What is conceptual learning in chemistry and why




- Slides: 50

What is conceptual learning in chemistry and why should we promote it? David Yaron+, Michael Karabinos+, Jodi Davenport*, Jordi Cuadros+ Department of Chemistry+ and Psychology*, Carnegie Mellon University Gaea Leinhardt, Jim Greeno, Karen Evans Learning Research and Development Center, University of Pittsburgh Laura Bartolo+, John Portman* Department of Information Science+ and Biology*, Kent State University CMU 2009 http: //www. chemcollective. org 1 W. Craig Carter, and Donald Sadoway

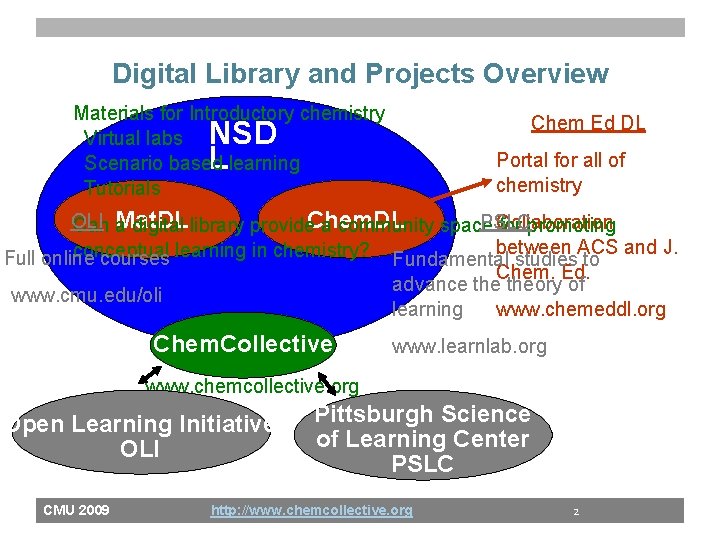
Digital Library and Projects Overview Materials for Introductory chemistry Virtual labs Scenario based learning Tutorials Chem Ed DL NSD L Portal for all of chemistry OLI Collaboration PSLC Can Mat. DL a digital library provide. Chem. DL a community space for promoting between ACS and J. conceptual Full online courses learning in chemistry? Fundamental studies to Chem. Ed. advance theory of www. cmu. edu/oli www. chemeddl. org learning Chem. Collective www. learnlab. org www. chemcollective. org Open Learning Initiative OLI CMU 2009 Pittsburgh Science of Learning Center PSLC http: //www. chemcollective. org 2

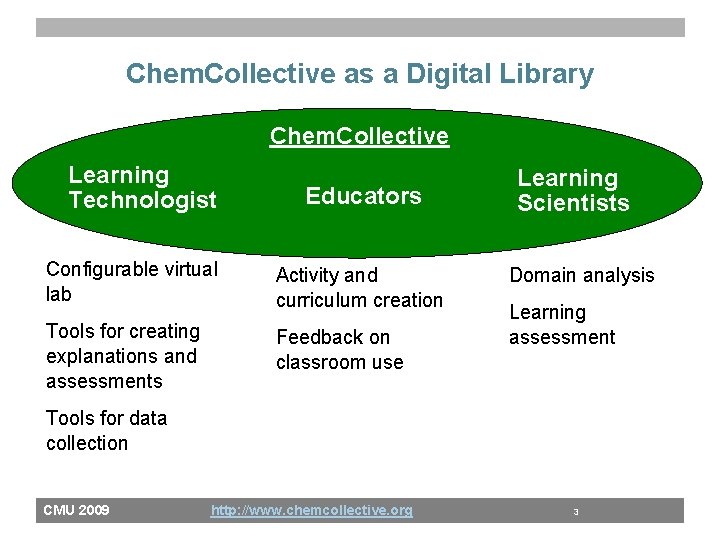
Chem. Collective as a Digital Library Chem. Collective Learning Technologist Educators Configurable virtual lab Activity and curriculum creation Tools for creating explanations and assessments Feedback on classroom use Learning Scientists Domain analysis Learning assessment Tools for data collection CMU 2009 http: //www. chemcollective. org 3

What is conceptual learning? • Physics’ Force Concept Inventory – Mathematical problem solving does not necessarily lead to ability to answer qualitative questions – Students learn what they practice. • Physics’ answer to “What is conceptual learning? ” – Non-conceptual instruction students struggle with hard problems – Conceptual instruction Couple mathematical problem solving with qualitative questions CMU 2009 http: //www. chemcollective. org 4

Conceptual learning • Being systematic about the goals of instruction and aligning the instruction to these goals • Four projects related to conceptual learning – – Virtual lab What is needed for scientific literacy? Teaching chemical equilibrium Molecular science across disciplines CMU 2009 http: //www. chemcollective. org 5

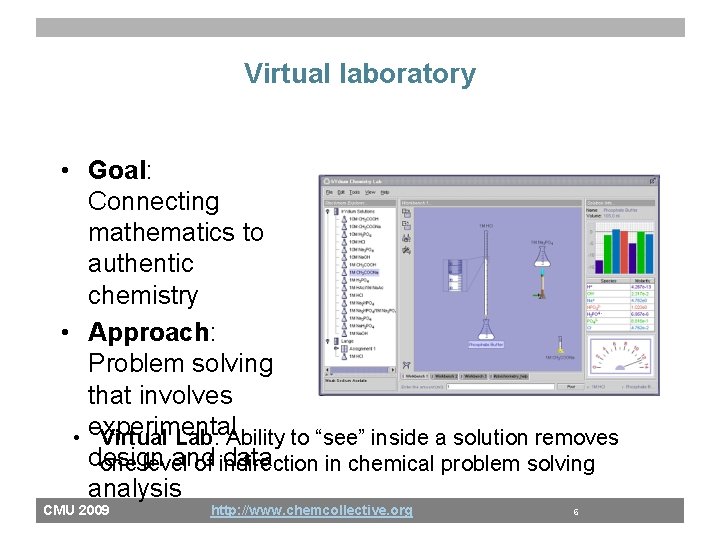
Virtual laboratory • Goal: Connecting mathematics to authentic chemistry • Approach: Problem solving that involves • experimental Virtual Lab: Ability to “see” inside a solution removes design and data one level of indirection in chemical problem solving analysis CMU 2009 http: //www. chemcollective. org 6

