Ionic Equations Whats really swimming around And whats



- Slides: 32

Ionic Equations. . What’s really swimming around. And what’s not swimming around. 1

Electrolytes: cmpds whose aqueous solutions conduct electricity. (refer to solubility rules). Electrolytic solutions must contain ions. Nonelectrolytes: cmpds whose solutions don’t conduct electricity. No ions are present in solution. For a RXN to occur, at least one product must a non-electrolyte. An insoluble solid (s) or a molecular substance such as a gas (g), or liquid (l) [as in H 2 O(l)] If everything remains aqueous (aq), no reaction occurs. All particles are ions and are spectators. 2

3

Double Replacement Reactions Or to impress your friends. Also called: Metathesis reactions 4

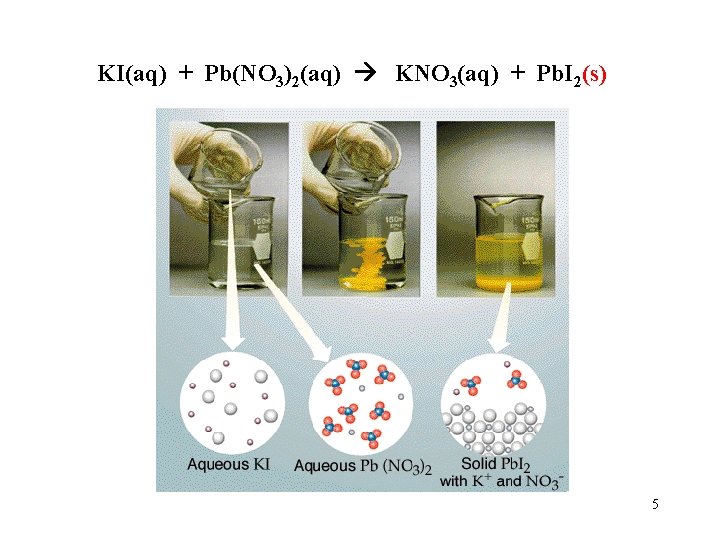
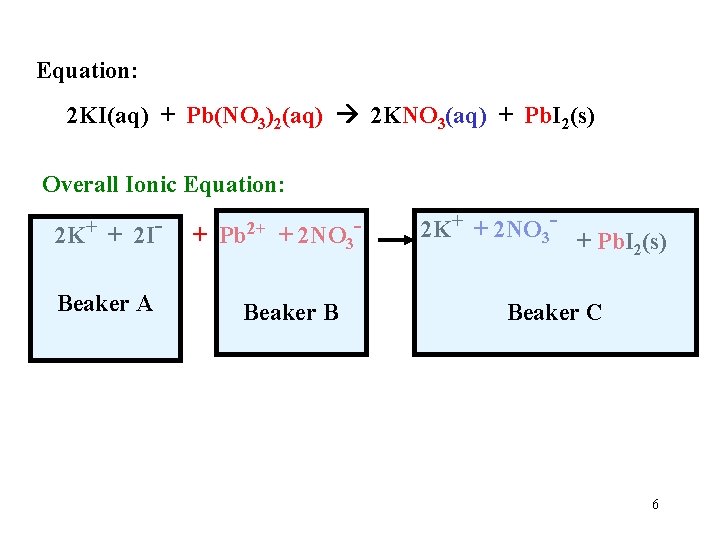
KI(aq) + Pb(NO 3)2(aq) KNO 3(aq) + Pb. I 2(s) 5

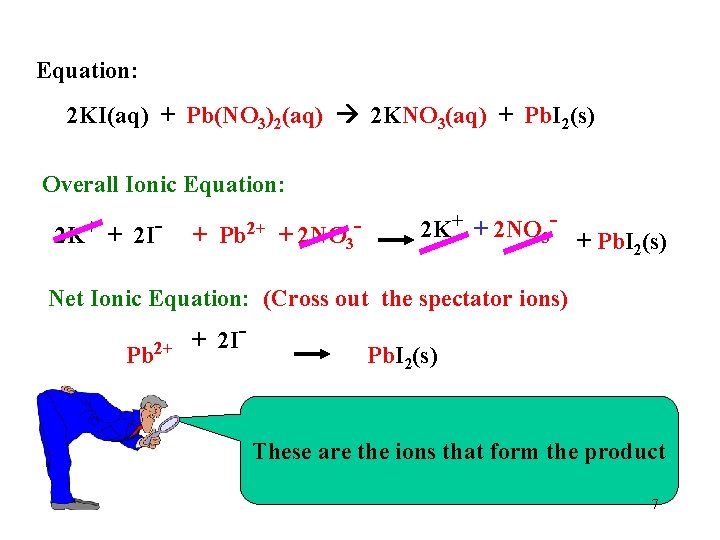
Equation: 2 KI(aq) + Pb(NO 3)2(aq) 2 KNO 3(aq) + Pb. I 2(s) Overall Ionic Equation: 2 K+ + 2 IBeaker A + Pb 2+ + 2 NO 3 - Beaker B 2 K+ + 2 NO 3 - + Pb. I (s) 2 Beaker C 6

