) 0 0 HEU without ASD n=42 30 (71) 6 [4 – 8] (2 – 16) 33 (79) 9 (21) 0 0 HUU with ASD n=42 30 (71) 6 [4 – 8] (2 – 16) 4 (10) 20 (48) 6 (14) 12 (29) HUU without ASD n=51 36 (71) 6 [4 – 9] (2 – 16) 0 5(10) 4 (8) 0 0 3 (21) 1 (7) 7 (50) 1 (7) 2 (14) 11 [6 – 35] (0 – 38) 0 2 (5) 1 (2) 26 (62) 2 (5) 10 (24) 33 [20 – 39] (0 – 41) 0 - 42 (82) - - - 13 (93) 42 (100)

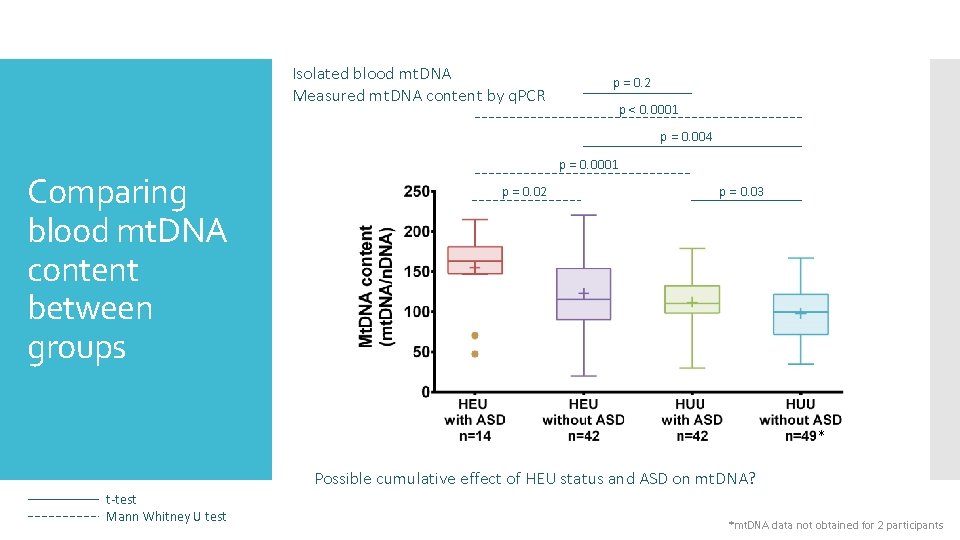
Isolated blood mt. DNA Measured mt. DNA content by q. PCR p = 0. 2 p < 0. 0001 p = 0. 004 Comparing blood mt. DNA content between groups p = 0. 0001 p = 0. 02 p = 0. 03 * Possible cumulative effect of HEU status and ASD on mt. DNA? t-test Mann Whitney U test *mt. DNA data not obtained for 2 participants

ASD may show a higher prevalence among HEU in our cohort – but could be subject to recruitment bias Mt. DNA effects are associated with both HEU status and ASD diagnosis Summary Did not detect any relationship with perinatal ARV exposure but our study sample is small HEU may get a “multi-hit”, perhaps increasing susceptibility There may be confounder(s) we were not able to consider

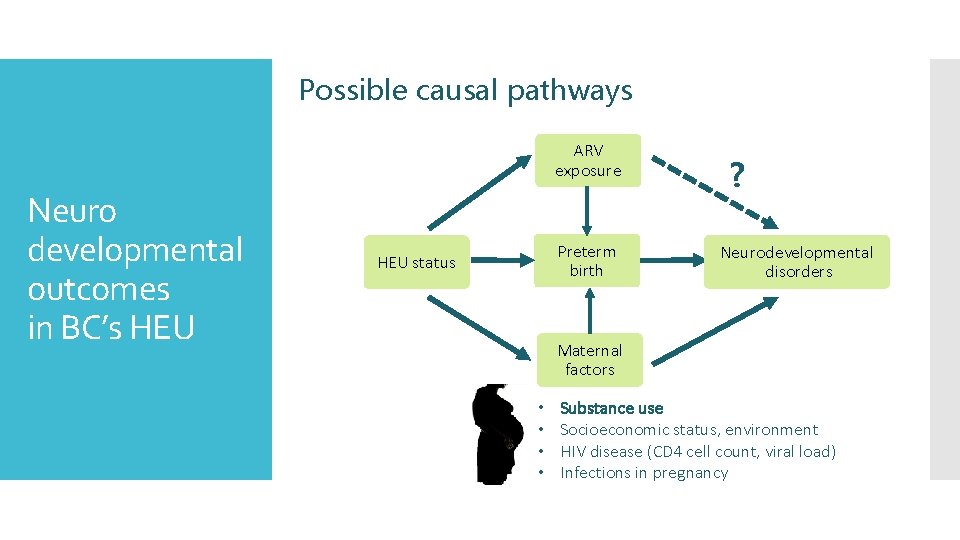
Possible causal pathways ARV exposure Neuro developmental outcomes in BC’s HEU Preterm birth HEU status ? Neurodevelopmental disorders Maternal factors • • Substance use Socioeconomic status, environment HIV disease (CD 4 cell count, viral load) Infections in pregnancy

