Chapter 8 Real Gases Real Gases Physical Chemistry



- Slides: 24

Chapter 8 Real Gases

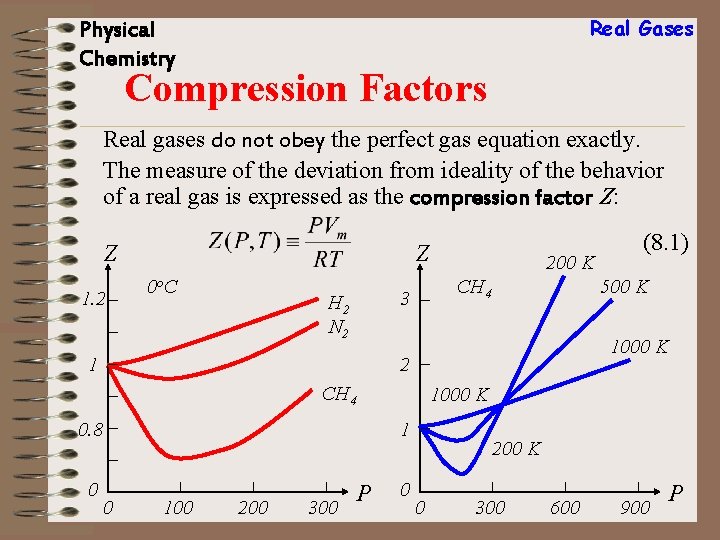
Real Gases Physical Chemistry Compression Factors Real gases do not obey the perfect gas equation exactly. The measure of the deviation from ideality of the behavior of a real gas is expressed as the compression factor Z: Z 1. 2 Z 0 o. C CH 4 3 H 2 N 2 1 500 K 1000 K 2 CH 4 0. 8 0 200 K 1000 K 1 0 100 200 300 P (8. 1) 0 200 K 0 300 600 900 P

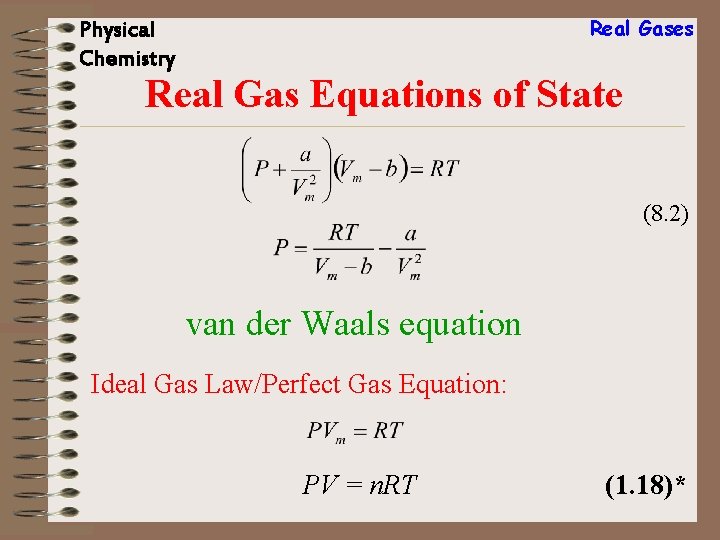
Real Gases Physical Chemistry Real Gas Equations of State (8. 2) van der Waals equation Ideal Gas Law/Perfect Gas Equation: PV = n. RT (1. 18)*

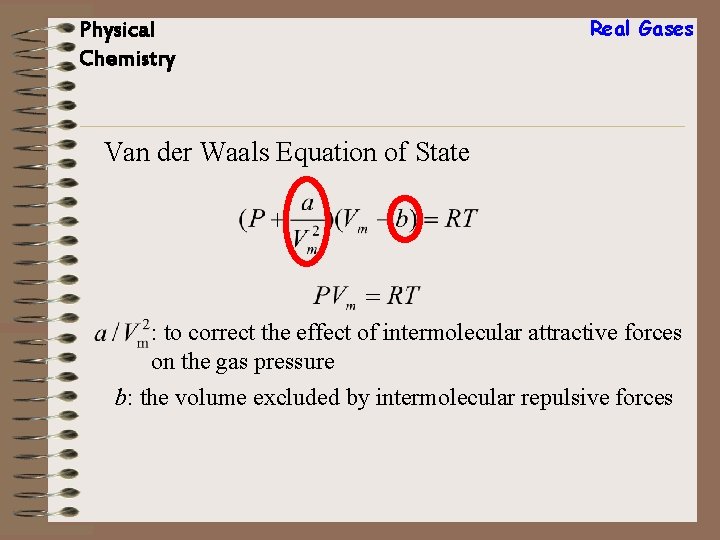
Physical Chemistry Real Gases Van der Waals Equation of State : to correct the effect of intermolecular attractive forces on the gas pressure b: the volume excluded by intermolecular repulsive forces