Equation: 2 KI(aq) + Pb(NO 3)2(aq) 2 KNO 3(aq) + Pb. I 2(s) Overall Ionic Equation: 2 K+ + 2 I- + Pb 2+ + 2 NO 3 - 2 K+ + 2 NO 3 - + Pb. I (s) 2 Net Ionic Equation: (Cross out the spectator ions) + 2 I Pb 2+ Pb. I 2(s) These are the ions that form the product 7

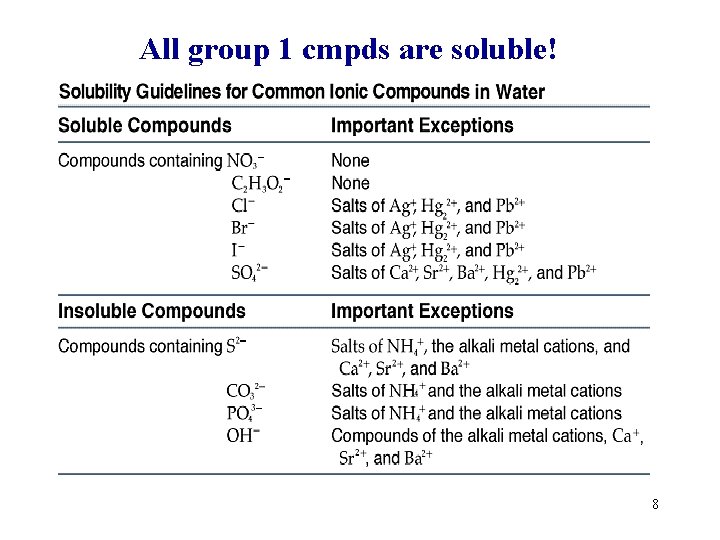
All group 1 cmpds are soluble! 8

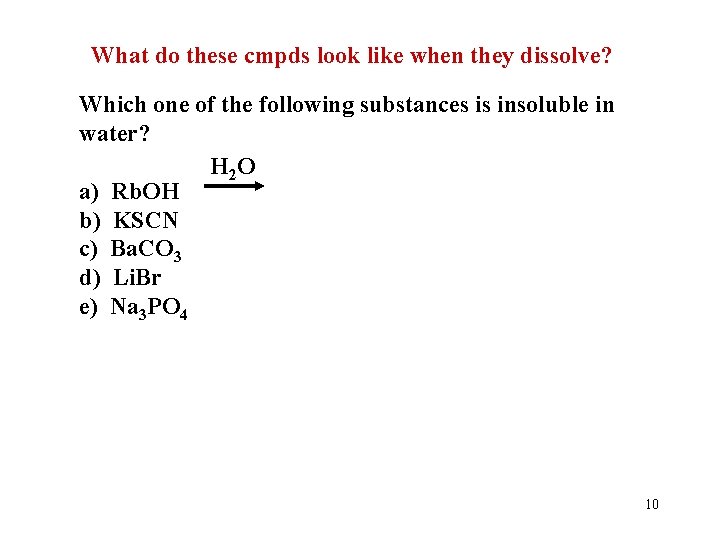
Let’s Take a Quiz!! Which one of the following substances is insoluble in water? a) b) c) d) e) Rb. OH KSCN Ba. CO 3 Li. Br Na 3 PO 4 What do these cmpds look like when they dissolve? 9

What do these cmpds look like when they dissolve? Which one of the following substances is insoluble in water? H 2 O a) Rb. OH b) KSCN c) Ba. CO 3 d) Li. Br e) Na 3 PO 4 10

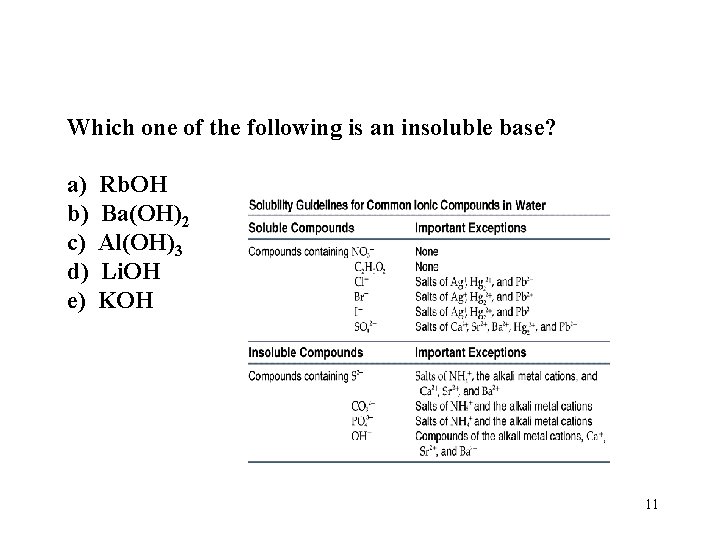
Which one of the following is an insoluble base? a) b) c) d) e) Rb. OH Ba(OH)2 Al(OH)3 Li. OH KOH 11

