Whats the Matter Matter is everything around you














- Slides: 31

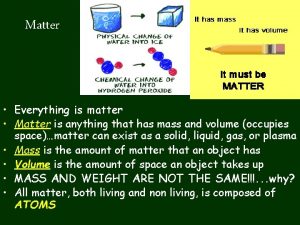
What’s the Matter? Matter is everything around you. The earth is one big mixture of gases, liquids and solids, all of which are different forms of matter. Matter is anything that has a mass & volume. Does air have mass? Does helium have mass?

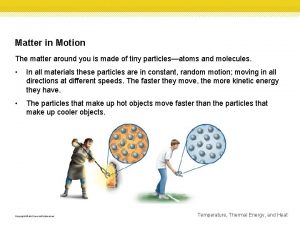
Kinetic Theory of Matter Kinetic-Relating to motion The theory explains of how particles in matter behave. 1. All matter is composed of small particles (atoms, molecules and ions). 2. These particles are in constant motion. 3. These particles are colliding with each other and the walls of their container.

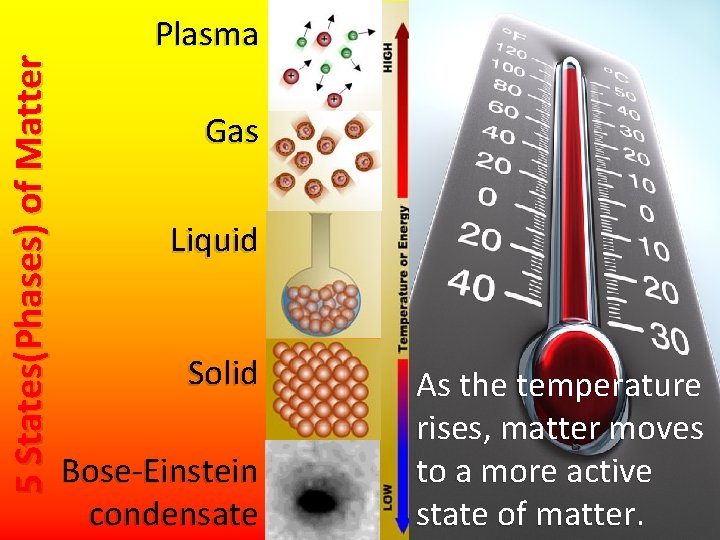
5 States(Phases) of Matter Plasma Gas Liquid Solid Bose-Einstein condensate As the temperature rises, matter moves to a more active state of matter.

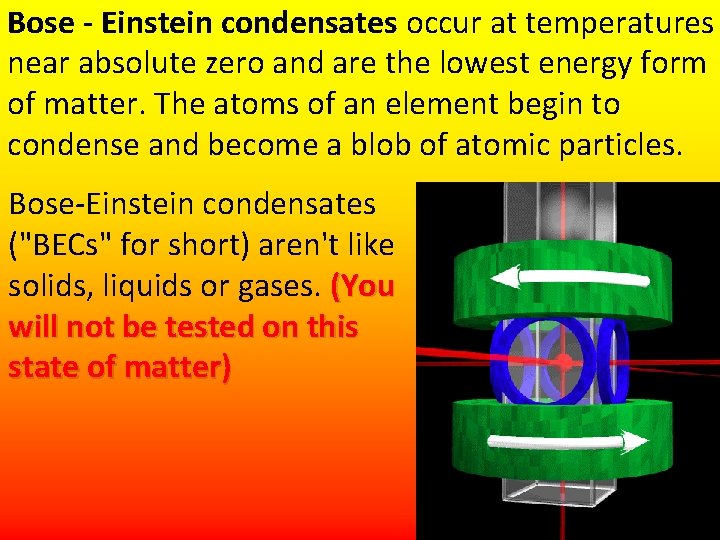
Bose - Einstein condensates occur at temperatures near absolute zero and are the lowest energy form of matter. The atoms of an element begin to condense and become a blob of atomic particles. Bose-Einstein condensates ("BECs" for short) aren't like solids, liquids or gases. (You will not be tested on this state of matter)

Warning This is the last time you will hear about Bose-Einstein Condensates in this class. You Do Not Talk About Bose-Einstein are not required to know about this state of Condensates Again. matter and you will not be tested on it! It Will Adversely Impact Your Grade!

