11 6 Real Gases Factors That Cause Deviation





- Slides: 29

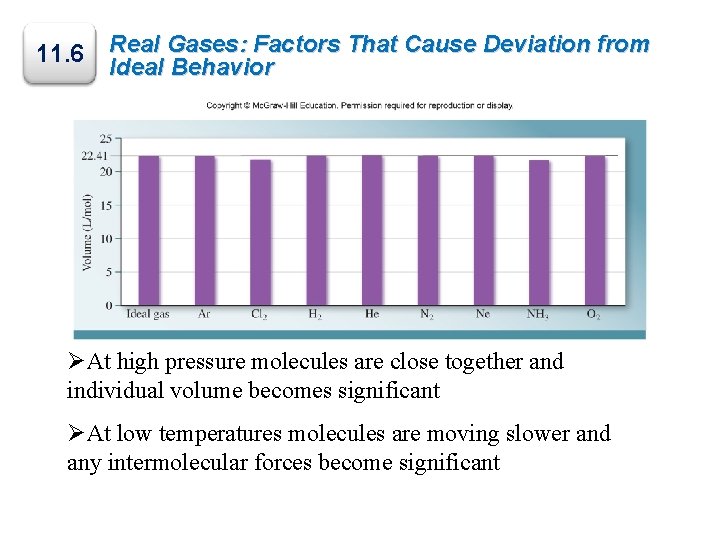
11. 6 Real Gases: Factors That Cause Deviation from Ideal Behavior ØAt high pressure molecules are close together and individual volume becomes significant ØAt low temperatures molecules are moving slower and any intermolecular forces become significant

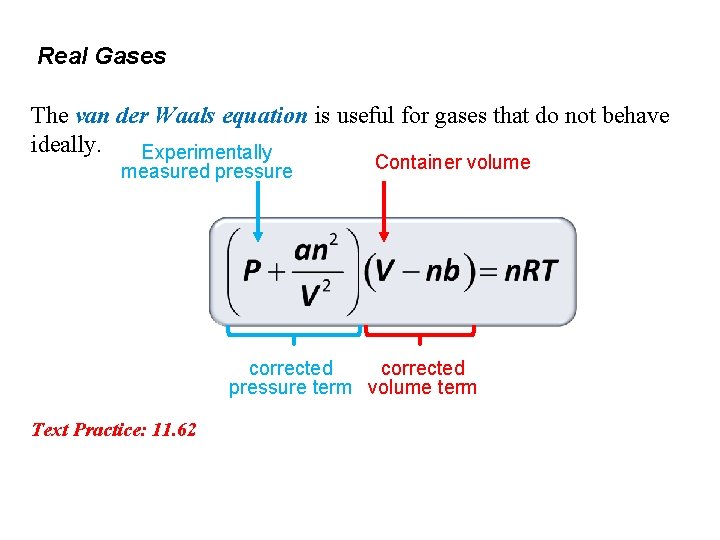
Real Gases The van der Waals equation is useful for gases that do not behave ideally. Experimentally measured pressure Container volume corrected pressure term volume term Text Practice: 11. 62

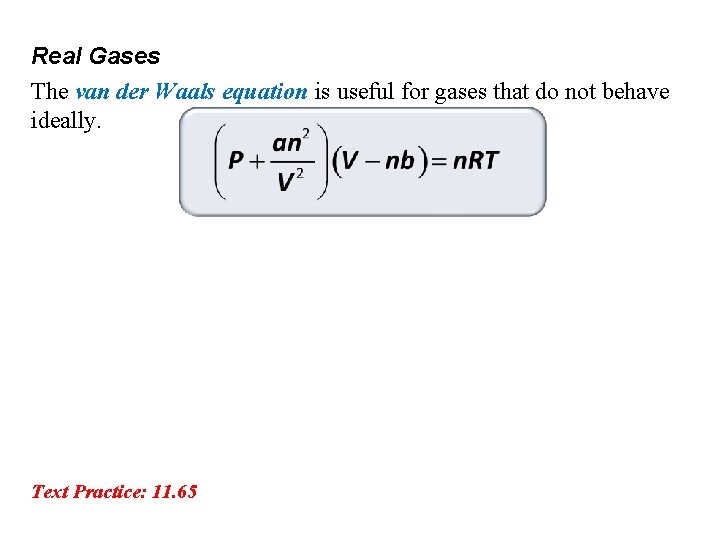
Real Gases The van der Waals equation is useful for gases that do not behave ideally. Text Practice: 11. 65