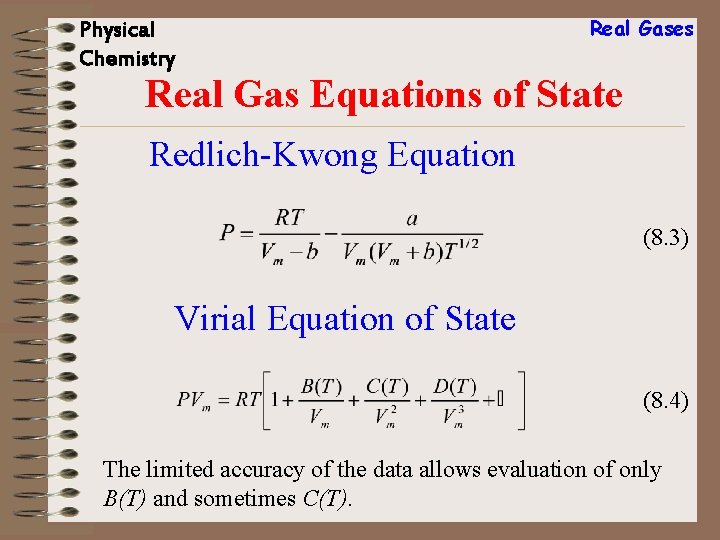
Physical Chemistry Real Gases Real Gas Equations of State Redlich-Kwong Equation (8. 3) Virial Equation of State (8. 4) The limited accuracy of the data allows evaluation of only B(T) and sometimes C(T).

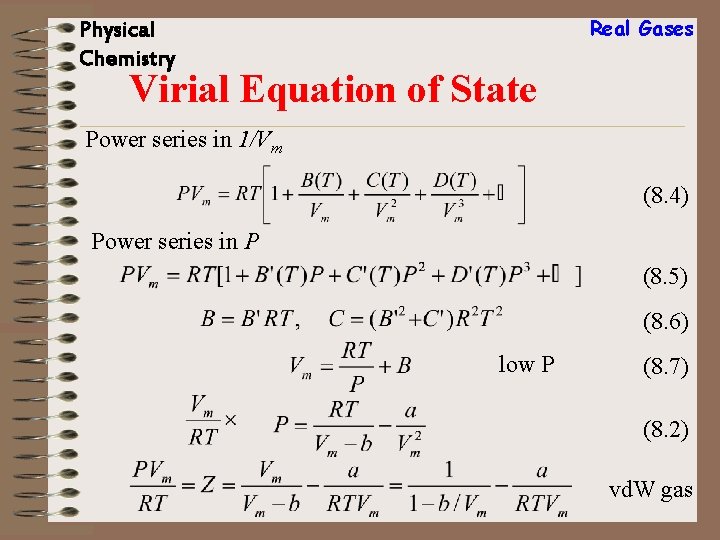
Real Gases Physical Chemistry Virial Equation of State Power series in 1/Vm (8. 4) Power series in P (8. 5) (8. 6) low P (8. 7) (8. 2) vd. W gas