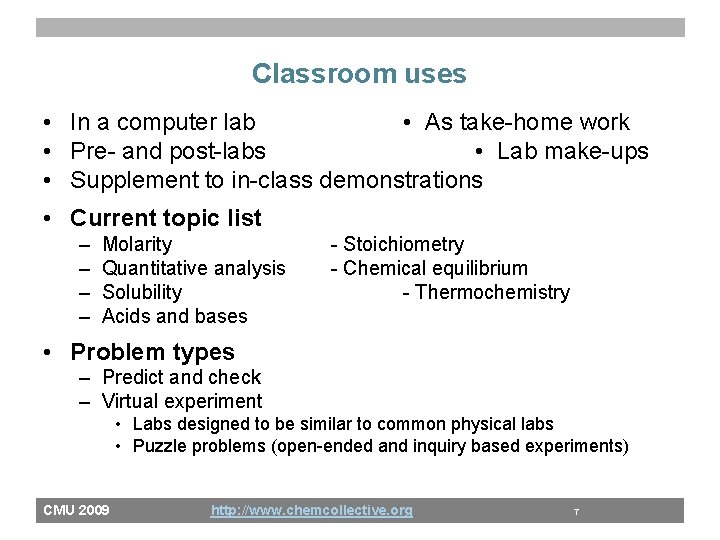
Classroom uses • In a computer lab • As take-home work • Pre- and post-labs • Lab make-ups • Supplement to in-class demonstrations • Current topic list – – Molarity Quantitative analysis Solubility Acids and bases - Stoichiometry - Chemical equilibrium - Thermochemistry • Problem types – Predict and check – Virtual experiment • Labs designed to be similar to common physical labs • Puzzle problems (open-ended and inquiry based experiments) CMU 2009 http: //www. chemcollective. org 7

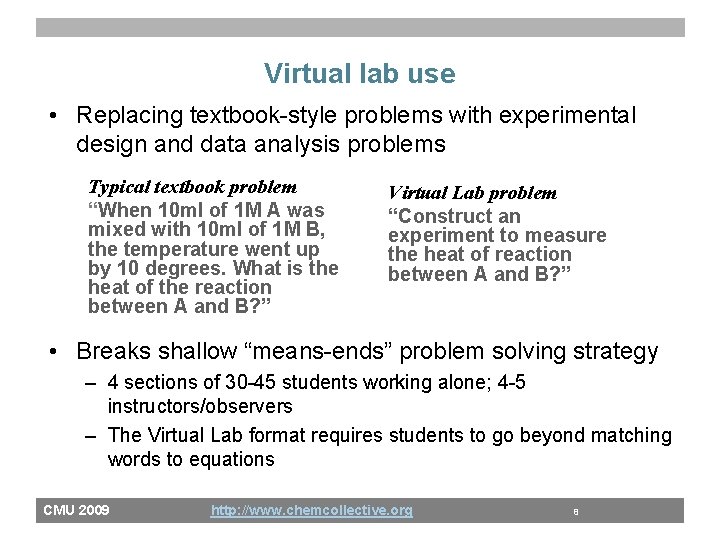
Virtual lab use • Replacing textbook-style problems with experimental design and data analysis problems Typical textbook problem “When 10 ml of 1 M A was mixed with 10 ml of 1 M B, the temperature went up by 10 degrees. What is the heat of the reaction between A and B? ” Virtual Lab problem “Construct an experiment to measure the heat of reaction between A and B? ” • Breaks shallow “means-ends” problem solving strategy – 4 sections of 30 -45 students working alone; 4 -5 instructors/observers – The Virtual Lab format requires students to go beyond matching words to equations CMU 2009 http: //www. chemcollective. org 8

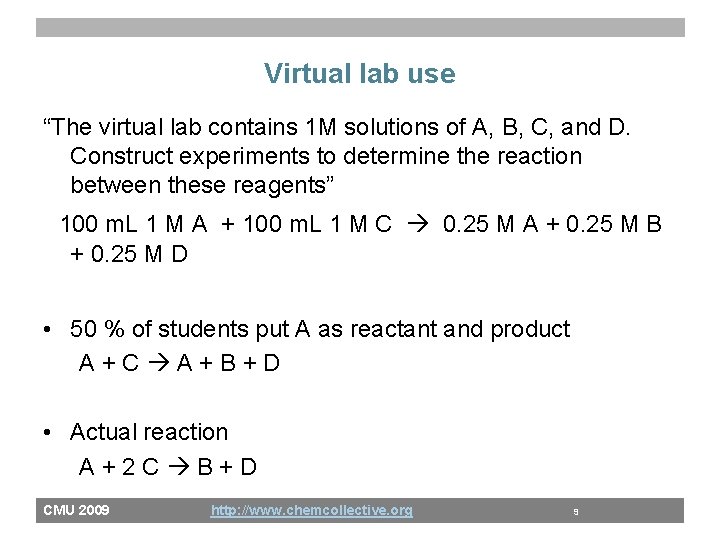
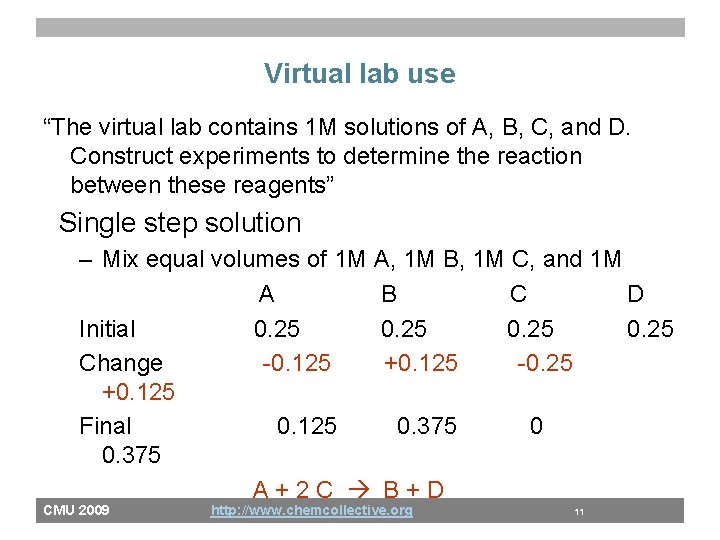
Virtual lab use “The virtual lab contains 1 M solutions of A, B, C, and D. Construct experiments to determine the reaction between these reagents” 100 m. L 1 M A + 100 m. L 1 M C 0. 25 M A + 0. 25 M B + 0. 25 M D • 50 % of students put A as reactant and product A+C A+B+D • Actual reaction A+2 C B+D CMU 2009 http: //www. chemcollective. org 9