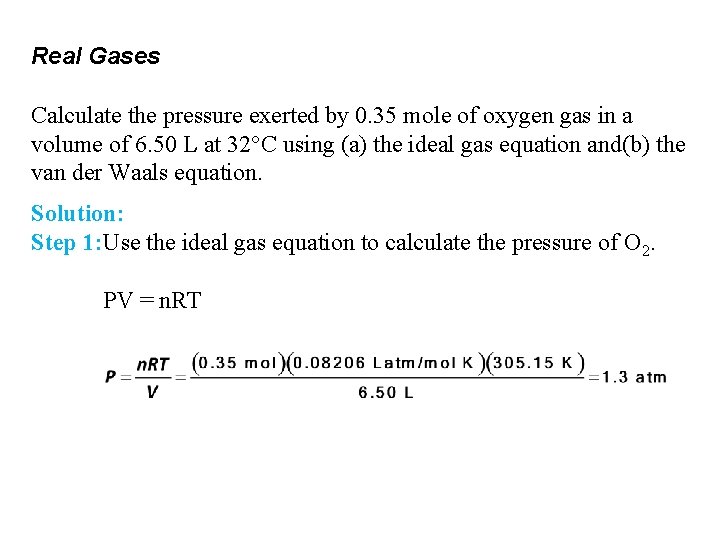
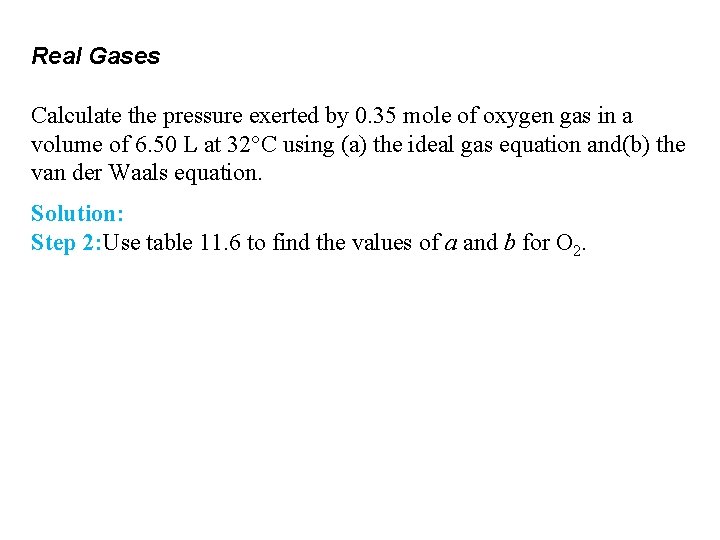
Real Gases Calculate the pressure exerted by 0. 35 mole of oxygen gas in a volume of 6. 50 L at 32°C using (a) the ideal gas equation and(b) the van der Waals equation. Solution: Step 1: Use the ideal gas equation to calculate the pressure of O 2. PV = n. RT

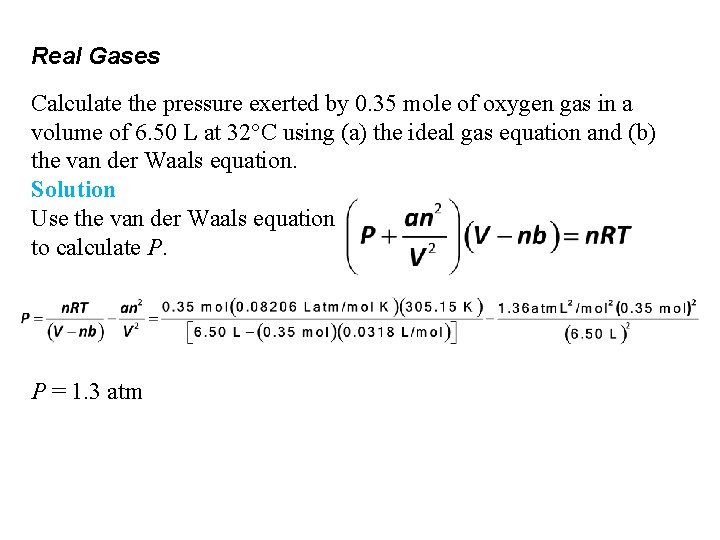
Real Gases Calculate the pressure exerted by 0. 35 mole of oxygen gas in a volume of 6. 50 L at 32°C using (a) the ideal gas equation and(b) the van der Waals equation. Solution: Step 2: Use table 11. 6 to find the values of a and b for O 2.