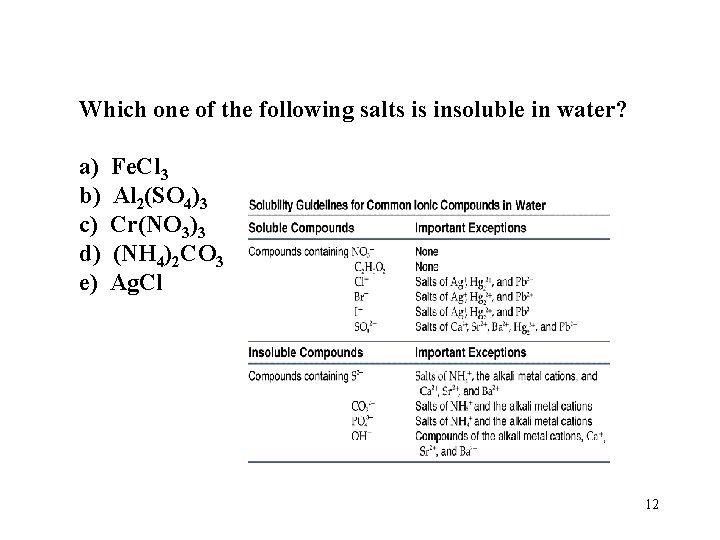
Which one of the following salts is insoluble in water? a) b) c) d) e) Fe. Cl 3 Al 2(SO 4)3 Cr(NO 3)3 (NH 4)2 CO 3 Ag. Cl 12

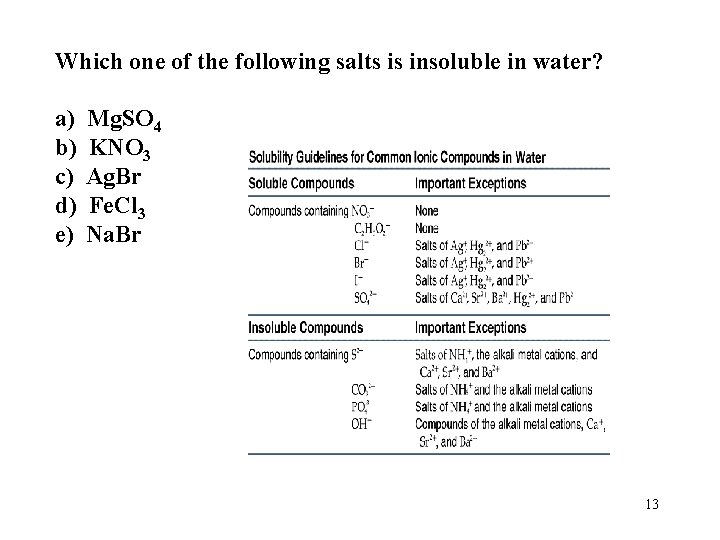
Which one of the following salts is insoluble in water? a) b) c) d) e) Mg. SO 4 KNO 3 Ag. Br Fe. Cl 3 Na. Br 13

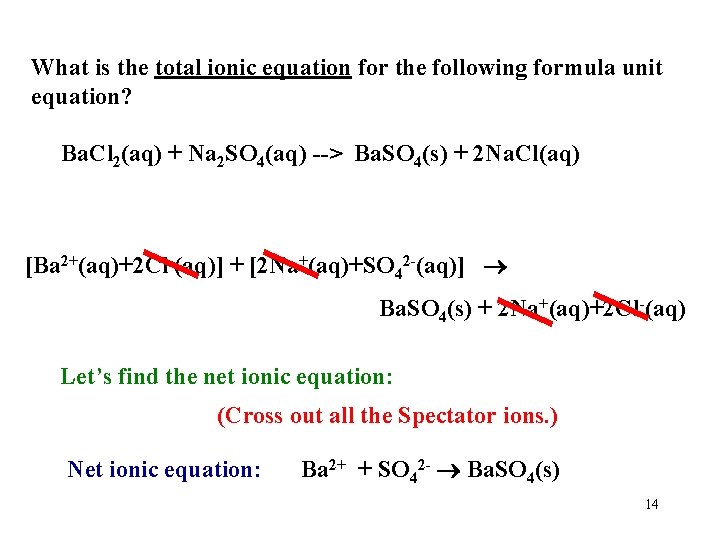
What is the total ionic equation for the following formula unit equation? Ba. Cl 2(aq) + Na 2 SO 4(aq) --> Ba. SO 4(s) + 2 Na. Cl(aq) [Ba 2+(aq)+2 Cl-(aq)] + [2 Na+(aq)+SO 42 -(aq)] Ba. SO 4(s) + 2 Na+(aq)+2 Cl-(aq) Let’s find the net ionic equation: (Cross out all the Spectator ions. ) Net ionic equation: Ba 2+ + SO 42 - Ba. SO 4(s) 14