Study objectives • To compare the frequency of neurodevelopmental disorders (ND) among HEU children compared to matched HUU • To examine possible associations with exposure to maternal ARVs Study design: retrospective controlled cohort study HEU (n = 446) Born in BC 1990 -2012 PH number on record matched 1: 3 HUU (n = 1323) Matched (98%) on: Age, Sex, Geocode • Dataset collected by • Data sources: BC ministry of Health (ICD-9 codes), Perinatal Service BC, Oak Tree Clinic, BC Vital Statistics

Population Data BC statement “All inferences, opinions, and conclusions drawn in this presentation are those of the authors, and do not reflect the opinions or policies of the Data Steward(s). ”

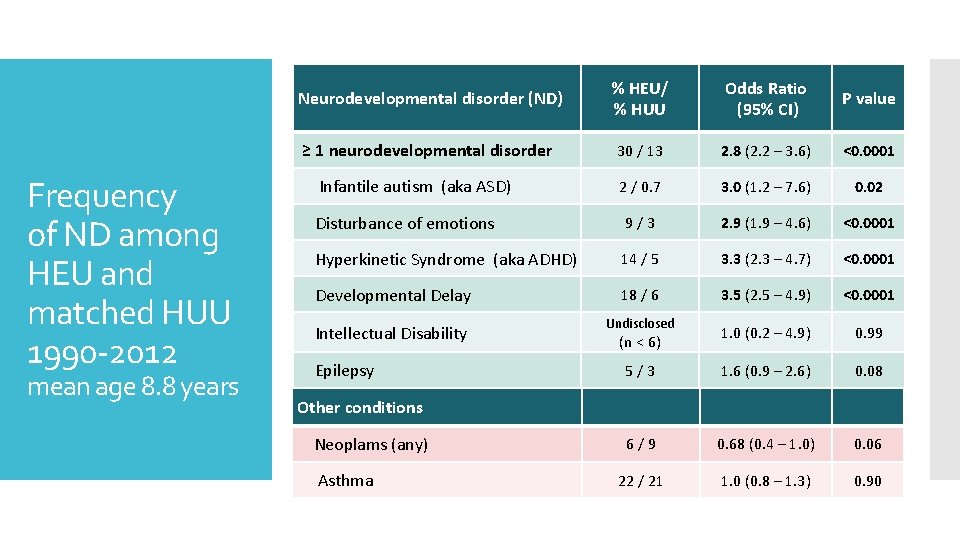
% HEU/ % HUU Odds Ratio (95% CI) ≥ 1 neurodevelopmental disorder 30 / 13 2. 8 (2. 2 – 3. 6) Infantile autism (aka ASD) 2 / 0. 7 3. 0 (1. 2 – 7. 6) 0. 02 Disturbance of emotions 9 / 3 2. 9 (1. 9 – 4. 6) <0. 0001 Hyperkinetic Syndrome (aka ADHD) 14 / 5 3. 3 (2. 3 – 4. 7) <0. 0001 Developmental Delay 18 / 6 3. 5 (2. 5 – 4. 9) <0. 0001 1. 0 (0. 2 – 4. 9) 0. 99 5 / 3 1. 6 (0. 9 – 2. 6) 0. 08 6 / 9 0. 68 (0. 4 – 1. 0) 0. 06 22 / 21 1. 0 (0. 8 – 1. 3) 0. 90 Neurodevelopmental disorder (ND) Frequency of ND among HEU and matched HUU 1990 -2012 mean age 8. 8 years Intellectual Disability Epilepsy Undisclosed (n < 6) P value <0. 0001 Other conditions Neoplams (any) Asthma