Real Gases Calculate the pressure exerted by 0. 35 mole of oxygen gas in a volume of 6. 50 L at 32°C using (a) the ideal gas equation and (b) the van der Waals equation. Solution Use the van der Waals equation to calculate P. P = 1. 3 atm

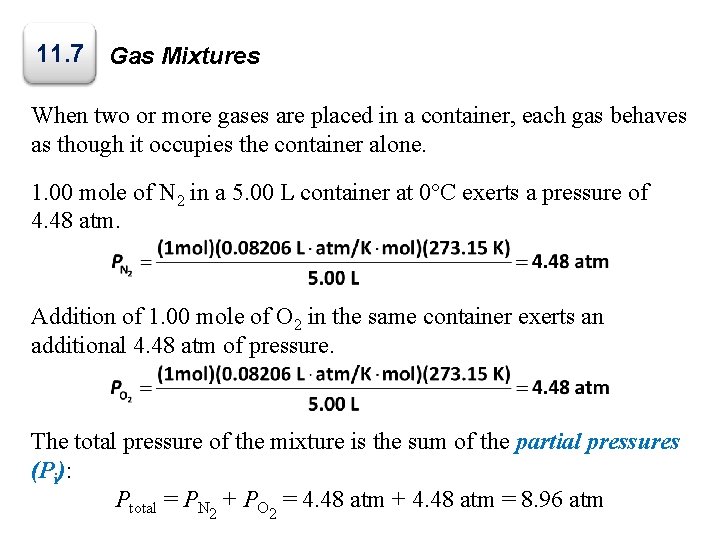
11. 7 Gas Mixtures When two or more gases are placed in a container, each gas behaves as though it occupies the container alone. 1. 00 mole of N 2 in a 5. 00 L container at 0°C exerts a pressure of 4. 48 atm. Addition of 1. 00 mole of O 2 in the same container exerts an additional 4. 48 atm of pressure. The total pressure of the mixture is the sum of the partial pressures (Pi): Ptotal = PN 2 + PO 2 = 4. 48 atm + 4. 48 atm = 8. 96 atm

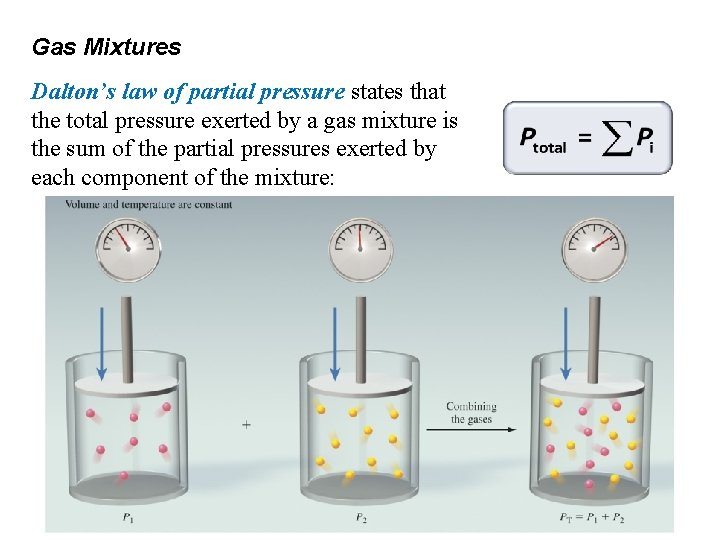
Gas Mixtures Dalton’s law of partial pressure states that the total pressure exerted by a gas mixture is the sum of the partial pressures exerted by each component of the mixture:

Gas Mixtures Determine the total pressure in a 2. 50 -L vessel containing the following mixture of gases at 15. 8°C: 0. 0194 mol He, 0. 0411 mol H 2, and 0. 169 mol Ne. Solution: Step 1: Since each gas behaves independently, calculate the partial pressure of each using the ideal gas equation: Text Practice: 11. 69

Chemistry: Atoms First Second Edition Julia Burdge & Jason Overby Chapter 12 Liquids and Solids M. Stacey Thomson Pasco-Hernando State College Copyright (c) The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

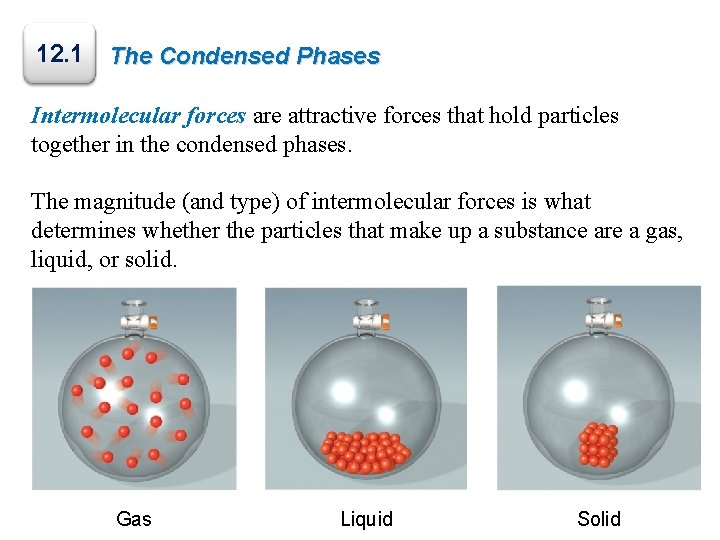
12. 1 The Condensed Phases Intermolecular forces are attractive forces that hold particles together in the condensed phases. The magnitude (and type) of intermolecular forces is what determines whether the particles that make up a substance are a gas, liquid, or solid. Gas Liquid Solid