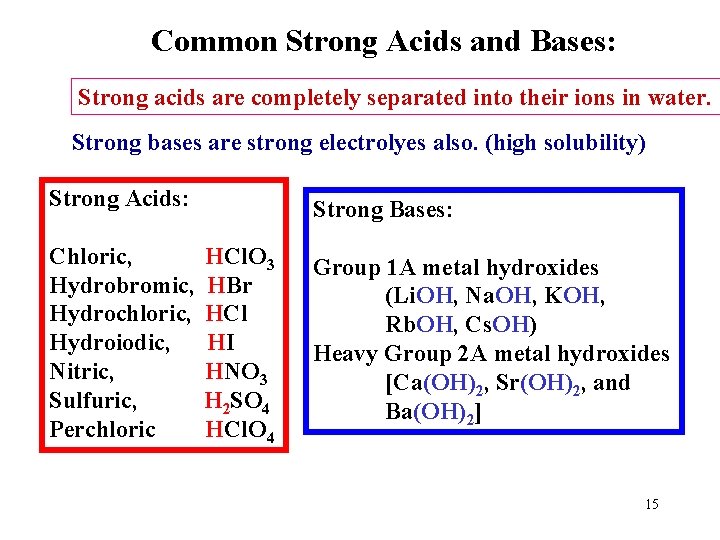
Common Strong Acids and Bases: Strong acids are completely separated into their ions in water. Strong bases are strong electrolyes also. (high solubility) Strong Acids: Chloric, Hydrobromic, Hydrochloric, Hydroiodic, Nitric, Sulfuric, Perchloric Strong Bases: HCl. O 3 HBr HCl HI HNO 3 H 2 SO 4 HCl. O 4 Group 1 A metal hydroxides (Li. OH, Na. OH, KOH, Rb. OH, Cs. OH) Heavy Group 2 A metal hydroxides [Ca(OH)2, Sr(OH)2, and Ba(OH)2] 15

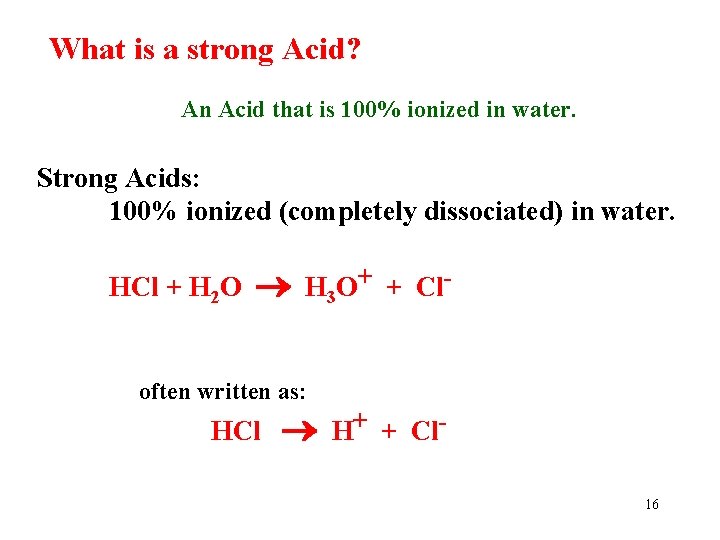
What is a strong Acid? An Acid that is 100% ionized in water. Strong Acids: 100% ionized (completely dissociated) in water. HCl + H 2 O H 3 O+ + Cl- often written as: HCl H+ + Cl 16

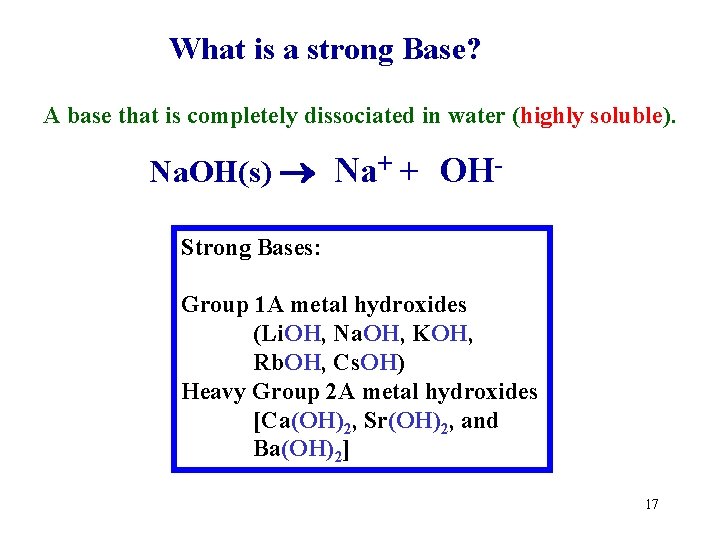
What is a strong Base? A base that is completely dissociated in water (highly soluble). Na. OH(s) Na+ + OHStrong Bases: Group 1 A metal hydroxides (Li. OH, Na. OH, KOH, Rb. OH, Cs. OH) Heavy Group 2 A metal hydroxides [Ca(OH)2, Sr(OH)2, and Ba(OH)2] 17

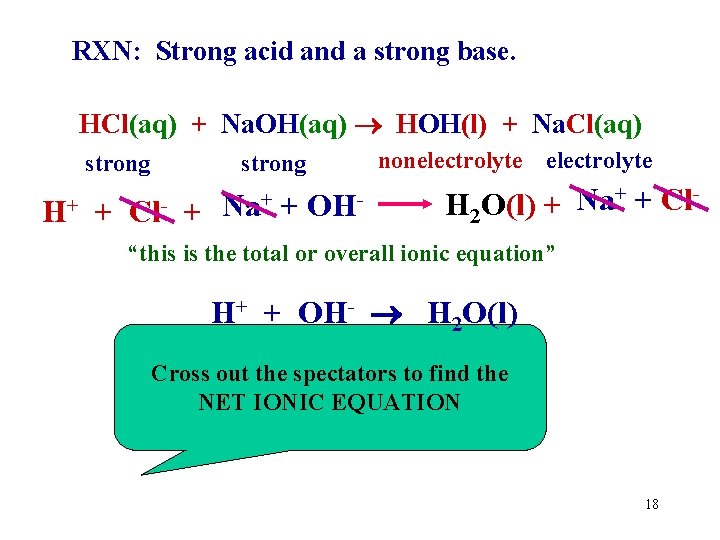
RXN: Strong acid and a strong base. HCl(aq) + Na. OH(aq) HOH(l) + Na. Cl(aq) strong H+ + strong Cl- + + OHNa + nonelectrolyte H 2 O(l) + Na+ + Cl- “this is the total or overall ionic equation” H+ + OH- H 2 O(l) Cross out the spectators to find the NET IONIC EQUATION 18