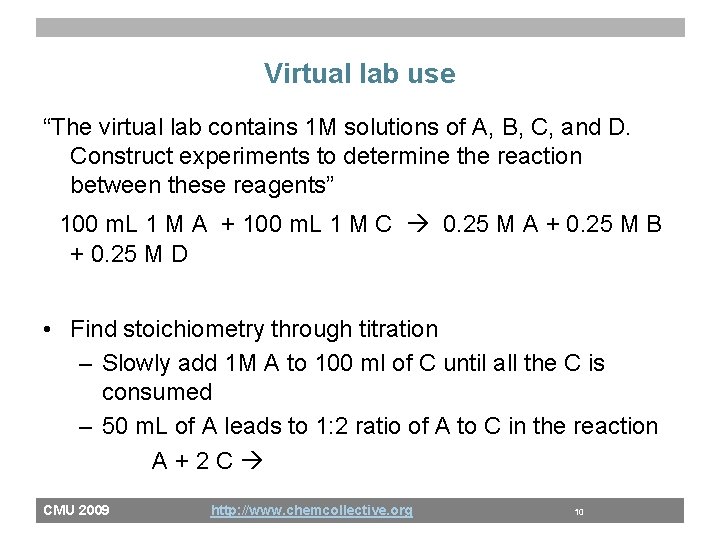
Virtual lab use “The virtual lab contains 1 M solutions of A, B, C, and D. Construct experiments to determine the reaction between these reagents” 100 m. L 1 M A + 100 m. L 1 M C 0. 25 M A + 0. 25 M B + 0. 25 M D • Find stoichiometry through titration – Slowly add 1 M A to 100 ml of C until all the C is consumed – 50 m. L of A leads to 1: 2 ratio of A to C in the reaction A+2 C CMU 2009 http: //www. chemcollective. org 10

Virtual lab use “The virtual lab contains 1 M solutions of A, B, C, and D. Construct experiments to determine the reaction between these reagents” Single step solution – Mix equal volumes of 1 M A, 1 M B, 1 M C, and 1 M A B C D Initial 0. 25 Change -0. 125 +0. 125 -0. 25 +0. 125 Final 0. 125 0. 375 0 0. 375 A+2 C B+D CMU 2009 http: //www. chemcollective. org 11

Assessment within a large lecture course • Study at Carnegie Mellon – Second semester intro course, 150 students • Information used – Pretest – 9 homework activities (virtual labs with templated feedback) – 3 hour exams – 2 pop exams (practice exam given 5 days before hour exam) – Final exam CMU 2009 http: //www. chemcollective. org 12

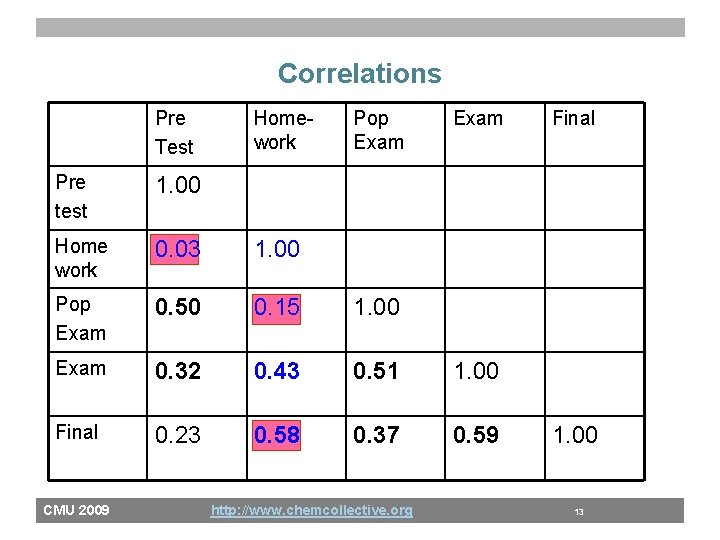
Correlations Pre Test Homework Pop Exam Pre test 1. 00 Home work 0. 03 1. 00 Pop Exam 0. 50 0. 15 1. 00 Exam 0. 32 0. 43 0. 51 1. 00 Final 0. 23 0. 58 0. 37 0. 59 CMU 2009 http: //www. chemcollective. org Final 1. 00 13

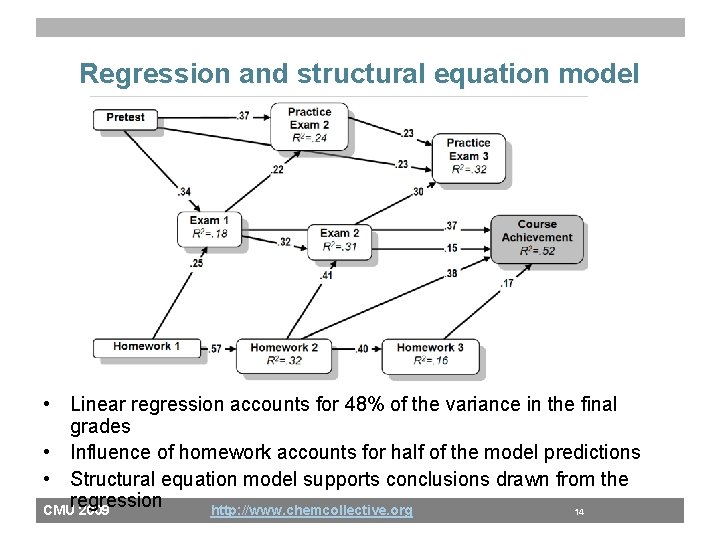
Regression and structural equation model • Linear regression accounts for 48% of the variance in the final grades • Influence of homework accounts for half of the model predictions • Structural equation model supports conclusions drawn from the regression CMU 2009 http: //www. chemcollective. org 14

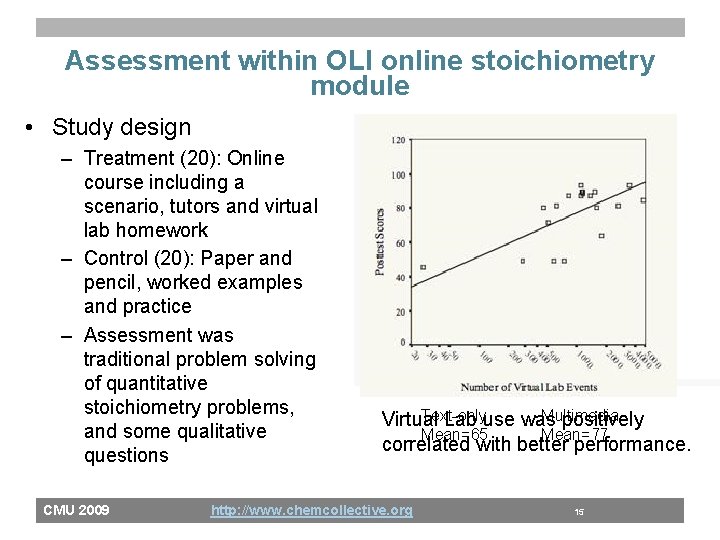
Assessment within OLI online stoichiometry module • Study design – Treatment (20): Online course including a scenario, tutors and virtual lab homework – Control (20): Paper and pencil, worked examples and practice – Assessment was traditional problem solving of quantitative stoichiometry problems, and some qualitative questions CMU 2009 Text-only Multimedia Virtual Lab use was positively Mean=65 Mean=77 correlated with better performance. http: //www. chemcollective. org 15