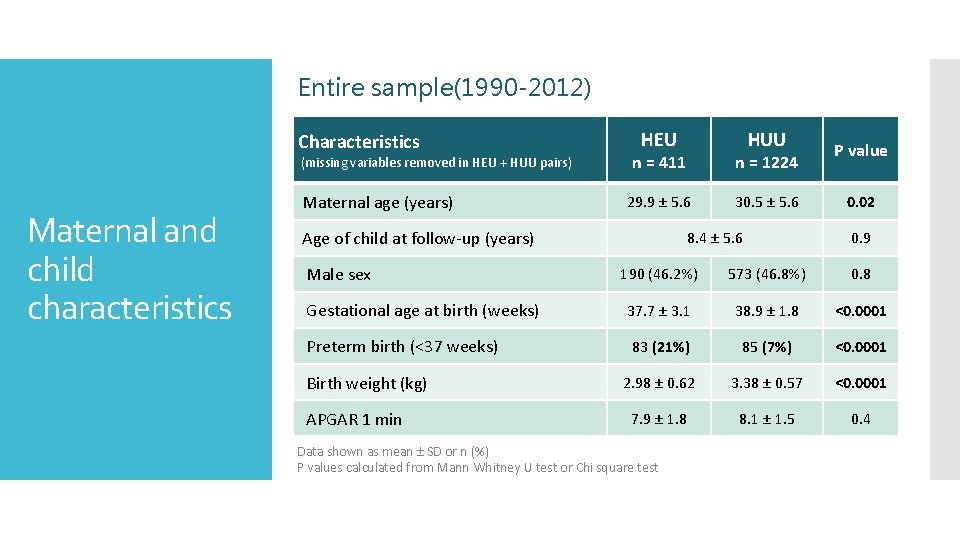
Entire sample(1990 -2012) Characteristics (missing variables removed in HEU + HUU pairs) Maternal and child characteristics Maternal age (years) HEU n = 411 n = 1224 29. 9 ± 5. 6 30. 5 ± 5. 6 Age of child at follow-up (years) Male sex HUU 8. 4 ± 5. 6 P value 0. 02 0. 9 190 (46. 2%) 573 (46. 8%) 0. 8 Gestational age at birth (weeks) 37. 7 ± 3. 1 38. 9 ± 1. 8 <0. 0001 Preterm birth (<37 weeks) 83 (21%) 85 (7%) <0. 0001 2. 98 ± 0. 62 3. 38 ± 0. 57 <0. 0001 7. 9 ± 1. 8 8. 1 ± 1. 5 0. 4 Birth weight (kg) APGAR 1 min Data shown as mean ± SD or n (%) P values calculated from Mann Whitney U test or Chi square test

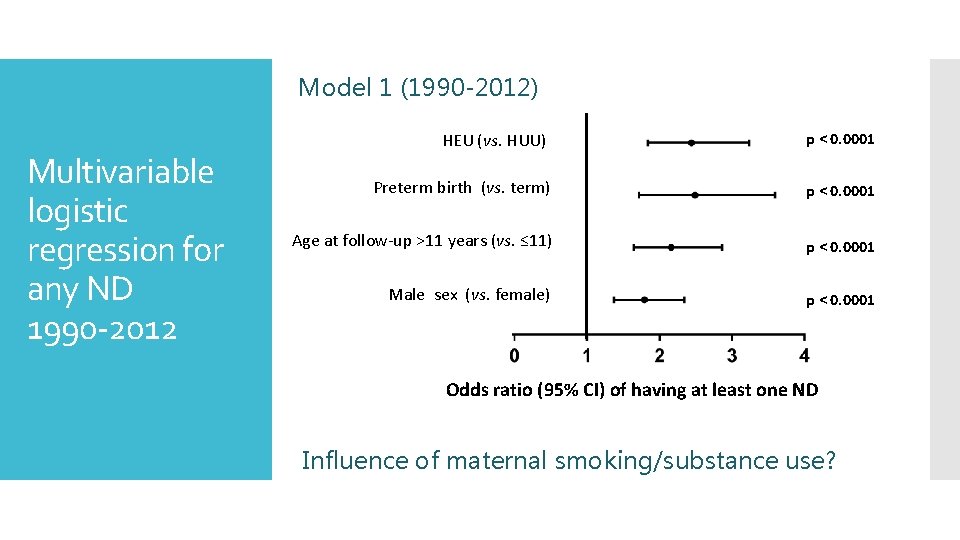
Model 1 (1990 -2012) Multivariable logistic regression for any ND 1990 -2012 HEU (vs. HUU) p < 0. 0001 Preterm birth (vs. term) p < 0. 0001 Age at follow-up >11 years (vs. ≤ 11) p < 0. 0001 Male sex (vs. female) p < 0. 0001 Odds ratio (95% CI) of having at least one ND Influence of maternal smoking/substance use?