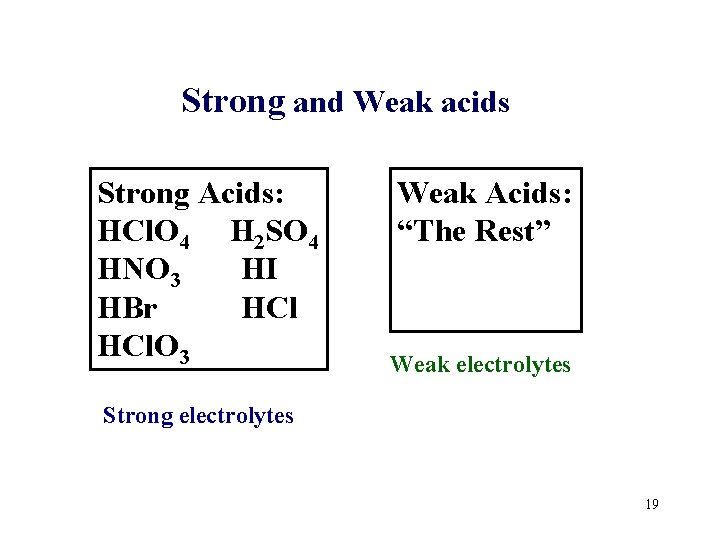
Strong and Weak acids Strong Acids: HCl. O 4 H 2 SO 4 HNO 3 HI HBr HCl. O 3 Weak Acids: “The Rest” Weak electrolytes Strong electrolytes 19

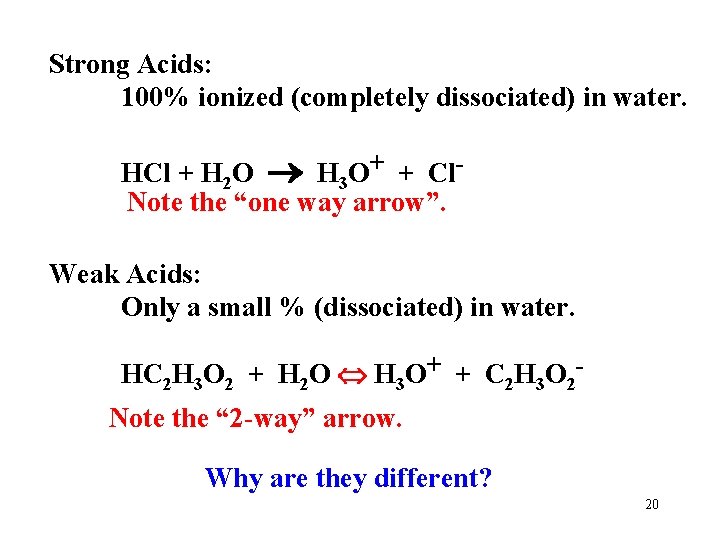
Strong Acids: 100% ionized (completely dissociated) in water. HCl + H 2 O H 3 O+ + Cl. Note the “one way arrow”. Weak Acids: Only a small % (dissociated) in water. HC 2 H 3 O 2 + H 2 O H 3 O+ + C 2 H 3 O 2 Note the “ 2 -way” arrow. Why are they different? 20

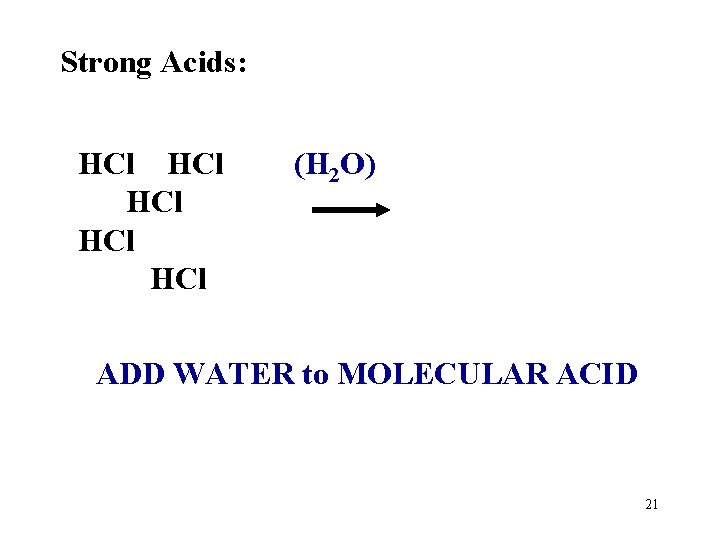
Strong Acids: HCl HCl HCl (H 2 O) ADD WATER to MOLECULAR ACID 21