Conceptual learning in chemistry: What is it? • Virtual laboratory – Connecting mathematics to authentic chemistry • What is needed for scientific literacy? • Teaching chemical equilibrium • Molecular science across disciplines CMU 2009 http: //www. chemcollective. org 16

Conceptual learning in chemistry: What is it? • Virtual laboratory – Connecting mathematics to authentic chemistry • What is needed for scientific literacy? • Teaching chemical equilibrium • Molecular science across disciplines CMU 2009 http: //www. chemcollective. org 17

Traditional high school course structure • CA state standards – – – • Standard 1 Atomic and Molecular Structure Standard 2 Chemical Bonds Standard 3 Conservation of Matter and Stoichiometry Standard 4 Gases and Their Properties Standard 5 Acids and Bases Standard 6 Solutions Standard 7 Chemical Thermodynamics Standard 8 Reaction Rates Standard 9 Chemical Equilibrium Standard 10 Organic Chemistry and Biochemistry Standard 11 Nuclear Processes Current chemistry AP exam guides are similarly structured around chemistry topic list CMU 2009 http: //www. chemcollective. org 18

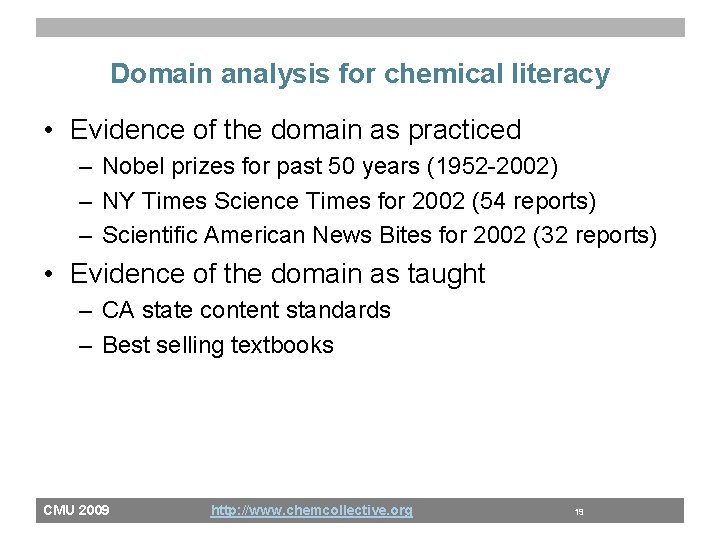
Domain analysis for chemical literacy • Evidence of the domain as practiced – Nobel prizes for past 50 years (1952 -2002) – NY Times Science Times for 2002 (54 reports) – Scientific American News Bites for 2002 (32 reports) • Evidence of the domain as taught – CA state content standards – Best selling textbooks CMU 2009 http: //www. chemcollective. org 19

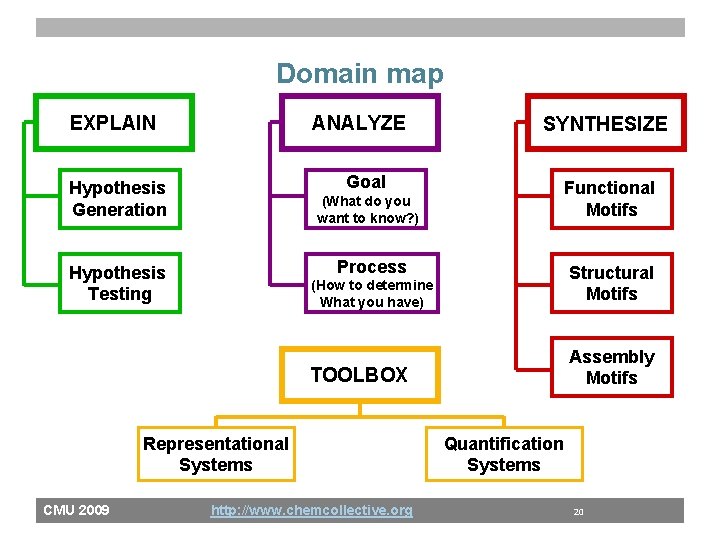
Domain map EXPLAIN ANALYZE Goal Hypothesis Generation (What do you want to know? ) Functional Motifs Process Hypothesis Testing (How to determine What you have) Structural Motifs TOOLBOX Assembly Motifs Representational Systems CMU 2009 SYNTHESIZE http: //www. chemcollective. org Quantification Systems 20

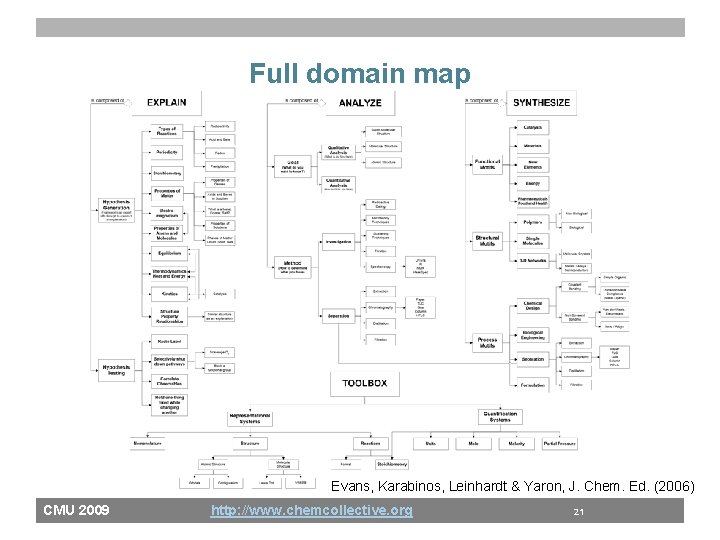
Full domain map Evans, Karabinos, Leinhardt & Yaron, J. Chem. Ed. (2006) CMU 2009 http: //www. chemcollective. org 21