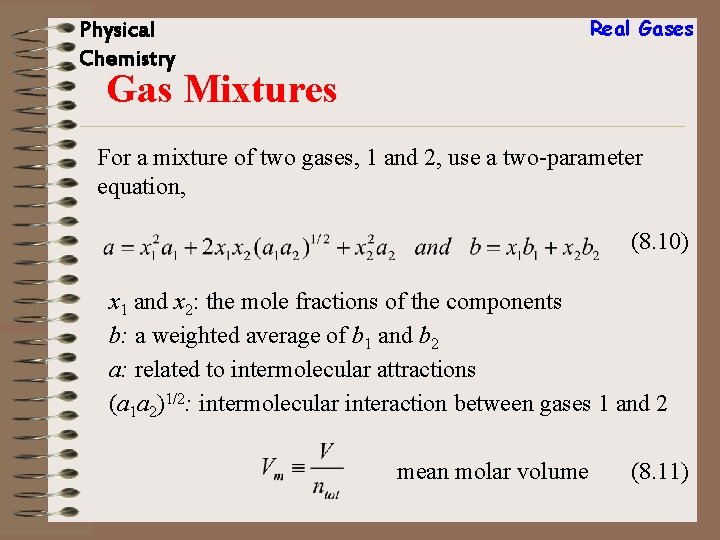
Real Gases Physical Chemistry Gas Mixtures For a mixture of two gases, 1 and 2, use a two-parameter equation, (8. 10) x 1 and x 2: the mole fractions of the components b: a weighted average of b 1 and b 2 a: related to intermolecular attractions (a 1 a 2)1/2: intermolecular interaction between gases 1 and 2 mean molar volume (8. 11)

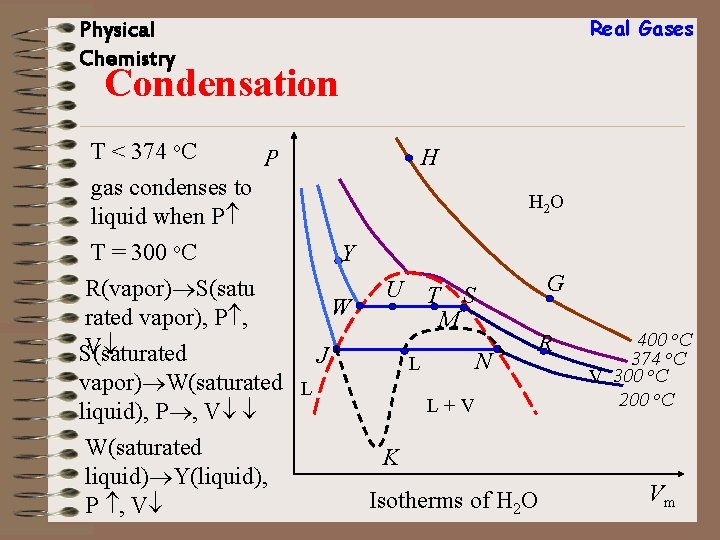
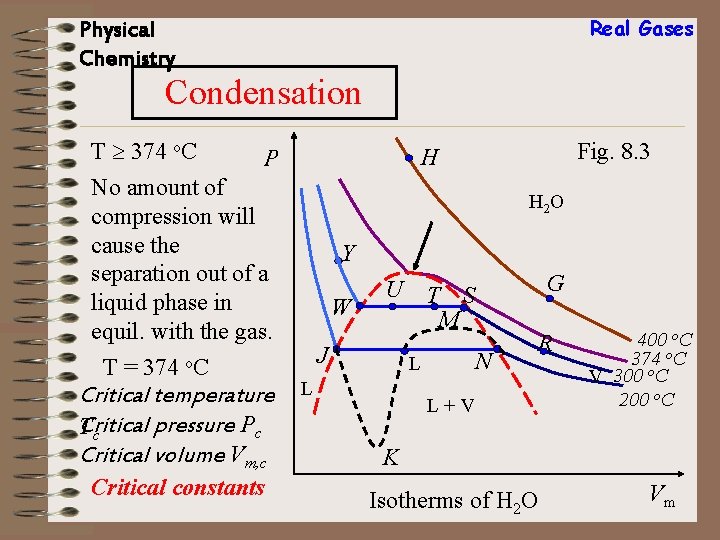
Real Gases Physical Chemistry Condensation T < 374 o. C H P gas condenses to liquid when P H 2 O T = 300 o. C R(vapor) S(satu rated vapor), P , V S(saturated Y W U J W(saturated liquid) Y(liquid), P , V N L vapor) W(saturated L liquid), P , V G T S M R L+V 400 o. C 374 o. C V 300 o. C 200 o. C K Isotherms of H 2 O Vm