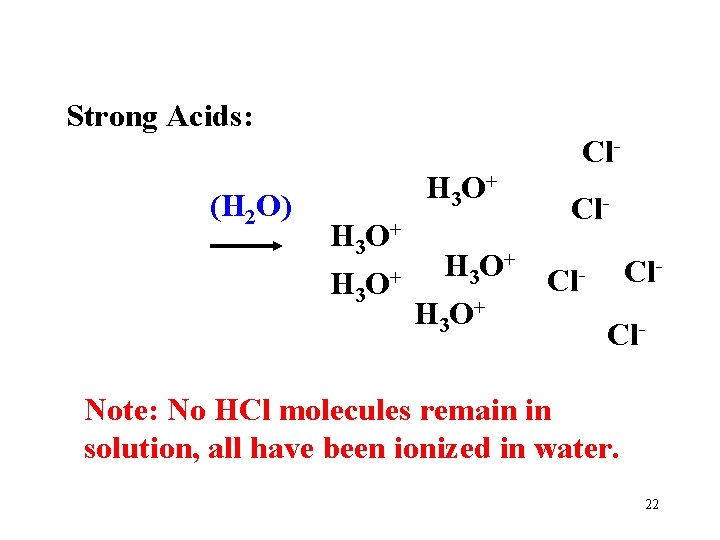
Strong Acids: Cl(H 2 O) H 3 O+ Cl- H 3 O+ Cl. Cl- Note: No HCl molecules remain in solution, all have been ionized in water. 22

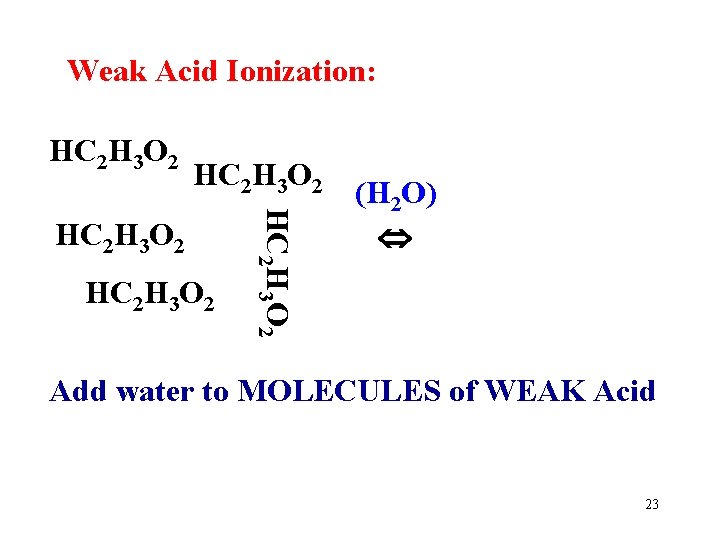
Weak Acid Ionization: HC 2 H 3 O 2 HC 2 H 3 O 2 (H 2 O) Add water to MOLECULES of WEAK Acid 23

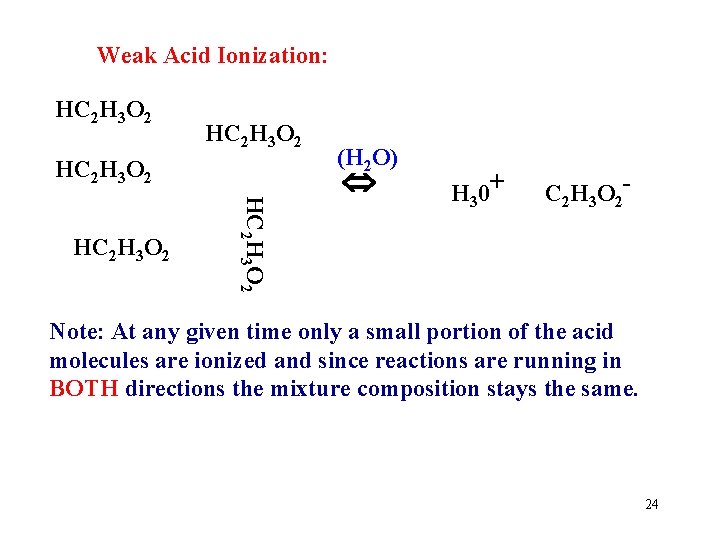
Weak Acid Ionization: HC 2 H 3 O 2 HC 2 H 3 O 2 (H 2 O) H 30+ C 2 H 3 O 2 - Note: At any given time only a small portion of the acid molecules are ionized and since reactions are running in BOTH directions the mixture composition stays the same. 24