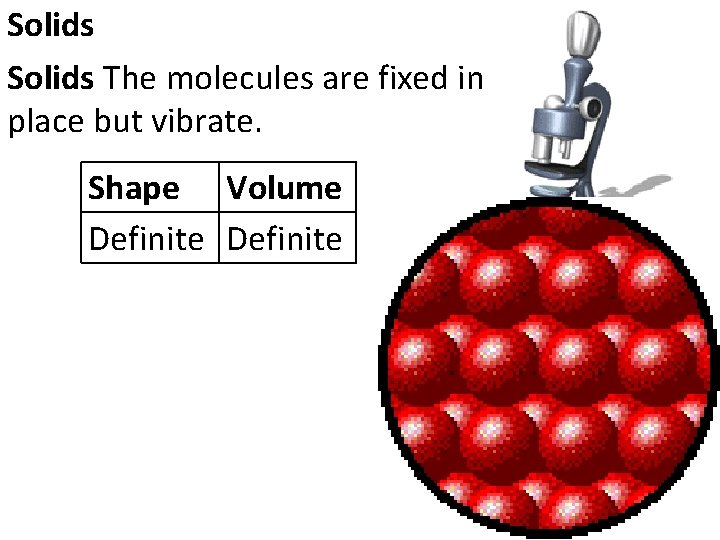
Solids The molecules are fixed in place but vibrate. Shape Volume Definite

Crystalline Solids Crystalline: A highly ordered threedimensional arrangement of particles. Ex. Salt, Metals, Diamond, Graphite & Sugar Amorphous Solids Amorphous: (without form) particles are randomly arranged in three dimensions. Ex. Obsidian, Glass, Coal, Plastic & rubber

Thermal Expansion Thermal expansion is the increase in volume of a substance in response to an increase in temperature. As a substance’s temperature increases, its particles move faster and spread out

Thermal Expansion Simile Suppose you have a lot of people dancing, think how the space increases between dancers as you get a faster song.

Thermal Expansion • Have you noticed a pattern of bumps when driving across a bridge? • These are expansion joints that allow the bridge to expand without breaking the bridge.

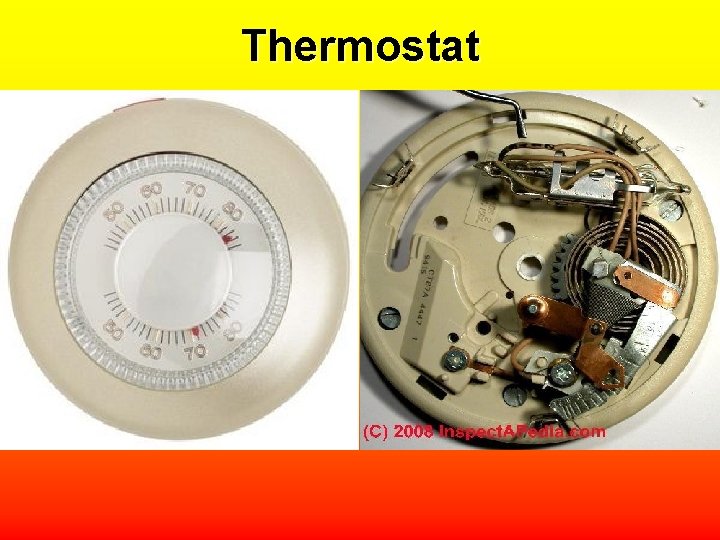
Thermostat

Thermometer • A thermometer works because of thermal expansion. The particles of the liquid in thermometer move faster and spread apart when placed in a warmer location.