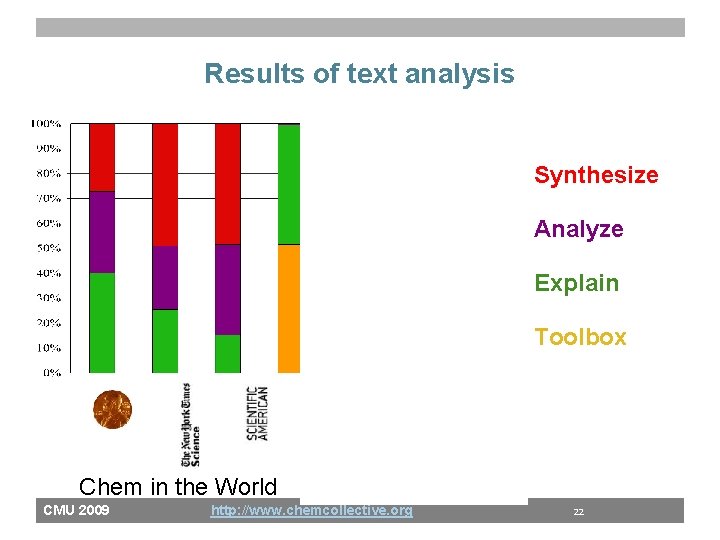
Results of text analysis Synthesize Analyze Explain Toolbox Chem in the World CMU 2009 Chem in Textbooks http: //www. chemcollective. org 22

Scenarios: Examples • Mixed reception (molecular weight, stoichiometry) • Cyanine dyes binding to DNA (equilibrium, Beer’s law) • Meals read-to-eat (thermochemistry) • Mission to mars (redox, thermochemistry) • Arsenic poisoning of wells in Bangladesh (stoichiometry, titration, analytical spectroscopy) • Ozone destruction (kinetics) CMU 2009 http: //www. chemcollective. org 23

Conceptual learning in chemistry: What is it? • Virtual laboratory – Connecting mathematics to authentic chemistry • What is needed for scientific literacy? – Replacing skills focus with knowledge of what chemists do • Teaching chemical equilibrium • Molecular science across disciplines CMU 2009 http: //www. chemcollective. org 24

Conceptual learning in chemistry: What is it? • Virtual laboratory – Connecting mathematics to authentic chemistry • What is needed for scientific literacy? – Replacing skills focus with knowledge of what chemists do • Teaching chemical equilibrium • Molecular science across disciplines CMU 2009 http: //www. chemcollective. org 25

Chemical equilibrium • Goal: Discovery why this topic is so difficult to learn, and try to fix it • Approach: – Domain analysis – Student talk alouds on traditional problems – Discovered “implicit knowledge” that could be made explicit in the instruction CMU 2009 http: //www. chemcollective. org 26

Chemical equilibrium • Goal: Discovery why this topic is so difficult to learn, and try to fix it • Approach: – Domain analysis 1. Utility of the knowledge 2. Detailed structure of the knowledge 3. Psychological aspects of the knowledge – Student talk alouds on traditional problems – Discovered “implicit knowledge” that could be made explicit in the instruction CMU 2009 http: //www. chemcollective. org 27

Chemical equilibrium / Acid-base chemistry 1) Utility of the knowledge – How is this knowledge used in organic chemistry and molecular biology 1) Compare p. H to p. Ka to determine ionization state 2) Buffers used to control p. H (qualitative not quantitative) 3) Titration as an analytical technique – Current instruction 1: Almost a footnote (in the p. H indicators section) 2 -3: Coverage may not be sufficiently qualitative CMU 2009 http: //www. chemcollective. org 28

Chemical equilibrium / Acid-base chemistry 2) Detailed structure of the knowledge – Need to be flexible with “progress of reaction” – General strategy (majority/minority species strategy) 3) Psychological aspects of the knowledge – Le. Chatlier (especially with addition/removal of a species) is most retained concept – Broad confusion regarding “progress of reaction” • Q (current state) vs. K (state towards which system tends) • Meaning of “initial” vs. “equilibrium” state CMU 2009 http: //www. chemcollective. org 29

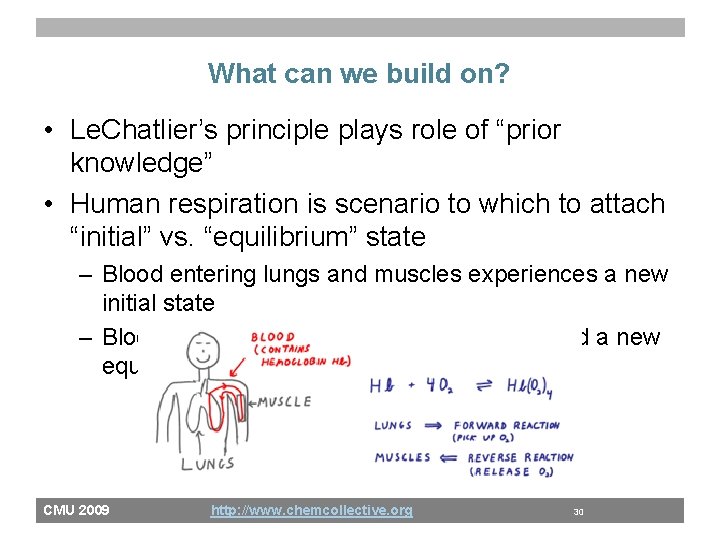
What can we build on? • Le. Chatlier’s principle plays role of “prior knowledge” • Human respiration is scenario to which to attach “initial” vs. “equilibrium” state – Blood entering lungs and muscles experiences a new initial state – Blood leaving lungs and muscles has reached a new equilibrium state CMU 2009 http: //www. chemcollective. org 30

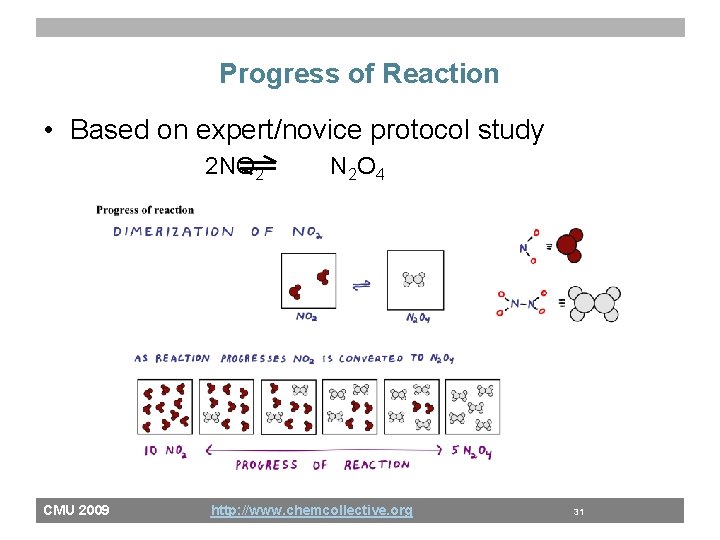
Progress of Reaction • Based on expert/novice protocol study 2 NO 2 CMU 2009 N 2 O 4 http: //www. chemcollective. org 31