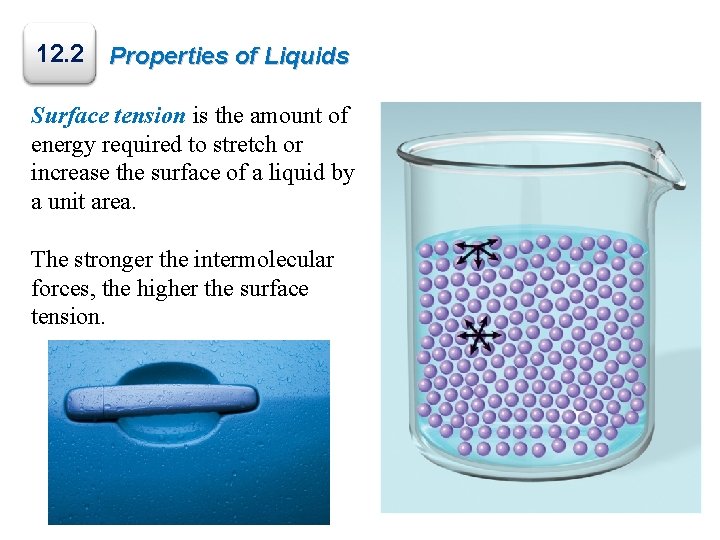
12. 2 Properties of Liquids Surface tension is the amount of energy required to stretch or increase the surface of a liquid by a unit area. The stronger the intermolecular forces, the higher the surface tension.

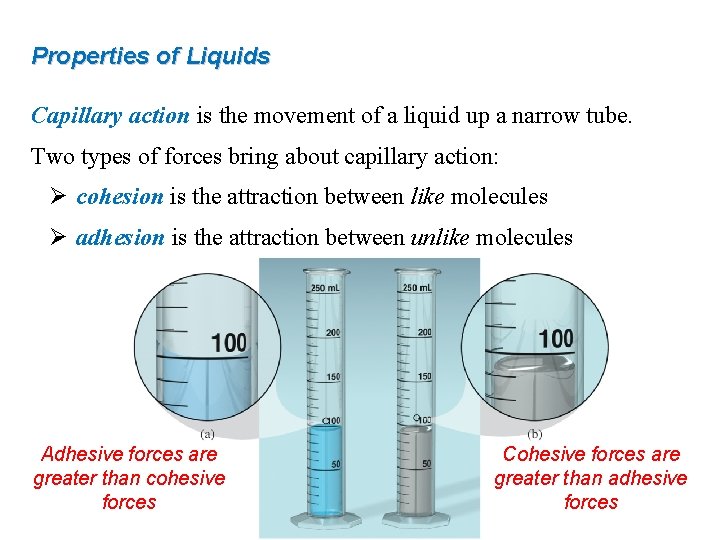
Properties of Liquids Capillary action is the movement of a liquid up a narrow tube. Two types of forces bring about capillary action: Ø cohesion is the attraction between like molecules Ø adhesion is the attraction between unlike molecules Adhesive forces are greater than cohesive forces Cohesive forces are greater than adhesive forces

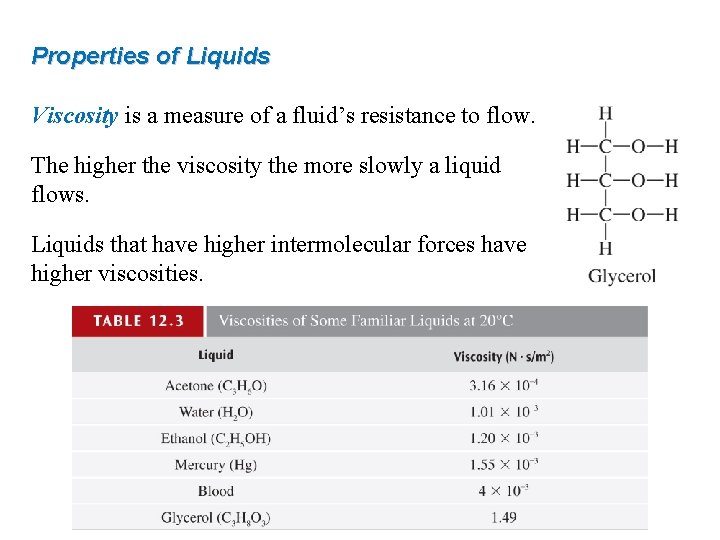
Properties of Liquids Viscosity is a measure of a fluid’s resistance to flow. The higher the viscosity the more slowly a liquid flows. Liquids that have higher intermolecular forces have higher viscosities.