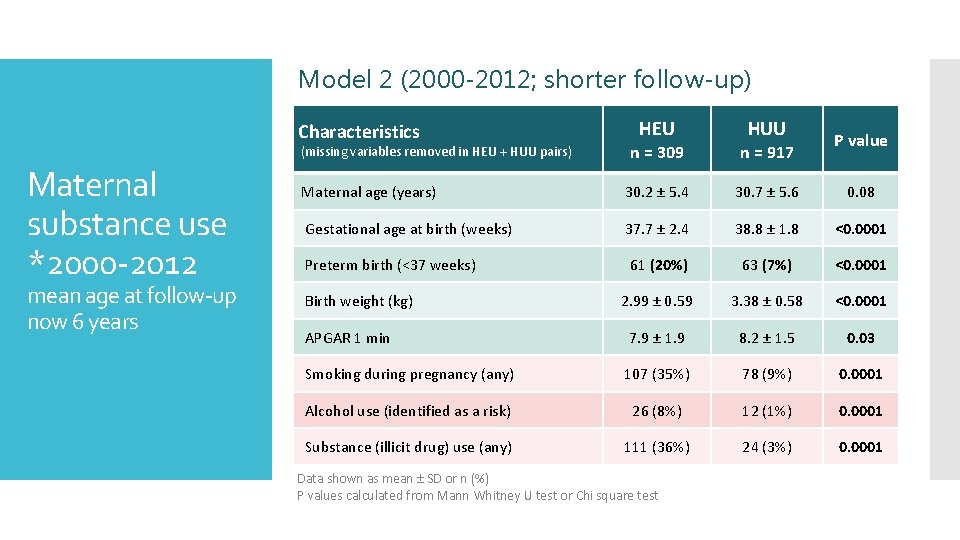
Model 2 (2000 -2012; shorter follow-up) Characteristics mean age at follow-up now 6 years HUU P value n = 309 n = 917 Maternal age (years) 30. 2 ± 5. 4 30. 7 ± 5. 6 Gestational age at birth (weeks) 37. 7 ± 2. 4 38. 8 ± 1. 8 <0. 0001 Preterm birth (<37 weeks) 61 (20%) 63 (7%) <0. 0001 2. 99 ± 0. 59 3. 38 ± 0. 58 <0. 0001 APGAR 1 min 7. 9 ± 1. 9 8. 2 ± 1. 5 0. 03 Smoking during pregnancy (any) 107 (35%) 78 (9%) 0. 0001 Alcohol use (identified as a risk) 26 (8%) 12 (1%) 0. 0001 Substance (illicit drug) use (any) 111 (36%) 24 (3%) 0. 0001 (missing variables removed in HEU + HUU pairs) Maternal substance use *2000 -2012 HEU Birth weight (kg) Data shown as mean ± SD or n (%) P values calculated from Mann Whitney U test or Chi square test 0. 08