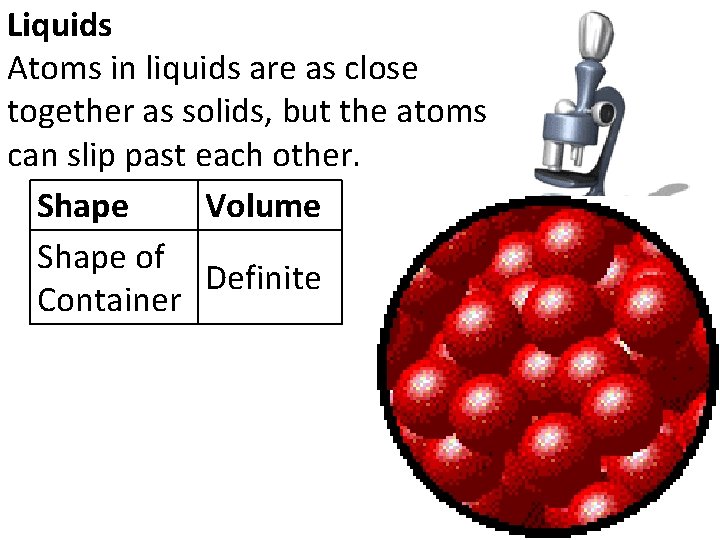
Liquids Atoms in liquids are as close together as solids, but the atoms can slip past each other. Shape Volume Shape of Definite Container

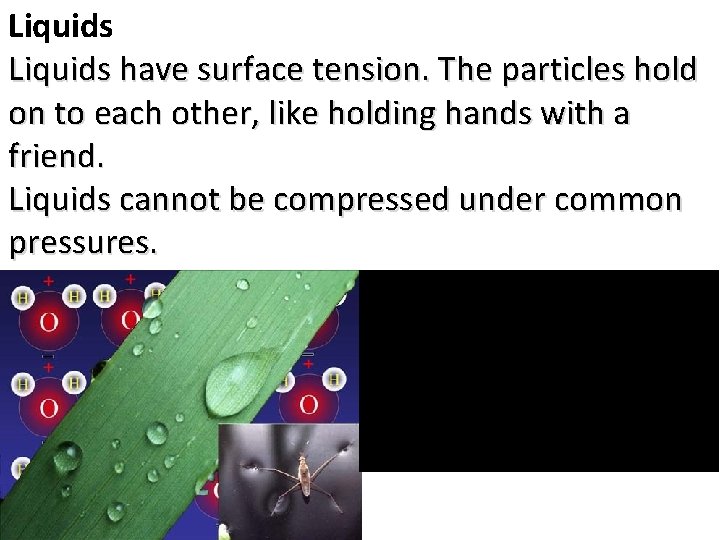
Liquids have surface tension. The particles hold on to each other, like holding hands with a friend. Liquids cannot be compressed under common pressures.

Viscosity A property of a fluid that offers resistance to flow. When you push a spoon with a small force through a bowl of water, it moves easily, but with the same force through mashed potatoes the spoon moves very slowly.


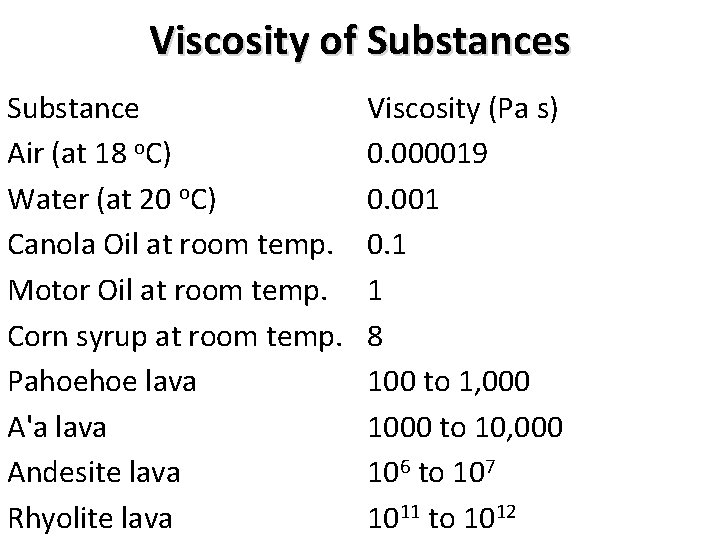
Viscosity of Substances Substance Air (at 18 o. C) Water (at 20 o. C) Canola Oil at room temp. Motor Oil at room temp. Corn syrup at room temp. Pahoehoe lava A'a lava Andesite lava Rhyolite lava Viscosity (Pa s) 0. 000019 0. 001 0. 1 1 8 100 to 1, 000 1000 to 10, 000 106 to 107 1011 to 1012