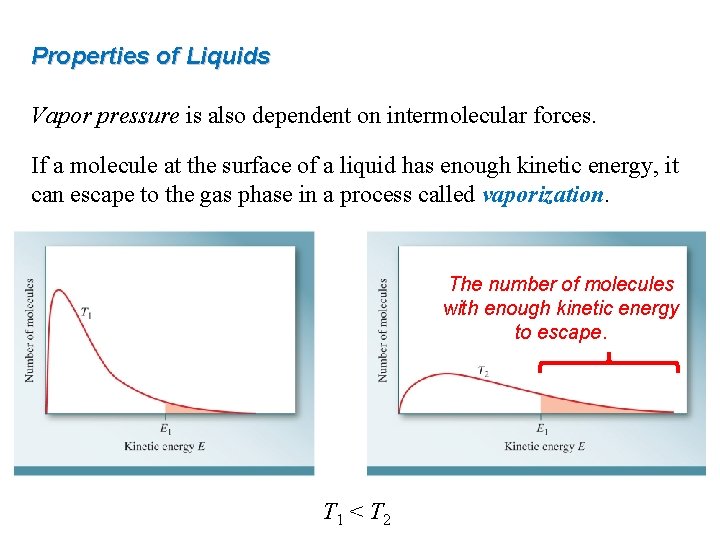
Properties of Liquids Vapor pressure is also dependent on intermolecular forces. If a molecule at the surface of a liquid has enough kinetic energy, it can escape to the gas phase in a process called vaporization. The number of molecules with enough kinetic energy to escape. T 1 < T 2

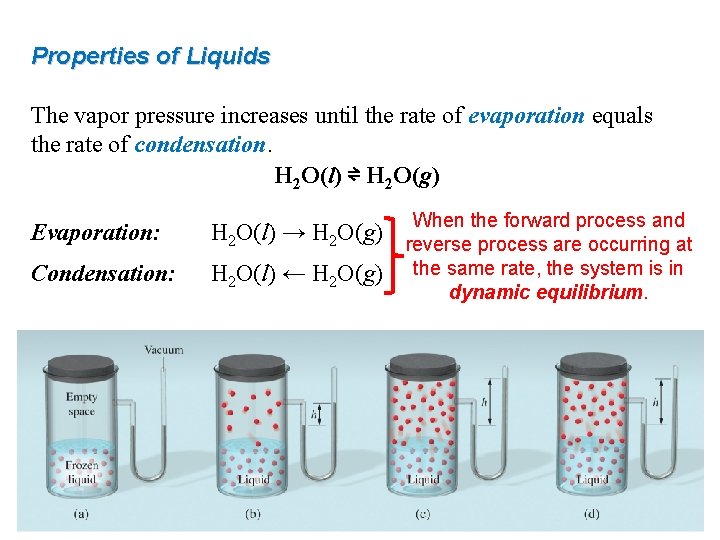
Properties of Liquids The vapor pressure increases until the rate of evaporation equals the rate of condensation. H 2 O(l) ⇌ H 2 O(g) Evaporation: H 2 O(l) → H 2 O(g) Condensation: H 2 O(l) ← H 2 O(g) When the forward process and reverse process are occurring at the same rate, the system is in dynamic equilibrium.

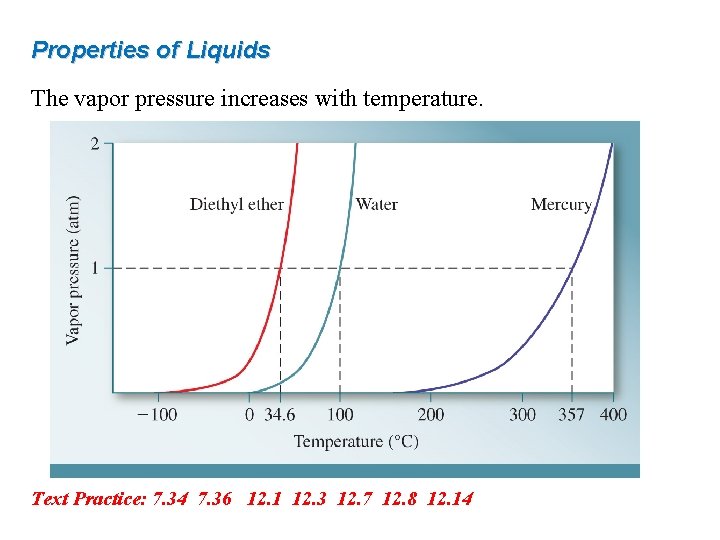
Properties of Liquids The vapor pressure increases with temperature. Text Practice: 7. 34 7. 36 12. 1 12. 3 12. 7 12. 8 12. 14