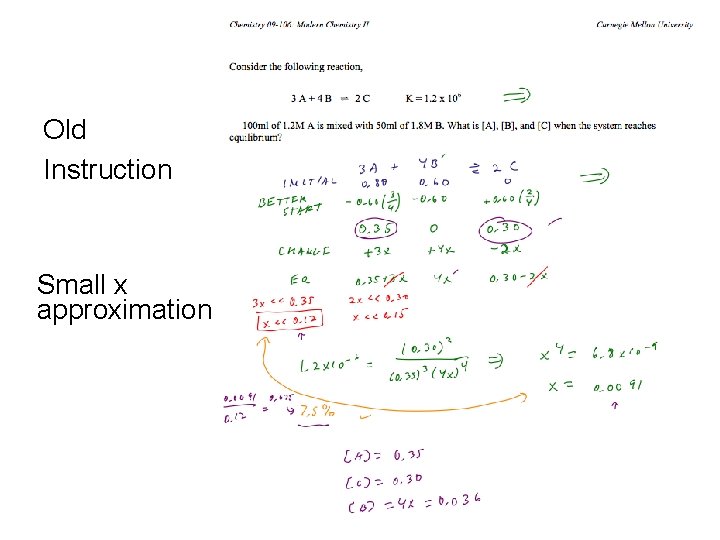
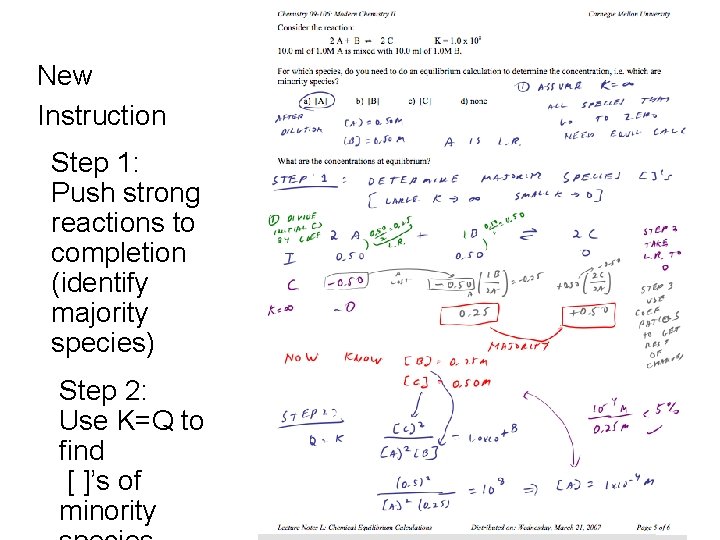
Majority / Minority Problem Solving Strategy • Old instruction – “Small x approximation” – Highly mathematical • New instruction – Majority/minority species strategy – Couples the problem solving steps to qualitative reasoning CMU 2009 http: //www. chemcollective. org 32

Old Instruction Small x approximation CMU 2009 http: //www. chemcollective. org 33

New Instruction Step 1: Push strong reactions to completion (identify majority species) Step 2: Use K=Q to find [ ]’s of CMU 2009 http: //www. chemcollective. org minority 34

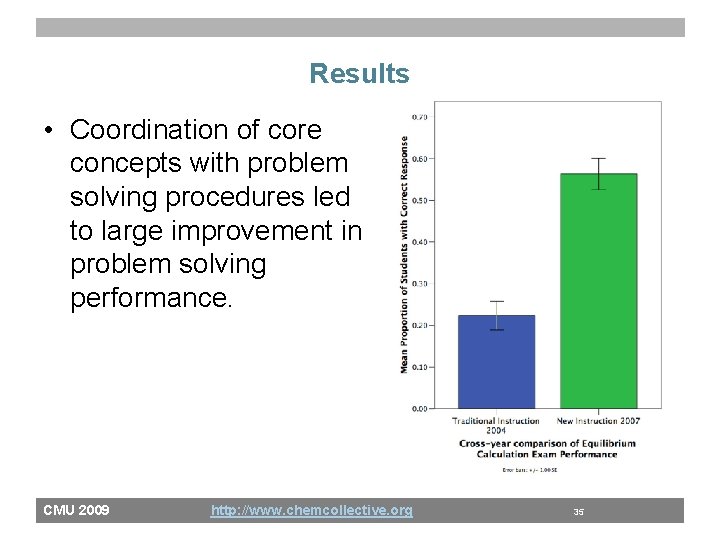
Results • Coordination of core concepts with problem solving procedures led to large improvement in problem solving performance. CMU 2009 http: //www. chemcollective. org 35

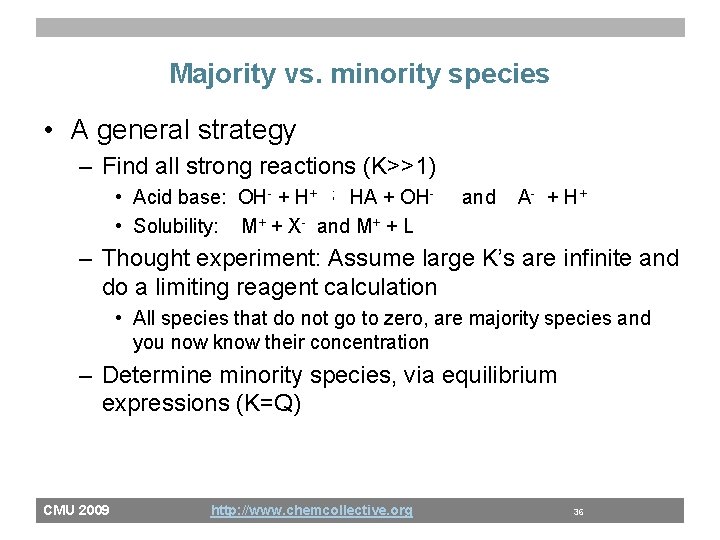
Majority vs. minority species • A general strategy – Find all strong reactions (K>>1) • Acid base: OH- + H+ ; HA + OH • Solubility: M+ + X- and M+ + L and A - + H+ – Thought experiment: Assume large K’s are infinite and do a limiting reagent calculation • All species that do not go to zero, are majority species and you now know their concentration – Determine minority species, via equilibrium expressions (K=Q) CMU 2009 http: //www. chemcollective. org 36

Conceptual learning in chemistry: What is it? • Virtual laboratory – Connecting mathematics to authentic chemistry • What is needed for scientific literacy? – Replacing skills focus with knowledge of what chemists do • Teaching chemical equilibrium – Connecting problem solving procedures to chemical concepts/mental models • Molecular science across disciplines CMU 2009 http: //www. chemcollective. org 37