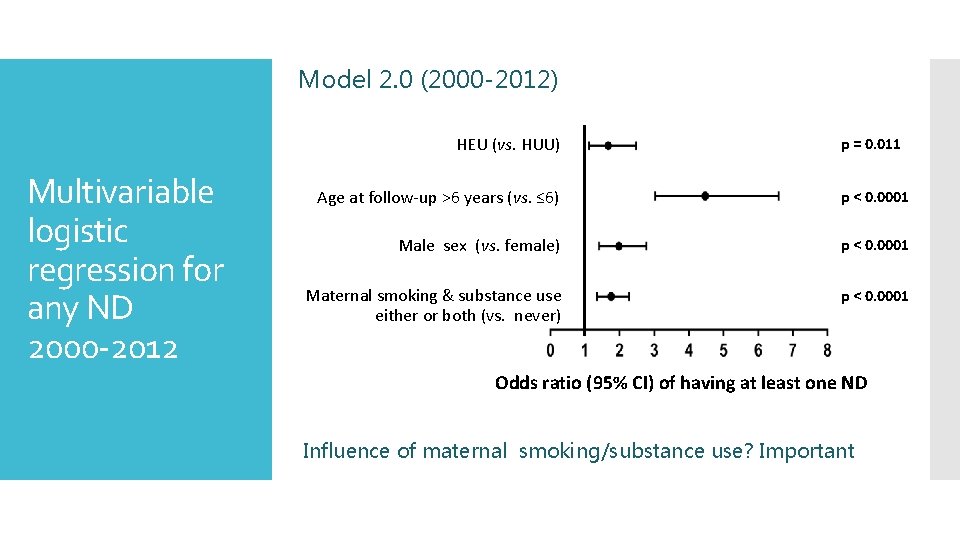
Model 2. 0 (2000 -2012) HEU (vs. HUU) Multivariable logistic regression for any ND 2000 -2012 p = 0. 011 Age at follow-up >6 years (vs. ≤ 6) p < 0. 0001 Male sex (vs. female) p < 0. 0001 Maternal smoking & substance use either or both (vs. never) p < 0. 0001 Odds ratio (95% CI) of having at least one ND Influence of maternal smoking/substance use? Important

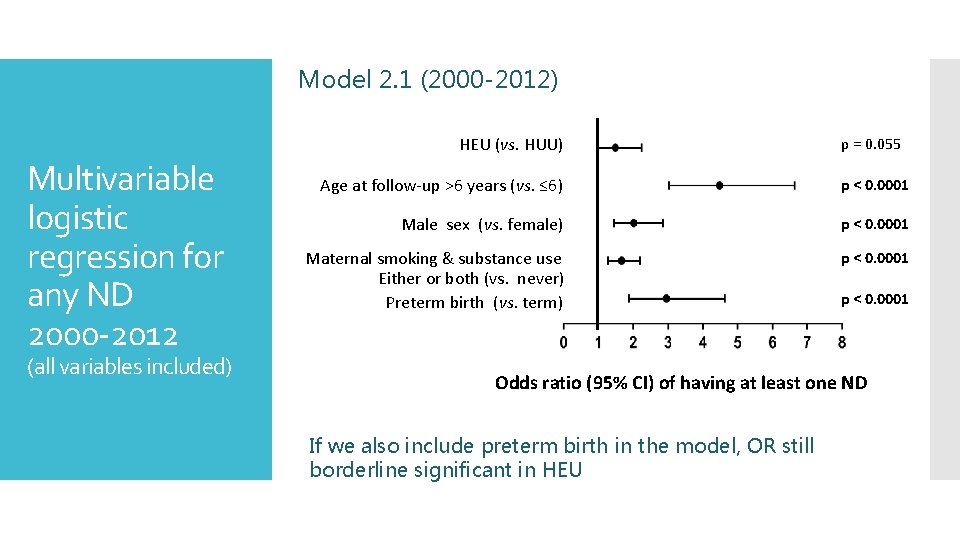
Model 2. 1 (2000 -2012) HEU (vs. HUU) Multivariable logistic regression for any ND 2000 -2012 (all variables included) p = 0. 055 Age at follow-up >6 years (vs. ≤ 6) p < 0. 0001 Male sex (vs. female) p < 0. 0001 Maternal smoking & substance use Either or both (vs. never) Preterm birth (vs. term) p < 0. 0001 Odds ratio (95% CI) of having at least one ND If we also include preterm birth in the model, OR still borderline significant in HEU