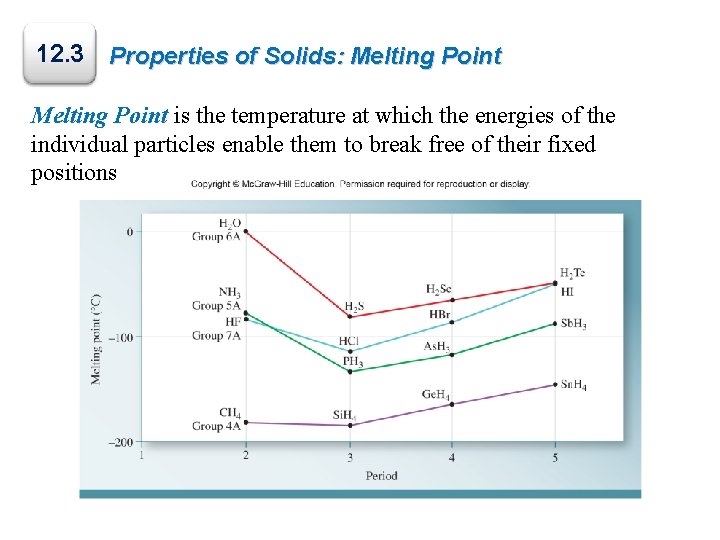
12. 3 Properties of Solids: Melting Point is the temperature at which the energies of the individual particles enable them to break free of their fixed positions

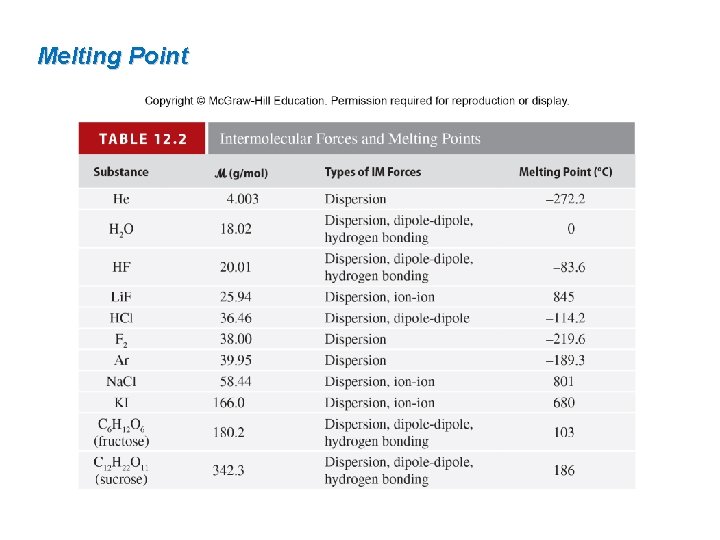
Melting Point

Vapor Pressure of Solids The vapor pressures of solids typically are very low at room temperature. A few exceptions are shown:

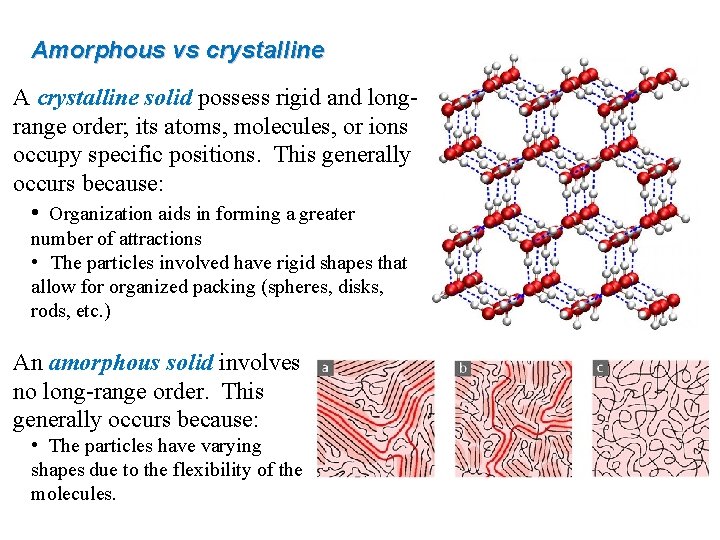
Amorphous vs crystalline A crystalline solid possess rigid and longrange order; its atoms, molecules, or ions occupy specific positions. This generally occurs because: • Organization aids in forming a greater number of attractions • The particles involved have rigid shapes that allow for organized packing (spheres, disks, rods, etc. ) An amorphous solid involves no long-range order. This generally occurs because: • The particles have varying shapes due to the flexibility of the molecules.