Gases Particles of gas, are spread apart and have too much energy to be attracted to other particles. Shape Volume Shape of Volume of Container

Gases can be compressed. They conform to the shape of the container in which they are held.

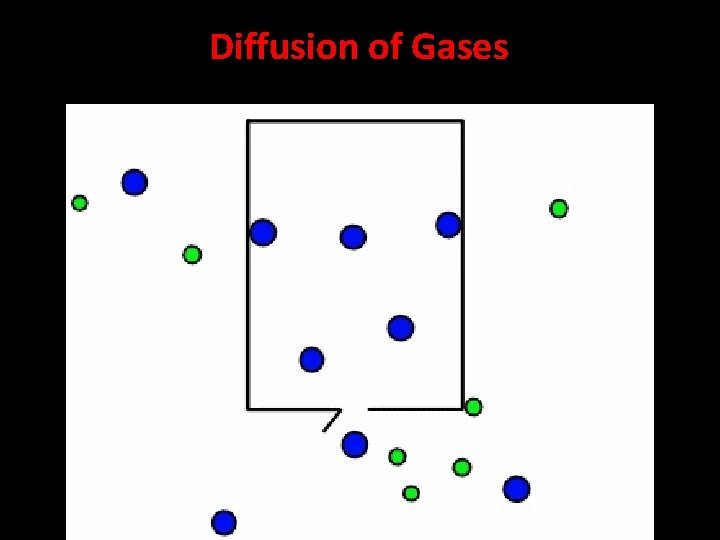
Diffusion If someone is cooking it doesn't take long for the smell to travel around the house to other rooms. This is because of diffusion. Diffusion in gases • When chemicals, like perfume or burning toast, are let loose in a room, the particles mix with the air particles. The particles of the gas are free to move quickly in all directions. They eventually spread through the whole room. • Diffusion in gases is quick because the particles in a gas move quickly. It happens even faster in hot gases

Diffusion of Gases

Plasmas The highest energy form of matter. Ionized form of gas which occurs at extremely high temperatures. The plasma state is not related to blood plasma. Shape Volume Shape of Volume of Container

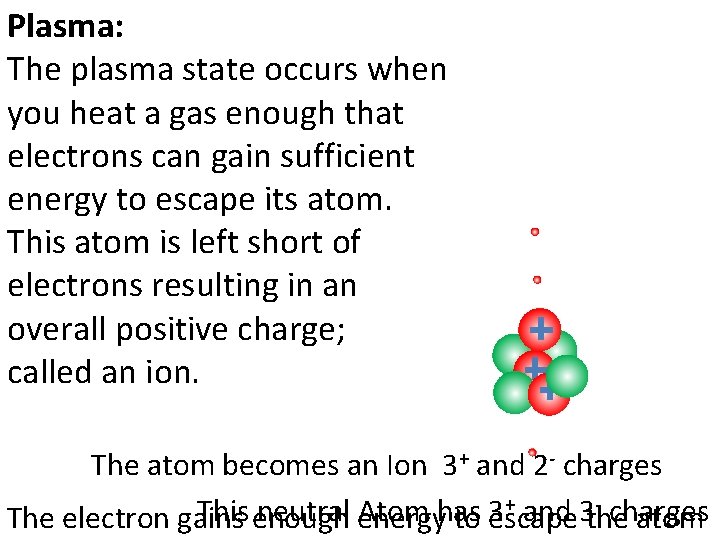
Plasma: The plasma state occurs when you heat a gas enough that electrons can gain sufficient energy to escape its atom. This atom is left short of electrons resulting in an overall positive charge; called an ion. The atom becomes an Ion 3+ and 2 - charges + and 3 - charges This neutral Atom has 3 The electron gains enough energy to escape the atom