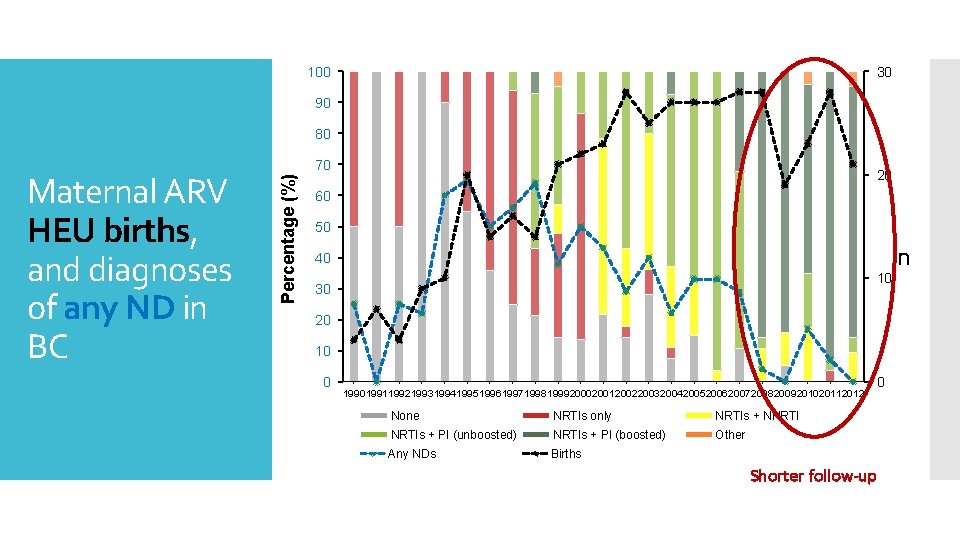
100 30 90 80 Percentage (%) Maternal ARV HEU births, and diagnoses of any ND in BC 70 20 60 50 n 40 10 30 20 10 0 19901991199219931994199519961997199819992000200120022003200420052006200720082009201020112012 None NRTIs only NRTIs + NNRTIs + PI (unboosted) NRTIs + PI (boosted) Other Any NDs Births 0 Shorter follow-up

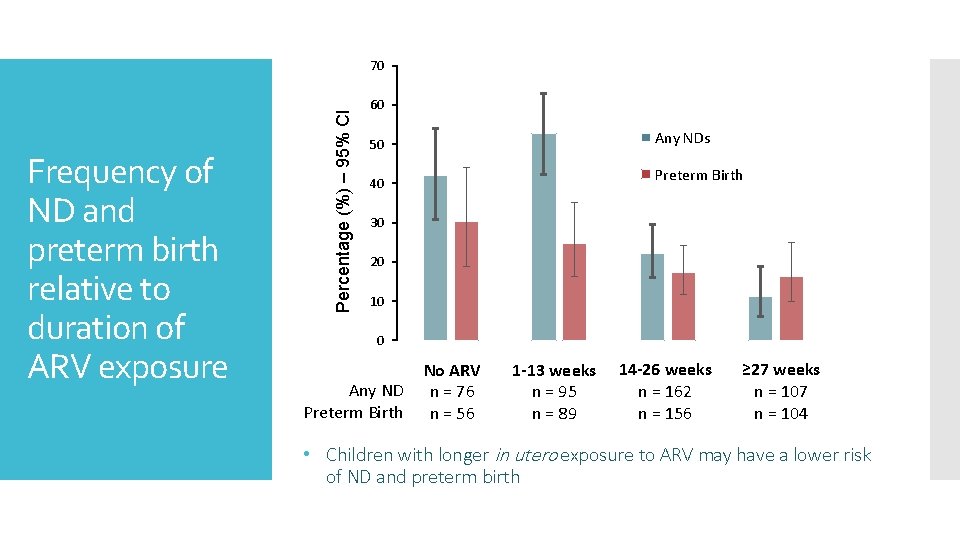
Frequency of ND and preterm birth relative to duration of ARV exposure Percentage (%) – 95% CI 70 60 Any NDs 50 Preterm Birth 40 30 20 10 0 Any ND Preterm Birth No ARV n = 76 n = 56 1 -13 weeks n = 95 n = 89 14 -26 weeks n = 162 n = 156 ≥ 27 weeks n = 107 n = 104 • Children with longer in utero exposure to ARV may have a lower risk of ND and preterm birth