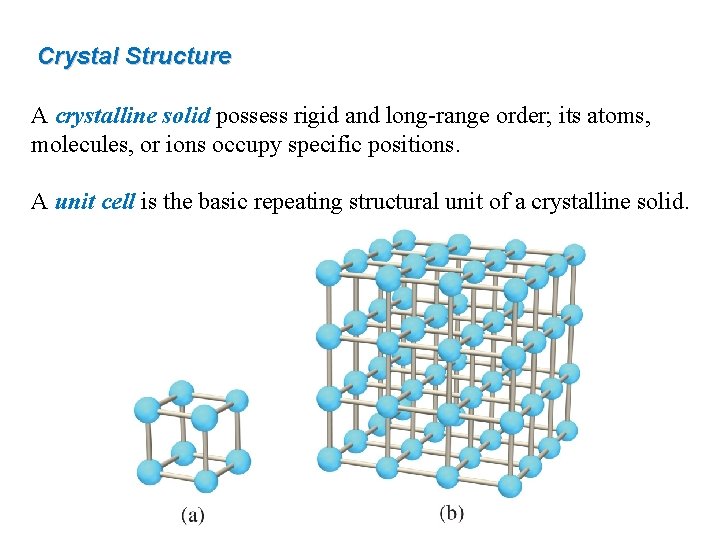
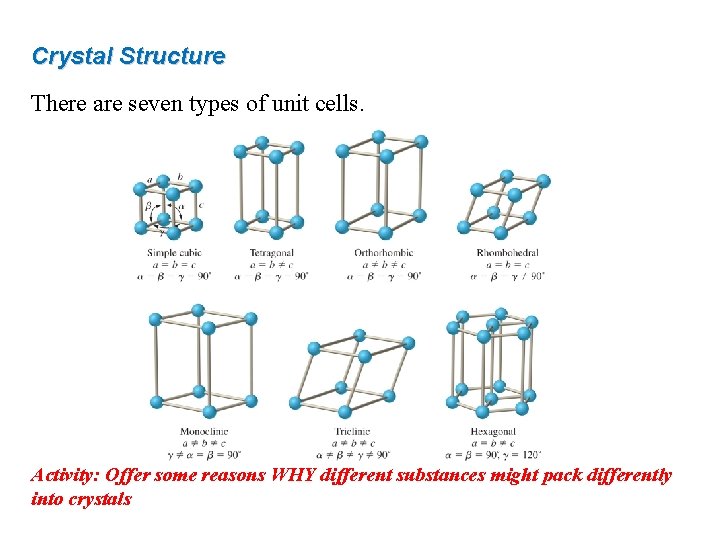
Crystal Structure A crystalline solid possess rigid and long-range order; its atoms, molecules, or ions occupy specific positions. A unit cell is the basic repeating structural unit of a crystalline solid.

Crystal Structure There are seven types of unit cells. Activity: Offer some reasons WHY different substances might pack differently into crystals

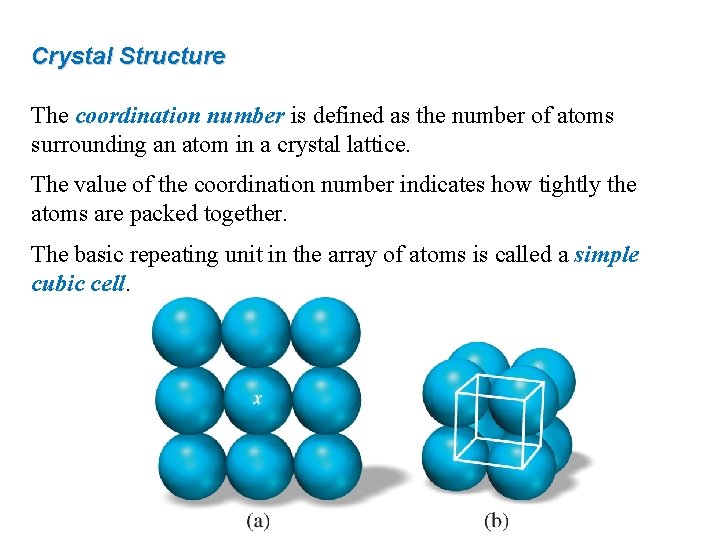
Crystal Structure The coordination number is defined as the number of atoms surrounding an atom in a crystal lattice. The value of the coordination number indicates how tightly the atoms are packed together. The basic repeating unit in the array of atoms is called a simple cubic cell.