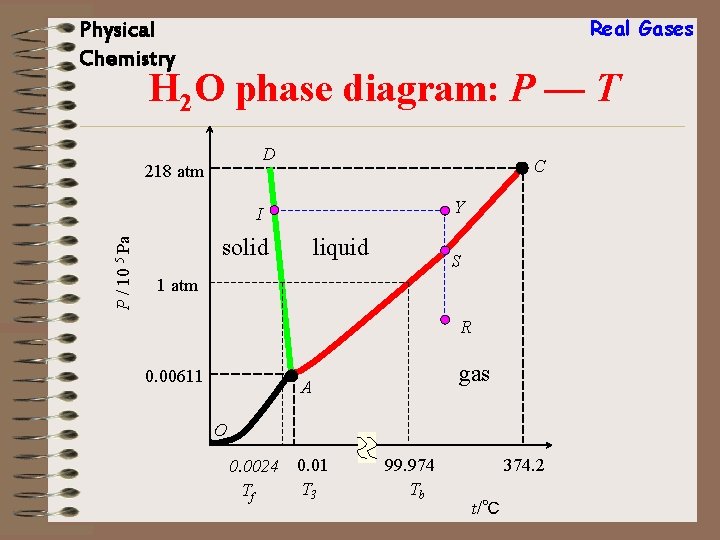
Real Gases Physical Chemistry H 2 O phase diagram: P — T D 218 atm C Y P / 10 5 Pa I solid liquid S 1 atm R 0. 00611 gas A O 0. 0024 Tf 0. 01 T 3 99. 974 Tb 374. 2 t/℃

Real Gases Physical Chemistry Condensation T 374 o. C No amount of compression will cause the separation out of a liquid phase in equil. with the gas. T = 374 o. C Critical temperature Critical pressure Pc T c Critical volume Vm, c Critical constants Fig. 8. 3 H P H 2 O Y W U J N L L G T S M R L+V 400 o. C 374 o. C V 300 o. C 200 o. C K Isotherms of H 2 O Vm

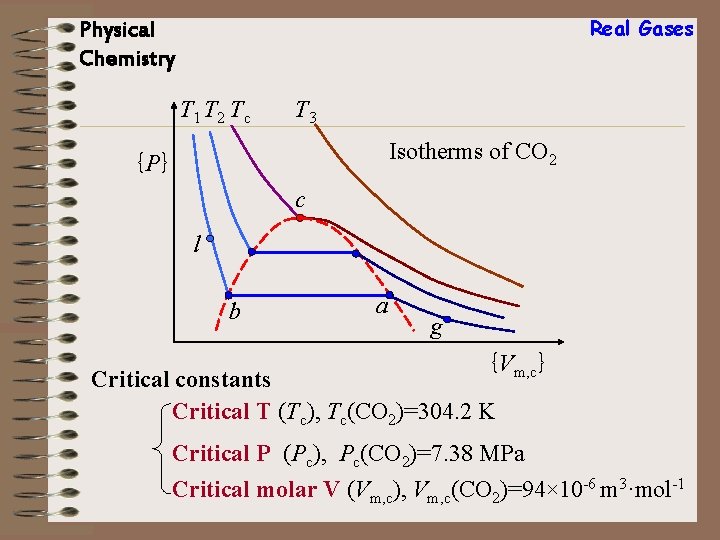
Real Gases Physical Chemistry T 1 T 2 Tc T 3 Isotherms of CO 2 {P} c l b a g {Vm, c} Critical constants Critical T (Tc), Tc(CO 2)=304. 2 K Critical P (Pc), Pc(CO 2)=7. 38 MPa Critical molar V (Vm, c), Vm, c(CO 2)=94× 10 -6 m 3·mol-1