Conceptual learning in chemistry: What is it? • Virtual laboratory – Connecting mathematics to authentic chemistry • What is needed for scientific literacy? – Replacing skills focus with knowledge of what chemists do • Teaching chemical equilibrium – Connecting problem solving procedures to chemical concepts/mental models • Molecular science across disciplines CMU 2009 http: //www. chemcollective. org 38

Conceptual frameworks that cross disciplines • Scope is molecular science – How molecular structure and motion lead to emergent macroscopic properties – The synthesis/engineering of structures with desirable properties • Build materials for discipline-specific courses, but that use a common core set of materials to show interdisciplinary connections • Experts from multiple domains (chemistry, materials science, biophysics) met to identify concepts/frameworks that are – Central to their domain – Have strong leverage – Are difficult to teach/learn CMU 2009 http: //www. chemcollective. org 39

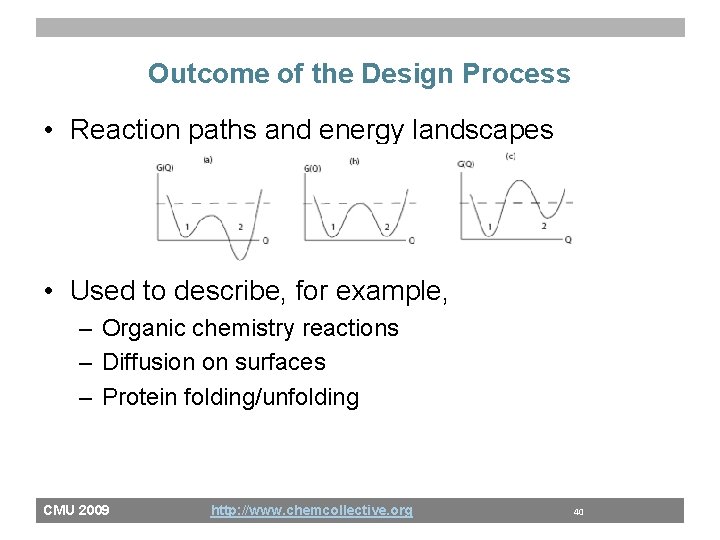
Outcome of the Design Process • Reaction paths and energy landscapes • Used to describe, for example, – Organic chemistry reactions – Diffusion on surfaces – Protein folding/unfolding CMU 2009 http: //www. chemcollective. org 40

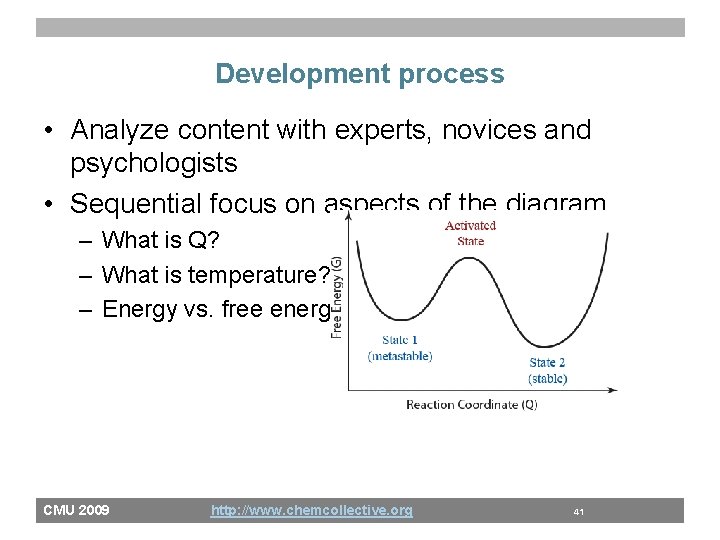
Development process • Analyze content with experts, novices and psychologists • Sequential focus on aspects of the diagram – What is Q? – What is temperature? – Energy vs. free energy CMU 2009 http: //www. chemcollective. org 41

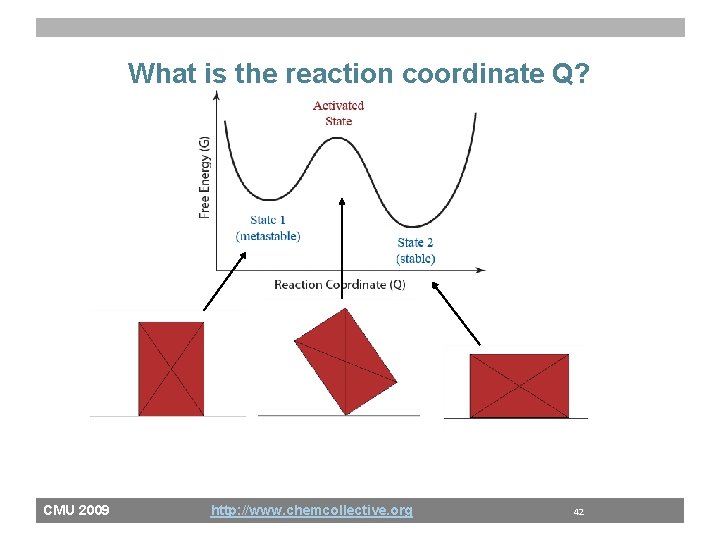
What is the reaction coordinate Q? CMU 2009 http: //www. chemcollective. org 42

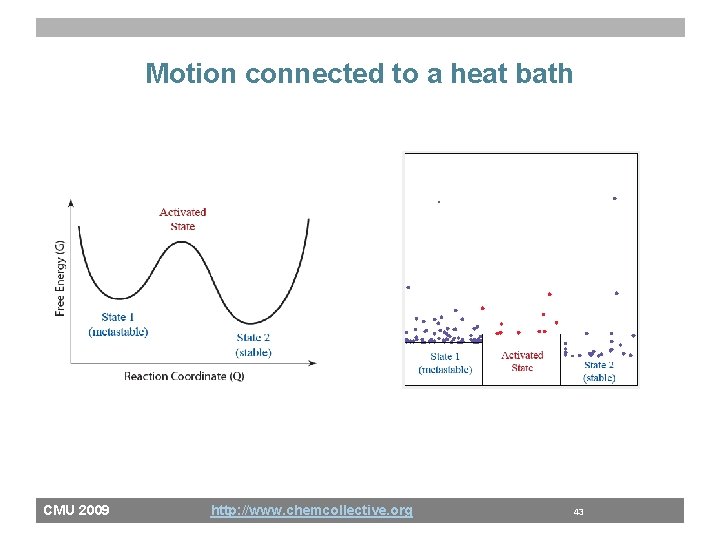
Motion connected to a heat bath CMU 2009 http: //www. chemcollective. org 43