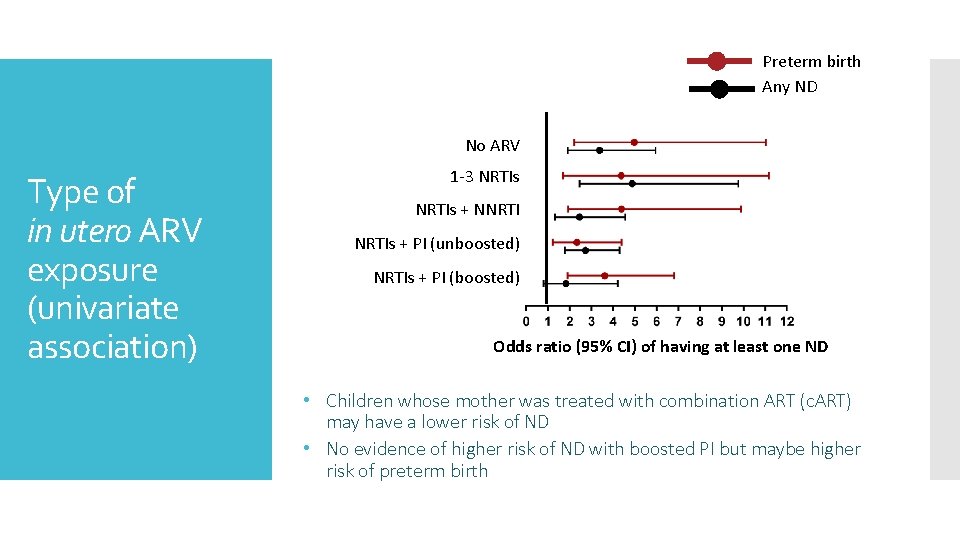
Preterm birth Any ND No ARV Type of in utero ARV exposure (univariate association) 1 -3 NRTIs + NNRTIs + PI (unboosted) NRTIs + PI (boosted) Odds ratio (95% CI) of having at least one ND • Children whose mother was treated with combination ART (c. ART) may have a lower risk of ND • No evidence of higher risk of ND with boosted PI but maybe higher risk of preterm birth

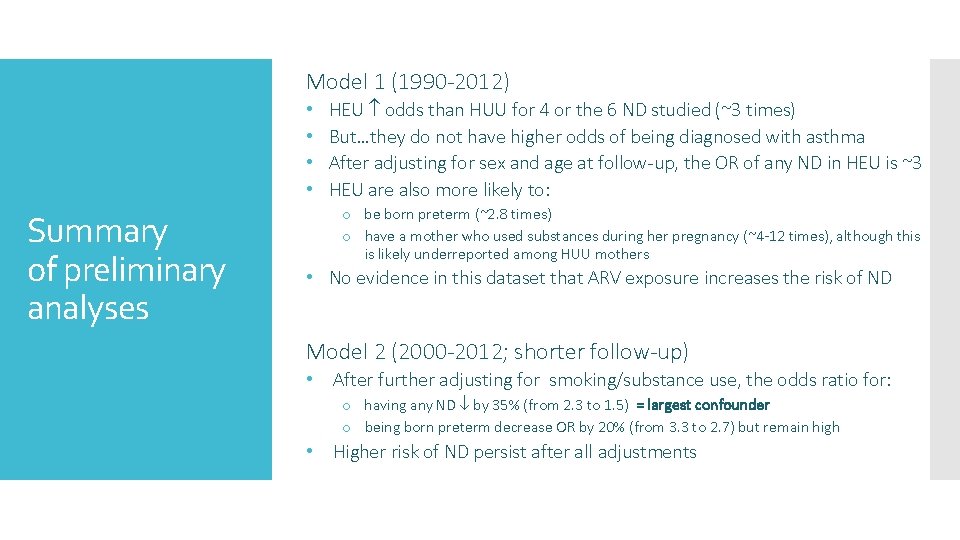
Model 1 (1990 -2012) • • Summary of preliminary analyses HEU odds than HUU for 4 or the 6 ND studied (~3 times) But…they do not have higher odds of being diagnosed with asthma After adjusting for sex and age at follow-up, the OR of any ND in HEU is ~3 HEU are also more likely to: o be born preterm (~2. 8 times) o have a mother who used substances during her pregnancy (~4 -12 times), although this is likely underreported among HUU mothers • No evidence in this dataset that ARV exposure increases the risk of ND Model 2 (2000 -2012; shorter follow-up) • After further adjusting for smoking/substance use, the odds ratio for: o having any ND by 35% (from 2. 3 to 1. 5) = largest confounder o being born preterm decrease OR by 20% (from 3. 3 to 2. 7) but remain high • Higher risk of ND persist after all adjustments