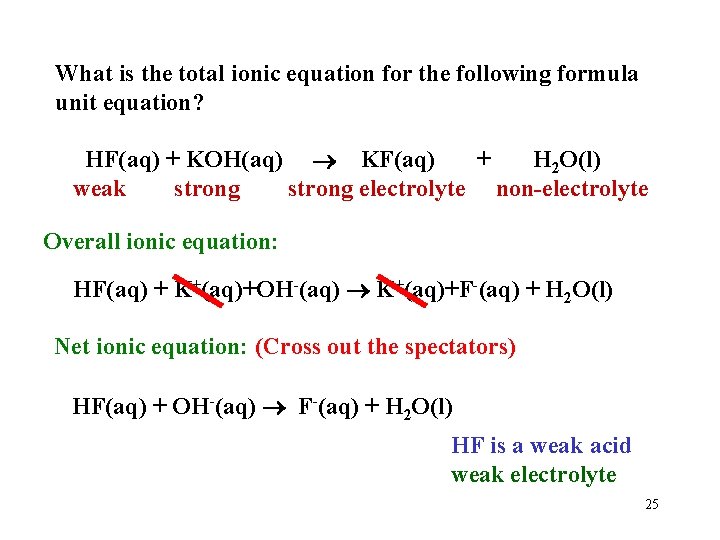
What is the total ionic equation for the following formula unit equation? HF(aq) + KOH(aq) KF(aq) + H 2 O(l) weak strong electrolyte non-electrolyte Overall ionic equation: HF(aq) + K+(aq)+OH-(aq) K+(aq)+F-(aq) + H 2 O(l) Net ionic equation: (Cross out the spectators) HF(aq) + OH-(aq) F-(aq) + H 2 O(l) HF is a weak acid weak electrolyte 25

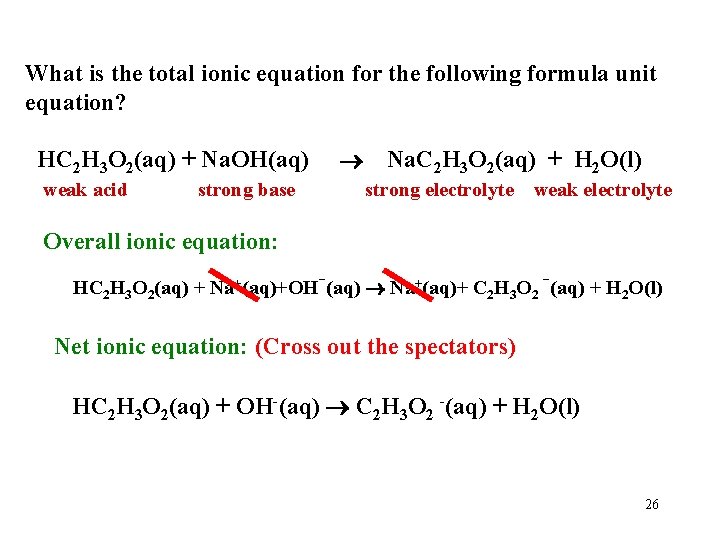
What is the total ionic equation for the following formula unit equation? HC 2 H 3 O 2(aq) + Na. OH(aq) weak acid strong base Na. C 2 H 3 O 2(aq) + H 2 O(l) strong electrolyte weak electrolyte Overall ionic equation: HC 2 H 3 O 2(aq) + Na+(aq)+OH-(aq) Na+(aq)+ C 2 H 3 O 2 -(aq) + H 2 O(l) Net ionic equation: (Cross out the spectators) HC 2 H 3 O 2(aq) + OH-(aq) C 2 H 3 O 2 -(aq) + H 2 O(l) 26

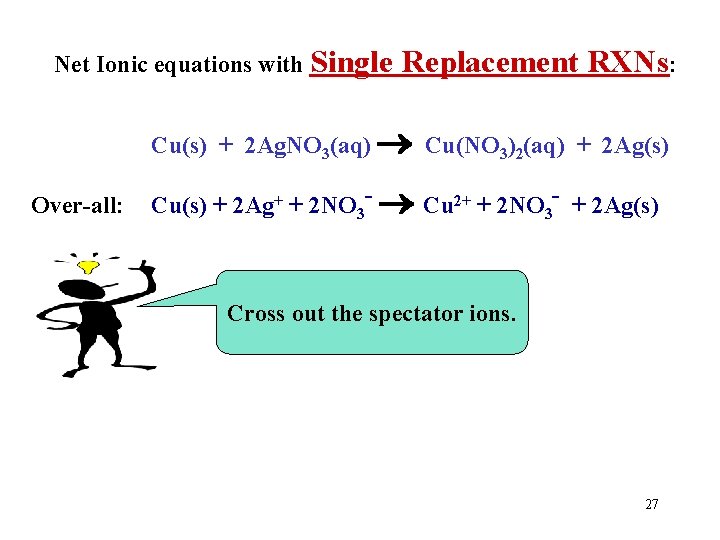
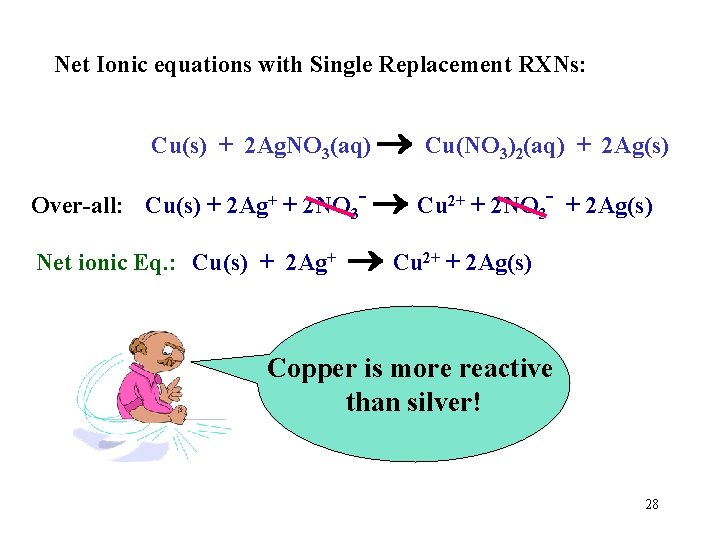
Net Ionic equations with Single Replacement RXNs: Cu(s) + 2 Ag. NO 3(aq) Cu(NO 3)2(aq) + 2 Ag(s) Over-all: Cu(s) + 2 Ag+ + 2 NO 3 - Cu 2+ + 2 NO 3 - + 2 Ag(s) Cross out the spectator ions. 27