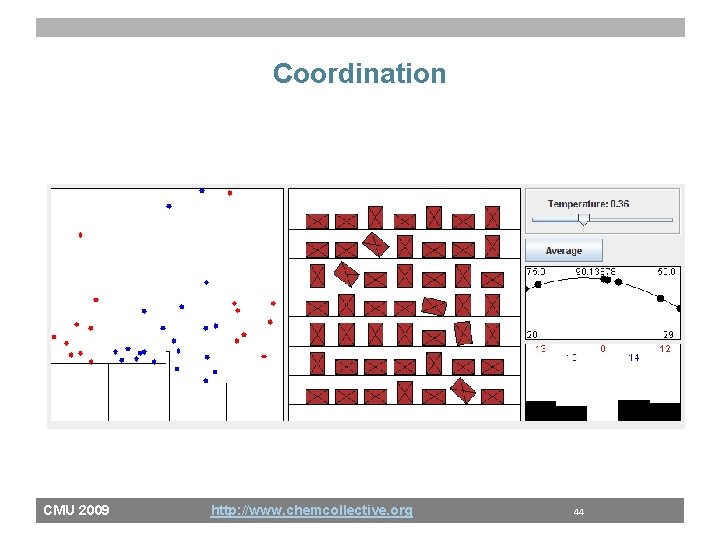
Coordination CMU 2009 http: //www. chemcollective. org 44

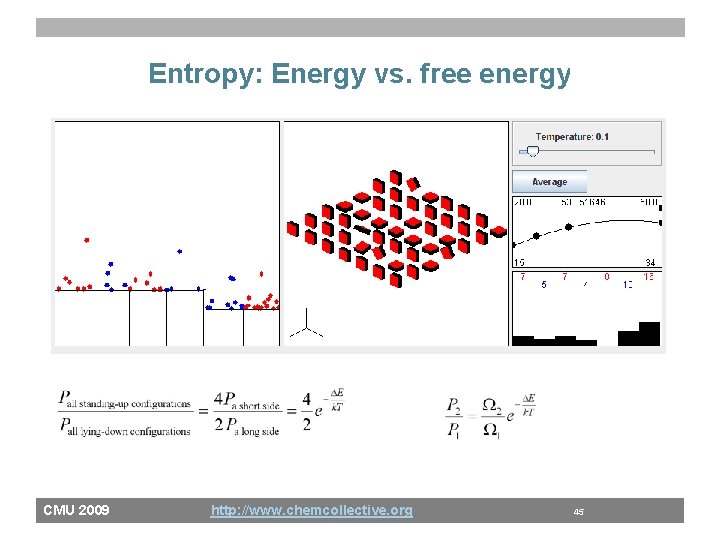
Entropy: Energy vs. free energy CMU 2009 http: //www. chemcollective. org 45

Other conceptual frameworks of molecular science • Reaction paths and energy landscapes • Molecular forces – e. g. Structure formation at different temperatures • Economies of exchange – Heat, proton (acid/base) and electron (redox) exchange • How natural and designed systems promote one chemical process over another – e. g. Kinetic vs. thermodynamic control CMU 2009 http: //www. chemcollective. org 46

Conceptual learning in chemistry: What is it? • Virtual laboratory – Connecting mathematics to authentic chemistry • What is needed for scientific literacy? – Replacing skills focus with knowledge of what chemists do • Teaching chemical equilibrium – Connecting problem solving procedures to chemical concepts/mental model • Molecular science across disciplines – Conceptual frameworks that have broad utility CMU 2009 http: //www. chemcollective. org 47

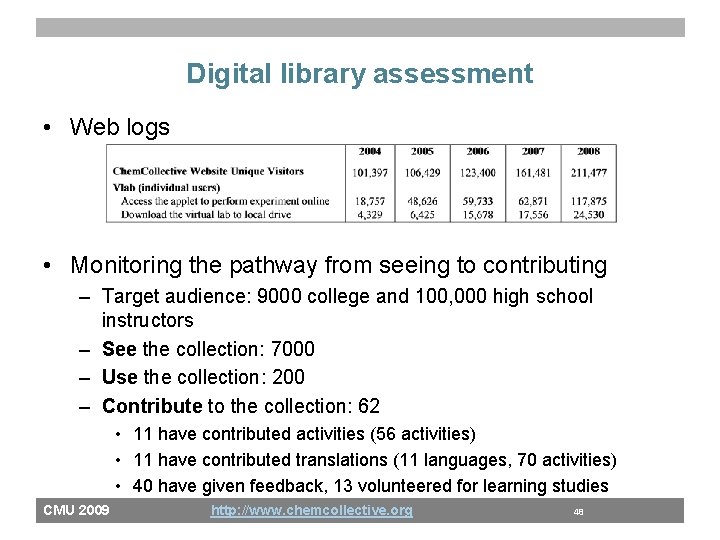
Digital library assessment • Web logs • Monitoring the pathway from seeing to contributing – Target audience: 9000 college and 100, 000 high school instructors – See the collection: 7000 – Use the collection: 200 – Contribute to the collection: 62 • 11 have contributed activities (56 activities) • 11 have contributed translations (11 languages, 70 activities) • 40 have given feedback, 13 volunteered for learning studies CMU 2009 http: //www. chemcollective. org 48

Closing comments • Can digital libraries serve as community spaces for promoting conceptual teaching and learning of chemistry? – Virtual lab does get reused and repurposed • Homework tool • Many instructors find the approach compelling – Chemical equilibrium and cross-disciplinary materials • Too soon to tell – Shifting high school chemistry from skills to literacy • No progress yet CMU 2009 http: //www. chemcollective. org 49

Thanks To • Erin Fried • Jason Chalecki Michael Karabinos • Greg Hamlin Jodi Davenport • Brendt Thomas Donovan Lange • Stephen Ulrich D. Jeff Milton • Jason Mc. Kesson Jordi Cuadros • Aaron Rockoff Rea Freeland • Jon Sung Emma Rehm • Jean Vettel William Mc. Cue • Rohith Ashok David H. Dennis • Joshua Horan Tim Palucka LRDC, University Jef Guarent • Gaea Leinhardt Amani Ahmed • Jim Greeno Giancarlo Dozzi • Karen Evans Katie Chang • Baohui Zhang Carnegie Mellon Funding • • • • CMU 2009 http: //www. chemcollective. org • • NSF: CCLI, NSDL, SLC William and Flora Hewlett Foundation Howard Hughes Medical Institute Dreyfus Foundation of Pittsburgh 50