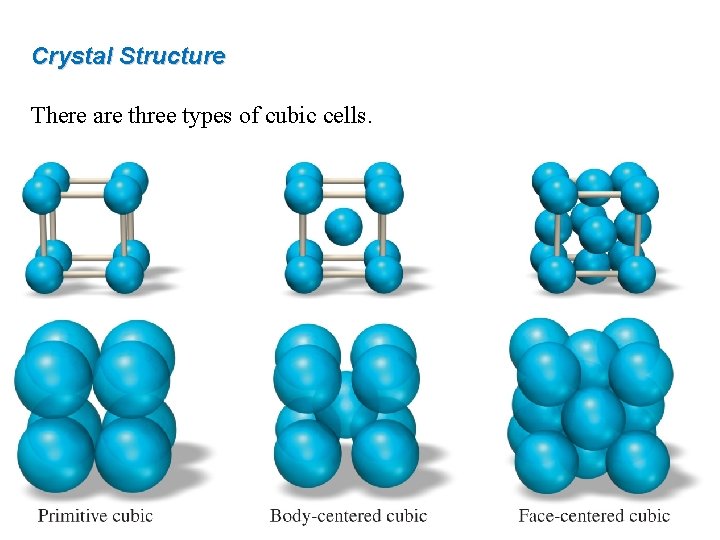
Crystal Structure There are three types of cubic cells.

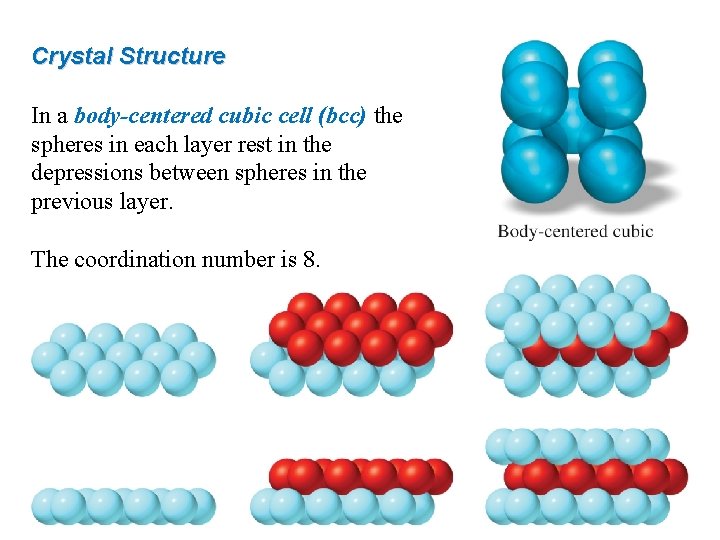
Crystal Structure In a body-centered cubic cell (bcc) the spheres in each layer rest in the depressions between spheres in the previous layer. The coordination number is 8.

Study Guide for Sections 11. 6 -11. 7, 12. 1 -12. 3 DAY 18, Terms to know: Sections 11. 6 -11. 7, 12. 1 -12. 3 van der Waals equation, partial pressure, Dalton’s law of partial pressure, surface tension, capillary action, cohesion, adhesion, viscosity, crystalline solid, unit cell DAY 18, Specific outcomes and skills that may be tested on exam 3: Sections 11. 6 -11. 7, 12. 1 -12. 3 • Be able to explain how an ideal gas behaves and what distinguishes an ideal gas from a real gas and under what conditions a real gas will behave ideally • Be able to use the van der Waals equation to determine the pressure of a gas given its volume, moles, temperature, and values for variables a and b • Be able to use the van der Waals equation to determine the temperature of a gas given its volume, moles, pressure, and values for variables a and b • Be able to determine overall pressure for a gas mixture given partial pressures or information that can be used to calculate partial pressures • Be able to explain what they following physical properties are and how the strength of intermolecular forces affects each physical property: surface tension, capillary action, viscosity, vapor pressure, boiling point • For a given set of molecules, rank them in increasing order for any of the following properties discussed : surface tension, viscosity, vapor pressure, boiling point • Be able to describe and explain the relationship between vapor pressure and temperature • Be able to describe and explain factors such as size and shape of molecules, strength of attraction between molecules, etc. that can affect the specific type of packing or unit cell formed in a crystalline solid

Extra Practice Problems for Sections 11. 6 -11. 7, 12. 1 -12. 3 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 11. 67 11. 71 11. 73 11. 79 11. 123 11. 139 12. 15 7. 37 7. 41 7. 43 7. 115 12. 97

Prep for Day 19 Must Watch videos: https: //www. youtube. com/watch? v=b_SXwf. HQ 774 (solids II, crash course chem) http: //www. youtube. com/watch? v=EZHm. UTm. Jt. F 8 (phases, Tyler De. Witt) https: //www. youtube. com/watch? v=Htc. Diy_RH 5 s (phase changes, Isaacs) Other helpful videos: http: //www. youtube. com/watch? v=Pu 0 D 1 XWB-wg (solids, UT-Austin) https: //www. youtube. com/watch? v=66 Dq. EGWD 20&list=PLq. OZ 6 FD_RQ 7 mco 4 Yb_a. YDD 8 w. HMPVYh. Qr. U (UC-Irvine, phase changes) Read Sections 12. 4 -12. 6