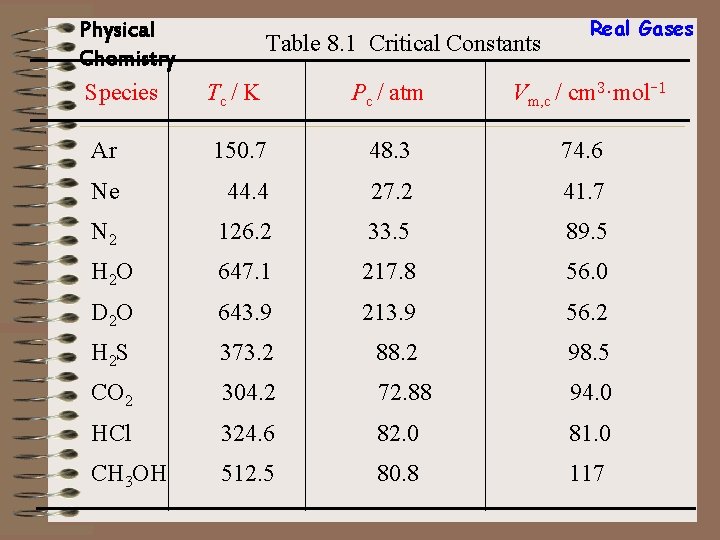
Physical Chemistry Species Table 8. 1 Critical Constants Real Gases Tc / K Pc / atm Vm, c / cm 3·mol-1 Ar 150. 7 48. 3 74. 6 Ne 44. 4 27. 2 41. 7 N 2 126. 2 33. 5 89. 5 H 2 O 647. 1 217. 8 56. 0 D 2 O 643. 9 213. 9 56. 2 H 2 S 373. 2 88. 2 98. 5 CO 2 304. 2 72. 88 94. 0 HCl 324. 6 82. 0 81. 0 CH 3 OH 512. 5 80. 8 117

Physical Chemistry Real Gases Fluid There is a continuity between the gaseous and the liquid states. In recognition of this continuity, the term fluid is used to mean either a liquid or a gas. An ordinary liquid can be viewed as a very dense gas. Only when both phases are present in the system is there a clear-cut distinction between liquid and gaseous states. For a single-phase liquid system it is customary to define as a liquid a fluid whose temperature is below Tc and whose molar volume is less than Vm, c. If these two conditions are not met, the liquid is called a gas. So a further distinction between gas and vapor can be made, but these two words are used interchangeably in this book.

Real Gases Physical Chemistry Supercritical fluid A supercritical fluid is one whose T and P satisfy T > Tc and P > Pc A supercritical fiquid usually has liquidlike density but its viscosity is much lower than typical for a liquid and diffusion coefficients in it are much higher than in liquids.

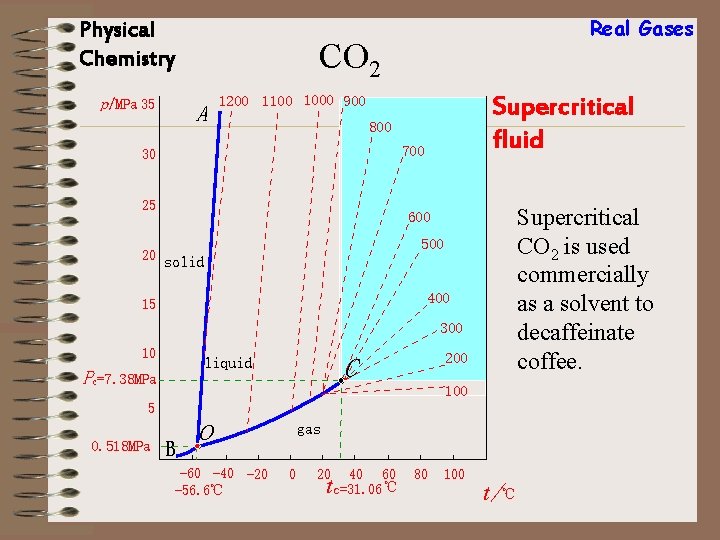
Physical Chemistry Real Gases CO 2 p/MPa 35 A Supercritical fluid 1200 1100 1000 900 800 700 30 25 Supercritical CO 2 is used commercially as a solvent to decaffeinate coffee. 600 500 20 solid 400 15 300 10 Pc=7. 38 MPa 100 5 0. 518 MPa 200 C liquid B o -60 -40 -20 -56. 6℃ gas 0 20 40 60 c=31. 06 ℃ t 80 100 t/℃