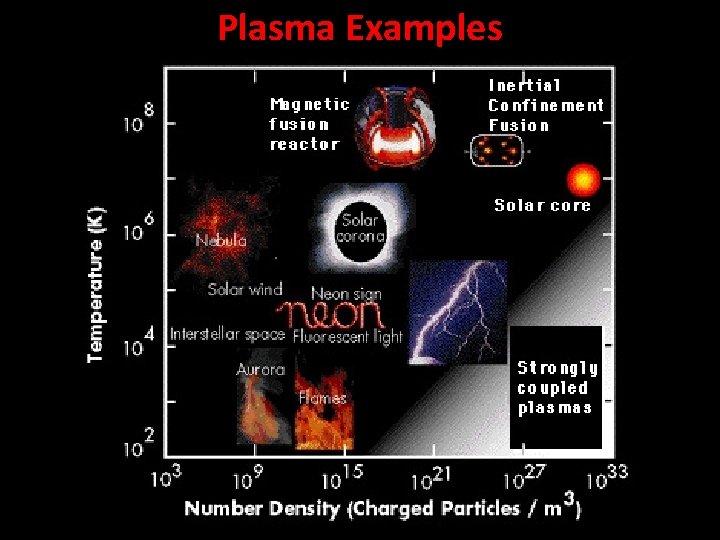
Plasma A plasma has a sufficient number of charged particles so that the gas, as a whole, exhibits a response to electric and magnetic fields and conducts electricity. Plasma is the most common state of matter in the universe.

Plasma Examples

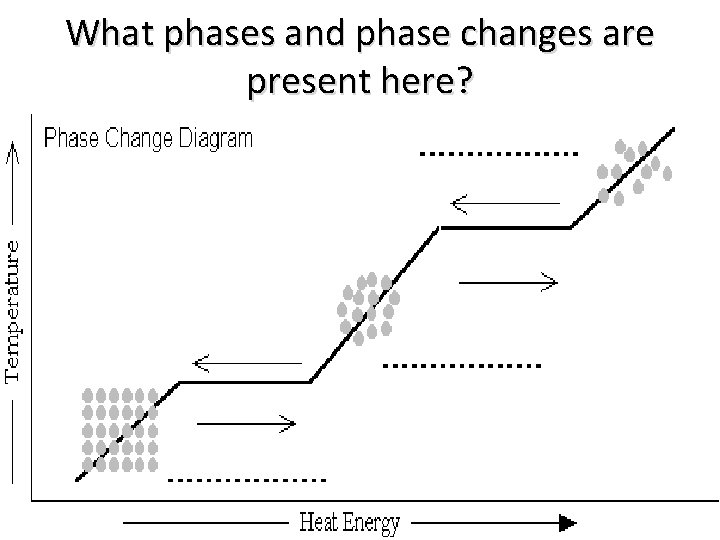
What phases and phase changes are present here?