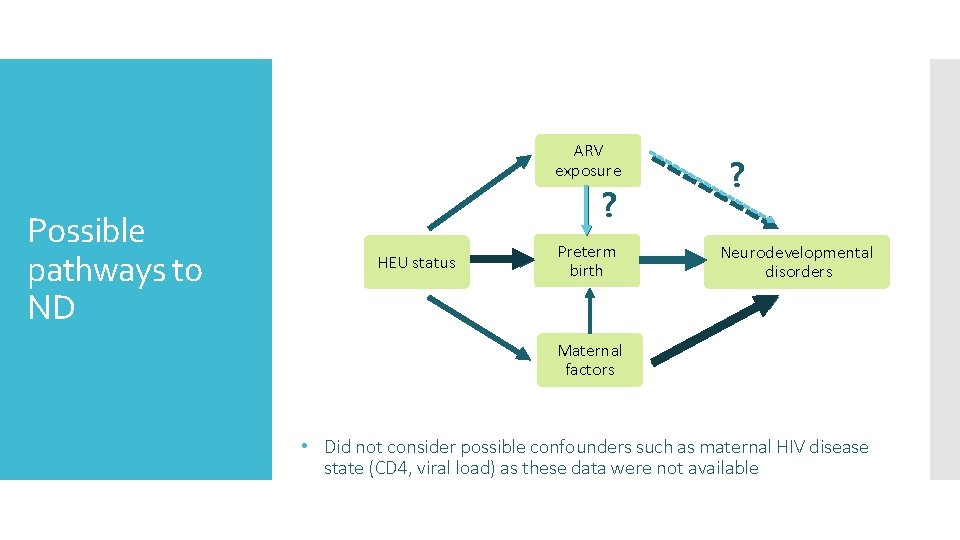
ARV exposure Possible pathways to ND ? HEU status Preterm birth ? Neurodevelopmental disorders Maternal factors • Did not consider possible confounders such as maternal HIV disease state (CD 4, viral load) as these data were not available

Cote Lab Matthew Budd Micah Piske Jason Brophy Lindy Samson Patricia Janssen Joel Singer CARMA participants Acknowledge ments Hugo Soudeyns Fatima Kakkar Valerie Lamarre Normand Lapointe Ari Bitnun Stanley Read CARMA Funding Ariane Alimenti John Forbes Laura Sauve Deborah Money Evelyn Maan

 Chapter 11 childhood and neurodevelopmental disorders
Chapter 11 childhood and neurodevelopmental disorders Neurocognitive disorder
Neurocognitive disorder Dsm 5 vs dsm 4
Dsm 5 vs dsm 4 Neurodevelopmental disorders
Neurodevelopmental disorders Reserch gate
Reserch gate Mitochondrial dna
Mitochondrial dna Leu heu
Leu heu Labelled diagram of mitochondria class 9
Labelled diagram of mitochondria class 9 Liver cirrhosis
Liver cirrhosis Melas syndrome
Melas syndrome Mitochondrial swelling
Mitochondrial swelling Mitochondrial vesicles
Mitochondrial vesicles Neurodevelopmental treatment for stroke
Neurodevelopmental treatment for stroke Robyn smith therapist
Robyn smith therapist Coding dna and non coding dna
Coding dna and non coding dna Dna polymerase function in dna replication
Dna polymerase function in dna replication Bioflix activity dna replication dna replication diagram
Bioflix activity dna replication dna replication diagram Replication process
Replication process Dna rna protein synthesis homework #2 dna replication
Dna rna protein synthesis homework #2 dna replication Unit 14 physiological disorders
Unit 14 physiological disorders Bipolar and other related disorders
Bipolar and other related disorders Bipolar and other related disorders
Bipolar and other related disorders Assistive technology for emotional disturbance
Assistive technology for emotional disturbance Puberty and autism spectrum disorders
Puberty and autism spectrum disorders Axis 1 and axis 2 disorders
Axis 1 and axis 2 disorders Neurotic stress-related and somatoform disorders
Neurotic stress-related and somatoform disorders Define a primary skin lesion and list three types
Define a primary skin lesion and list three types Physical disorders and health psychology
Physical disorders and health psychology