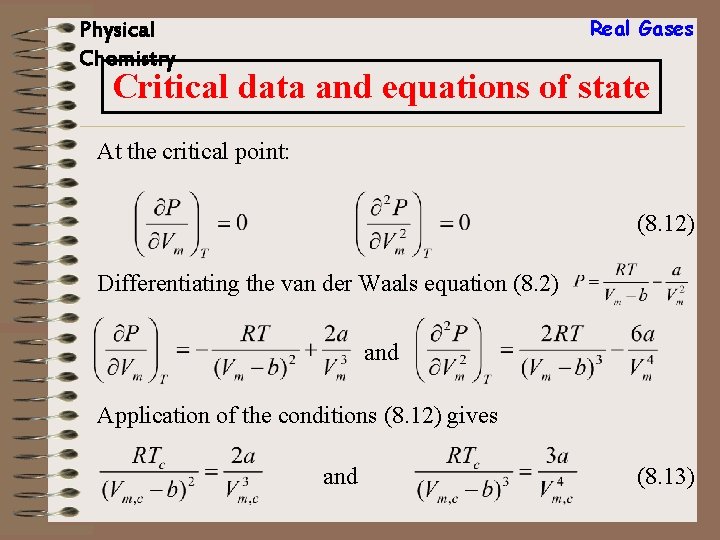
Real Gases Physical Chemistry Critical data and equations of state At the critical point: (8. 12) Differentiating the van der Waals equation (8. 2) and Application of the conditions (8. 12) gives and (8. 13)

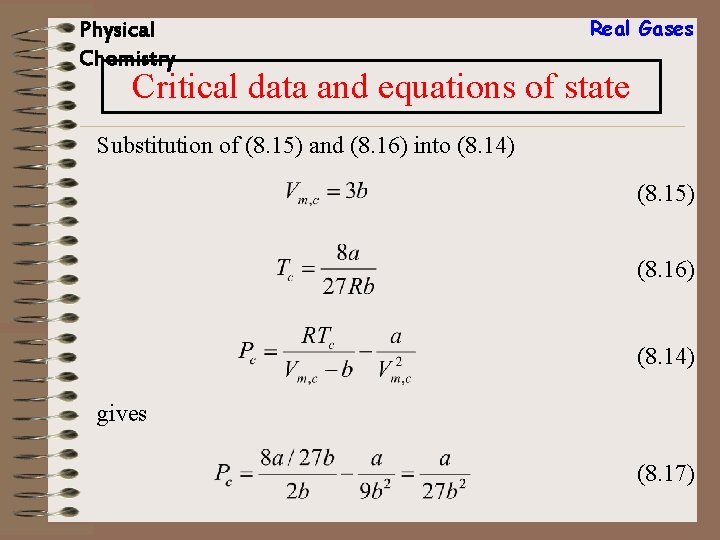
Real Gases Physical Chemistry Critical data and equations of state From van der Waals equation: (8. 14) Division of the first equation in (8. 13) by the second yields (8. 15) Use of (8. 15) in the first equation in (8. 13) gives and (8. 16)

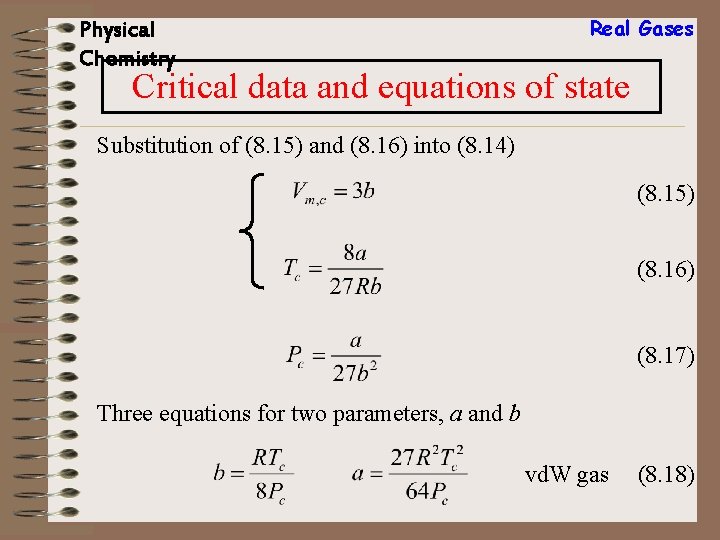
Physical Chemistry Real Gases Critical data and equations of state Substitution of (8. 15) and (8. 16) into (8. 14) (8. 15) (8. 16) (8. 14) gives (8. 17)

Physical Chemistry Real Gases Critical data and equations of state Substitution of (8. 15) and (8. 16) into (8. 14) (8. 15) (8. 16) (8. 17) Three equations for two parameters, a and b vd. W gas (8. 18)

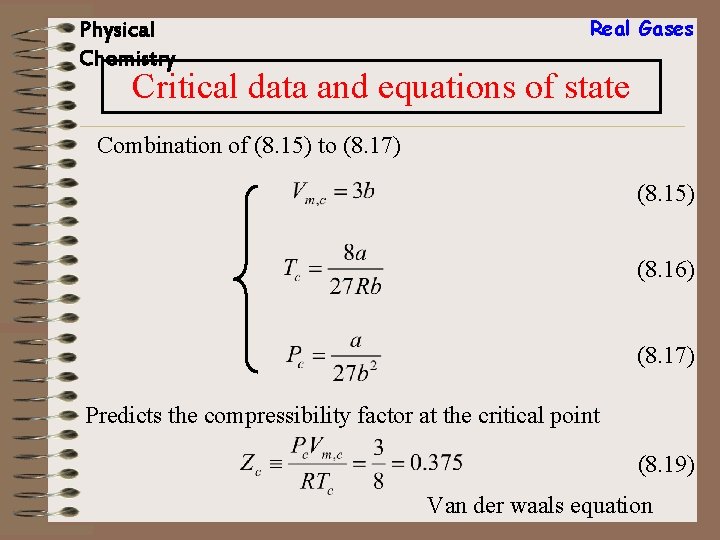
Physical Chemistry Real Gases Critical data and equations of state Combination of (8. 15) to (8. 17) (8. 15) (8. 16) (8. 17) Predicts the compressibility factor at the critical point (8. 19) Van der waals equation

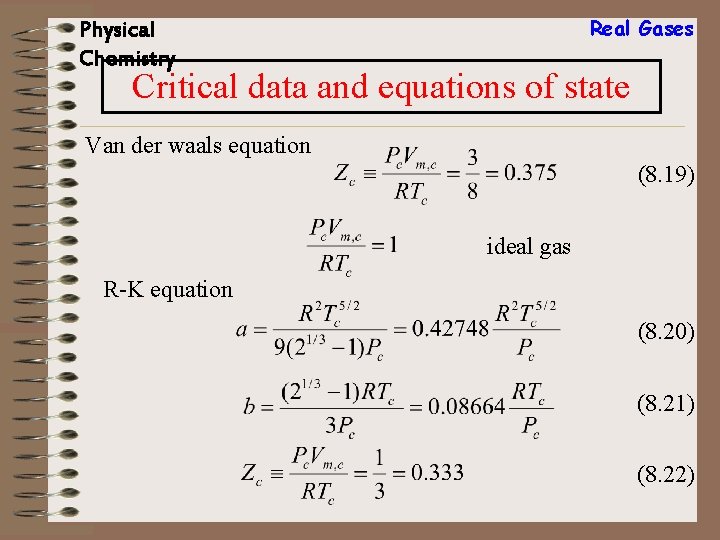
Real Gases Physical Chemistry Critical data and equations of state Van der waals equation (8. 19) ideal gas R-K equation (8. 20) (8. 21) (8. 22)

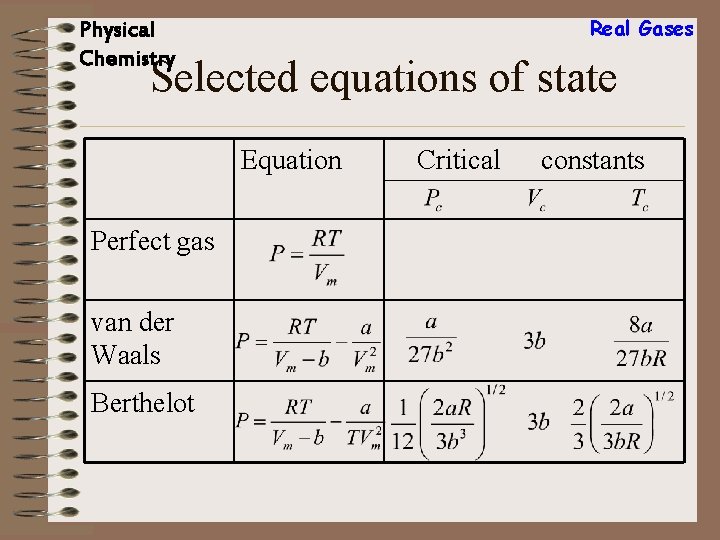
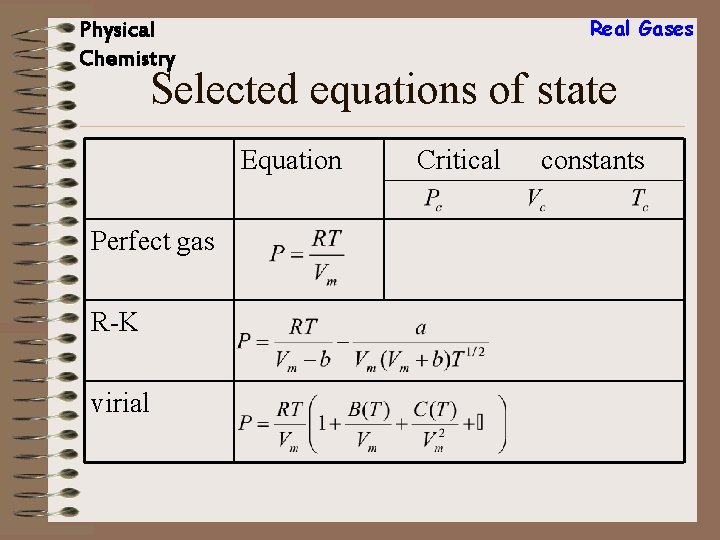
Real Gases Physical Chemistry Selected equations of state Equation Perfect gas van der Waals Berthelot Critical constants

Real Gases Physical Chemistry Selected equations of state Equation Perfect gas R-K virial Critical constants

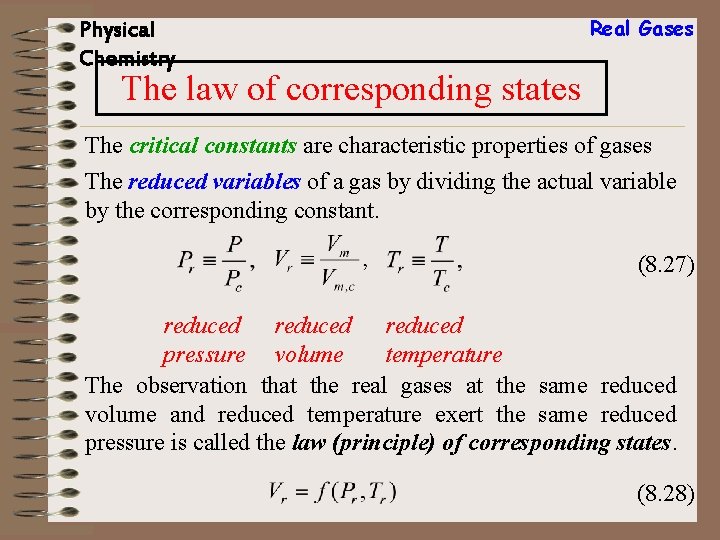
Physical Chemistry Real Gases The law of corresponding states The critical constants are characteristic properties of gases The reduced variables of a gas by dividing the actual variable by the corresponding constant. (8. 27) reduced pressure volume temperature The observation that the real gases at the same reduced volume and reduced temperature exert the same reduced pressure is called the law (principle) of corresponding states. (8. 28)
 Chemistry chapter 11 gases test
Chemistry chapter 11 gases test Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases Physical characteristics of gases
Physical characteristics of gases What are the different properties of gas?
What are the different properties of gas? Ib organic chemistry
Ib organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Internal energy of real gas
Internal energy of real gas Fugacity of real gases
Fugacity of real gases Ostwald viscometer
Ostwald viscometer Subatomic particles can usually pass undeflected
Subatomic particles can usually pass undeflected Physical chemistry crash course
Physical chemistry crash course Transport number in chemistry
Transport number in chemistry Physical chemistry problems
Physical chemistry problems Scope of physical chemistry
Scope of physical chemistry What is physical change and chemical change
What is physical change and chemical change Chapter 11 review gases section 1
Chapter 11 review gases section 1 Lussac's law worksheet answer key
Lussac's law worksheet answer key Chapter 14 the behavior of gases
Chapter 14 the behavior of gases Section 13.2 the combined gas law and avogadro's principle
Section 13.2 the combined gas law and avogadro's principle Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Rate fences services marketing
Rate fences services marketing Physical fitness components and tests grade 9
Physical fitness components and tests grade 9 Applications of molality in pharmacy
Applications of molality in pharmacy