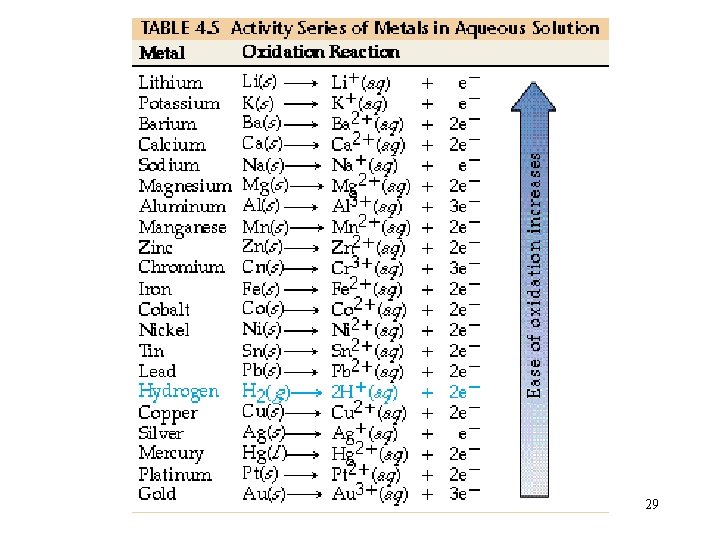
Net Ionic equations with Single Replacement RXNs: Cu(s) + 2 Ag. NO 3(aq) Cu(NO 3)2(aq) + 2 Ag(s) Over-all: Cu(s) + 2 Ag+ + 2 NO 3 - Cu 2+ + 2 NO 3 - + 2 Ag(s) Net ionic Eq. : Cu(s) + 2 Ag+ Cu 2+ + 2 Ag(s) Copper is more reactive than silver! 28

29

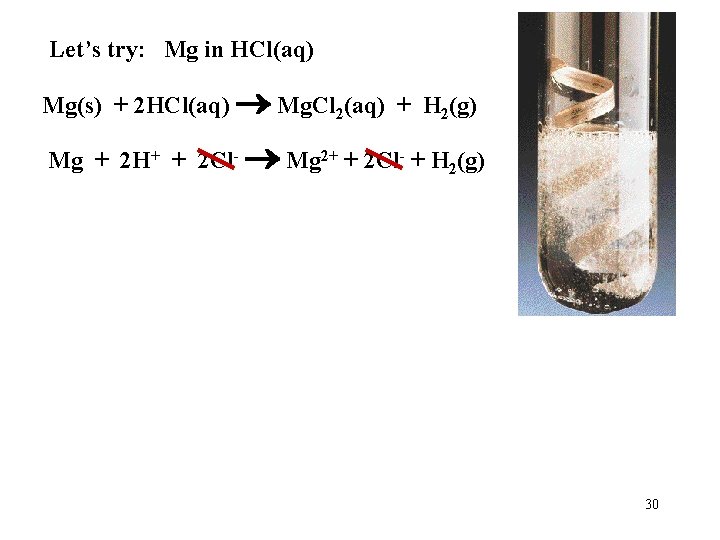
Let’s try: Mg in HCl(aq) Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g) Mg + 2 H+ + 2 Cl- Mg 2+ + 2 Cl- + H 2(g) 30

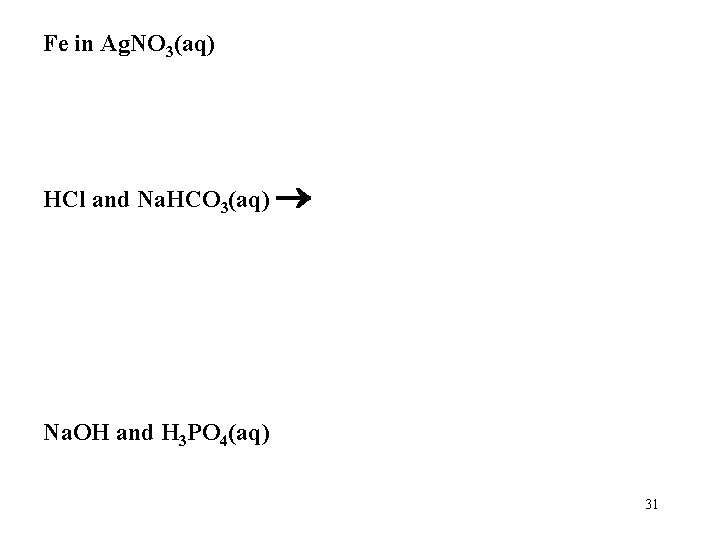
Fe in Ag. NO 3(aq) HCl and Na. HCO 3(aq) Na. OH and H 3 PO 4(aq) 31

Study for a Quiz! 32