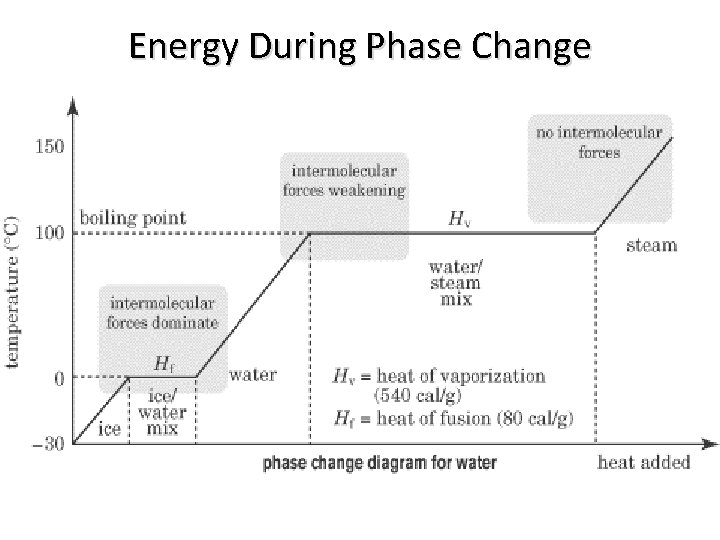
Think about this question During each phase change the temperature stays the same, even though thermal energy is still being added to the substance. So, if the energy is not being used to make the particles move faster (i. e. increase temperature), then what affect does the added thermal energy have?

Energy During Phase Change

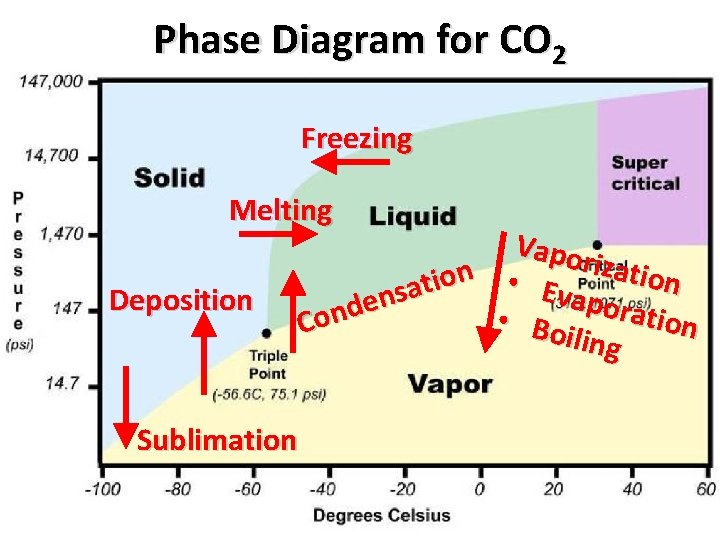
Phase Diagram for CO 2 Freezing Melting Deposition Sublimation Vapo rizati n o o i n • t a E s v apora n e d tion • Bo Con iling

Examples of Phase Changes • Melting: Ice becoming Water • Evaporation: A puddle becoming water vapor • Boiling: A pot of water on the stove becoming steam • Condensing: Water droplets forming outside a glass of ice water • Freezing: Water becoming snow • Depositon: Frost • Sublimation: Dry Ice becoming a gas without going through the liquid phase

Challenge Questions: 1. How many states of matter are present in a can of soda? 2. How many states of matter make up your body?
 Everything around you is made up of
Everything around you is made up of Everything around you is made up of
Everything around you is made up of Everything around us
Everything around us Everything that surrounds us is our
Everything that surrounds us is our We will not be moved you're standing with us
We will not be moved you're standing with us Jesus you are my lord
Jesus you are my lord كما تدين تدان english
كما تدين تدان english Martin luther king of hinduism
Martin luther king of hinduism Lord i lift everything to you
Lord i lift everything to you How to put god first in everything you do
How to put god first in everything you do Everything you need to know about the odyssey
Everything you need to know about the odyssey Everything that lives and moves will be food for you
Everything that lives and moves will be food for you Steven johnson everything bad is good for you
Steven johnson everything bad is good for you Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Tư thế worm breton là gì
Tư thế worm breton là gì Hát lên người ơi alleluia
Hát lên người ơi alleluia Các môn thể thao bắt đầu bằng tiếng đua
Các môn thể thao bắt đầu bằng tiếng đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống